Supplemental Digital Content is available in the text
Abstract
Nuclear factor-kappaB (NF-κB) is a key inflammatory transcription factor expressed frequently in tumors. Numerous studies have investigated the correlation between NF-κB expression and prognosis in solid tumors, but the conclusions are still in contradiction. Here, we conduct a meta-analysis to explore the overall association of NF-κB overexpression and survival in human solid tumors.
Pubmed and EBSCO databases were searched for studies evaluating expression of NF-κB (as measured by immunohistochemistry) and overall survival (OS) and disease-free survival (DFS) in solid tumors. Published data were extracted and computed into odds ratios (ORs) for death at 3, 5, and 10 years. Data were pooled using the Mantel–Haenszel random-effect model. All statistical tests were two-sided.
Forty-four studies with a total of 4418 patients were included in this meta-analysis. NF-κB overexpression was associated with worse OS at 3 years (OR = 3.40, 95% confidence interval [CI] = 2.41–4.79, P < 0.00001), 5 years (OR = 2.72, 95% CI = 1.92–3.85, P < 0.00001), and 10 years (OR = 2.63, 95% CI = 1.34–5.16, P = 0.005) of solid tumors. Results for 3- and 5-year DFS were similar. NF-κB expression was associated with poor 3-year OS in both Tumor, Lymph Node, Metastasis stage I-II (OR = 9.11, 95% CI = 2.90–28.68, P = 0.0002) and III-IV (OR = 2.59, 95% CI = 1.61–4.15, P < 0.0001). There is no correlation between cellular localization of NF-kB overexpression and OS of solid tumors. Among the tumor types, NF-κB was associated with worse 3 year-OS of colorectal cancer (OR = 2.70, 95% CI = 1.64–4.46, P < 0.0001), esophageal carcinoma (OR = 6.00, 95% CI = 3.29–10.94, P < 0.0001) and worse 5 year-OS of colorectal cancer (OR = 2.72, 95% CI = 1.92–3.85, P < 0.00001), esophageal carcinoma (OR = 5.96, 95% CI = 3.48–10.18, P = 0.03), and nonsmall cell lung cancer (OR = 1.69, 95% CI = 1.20–2.38, P = 0.002).
Expression of NF-κB is associated with worse survival in most solid tumors irrespective of NF-κB localization.
INTRODUCTION
Cancer is an extremely complex fatal disease with accumulation of abnormal genetic/epigenetic and inflammatory alterations during multistep development.1 Inflammatory transcriptional factors, such as signal transducer and activator of transcription 3 and nuclear factor-kappaB (NF-κB), are considered to play a pivotal role in tumor initiation and progression.2,3 Accumulating evidence has demonstrated that persistent activation of inflammatory transcriptional factors facilitate tumor development through various mechanisms, such as providing proliferation and survival advantages, and promoting tumor-related inflammation.4
NF-κB, a transcriptional factor involved in multicellular biology response, was first discovered by Sen and Baltimore in 1986.5 The mammalian NF-κB family consists of 5 protein members: NF-κB1 (p50 and its precursor p105), NF-κB2 (p52 and its precursor p100), RelA (p65), RelB, and c-Rel.6 NF-kB transcriptional factor, as an inactive complex with inhibitory proteins called I-κB in the cytoplasm in normal resting cells,7 can be activated to enter the nucleus where it regulates the expression of diverse genes encoding cytokines, growth factors, cell adhesion molecules, and apoptotic-related proteins.8,9 Aberrant activation of NF-κB has been linked to inflammatory and autoimmune diseases, infection and cancer.10 The first hint to a link between NF-κB and cancer has emerged with the discovery of RelA/p65, and the realization of the close kinship to c-Rel and its oncogenic avian homolog v-Rel.11 Following studies reported that NF-κB was constitutively activated in various malignancies such as lymphoma,12 gastrointestinal tumor,13–15 genitourinary,16,17 gynecological,18,19 thoracic,20 head, and neck tumors.21,22 Notably, the presence of activated NF-κB in a tumor is not necessarily causal. NF-κB is involved in most if not all cellular processes in tumor evolution including inflammation, transformation, proliferation, angiogenesis, invasion, metastasis, chemoresistance, and radioresistance.23 Given the tumor promoting role of NF-κB, targeting NF-κB for tumor prevention and therapy might be beneficial. A myriad of studies have investigated the correlation between NF-κB overexpression and prognosis in solid tumors. However, the prognostic value of NF-κB overexpression across different solid tumors is still in contradiction.
We therefore conducted an exhaustive meta-analysis combining evidence to evaluate the prognostic impact of NF-κB overexpression in solid tumors. We also evaluated whether the outcome differs between nuclear and cytoplasmic NF-κB expression and between different tumor types. This meta-analysis aimed to evaluate the role of NF-κB in relation to survival in solid tumors, thereby supporting more rational development of therapeutic strategies against NF-κB.
MATERIAL AND METHODS
This meta-analysis was conducted according to preferred reporting items for systematic reviews and meta-analyses statement.24 This study based on summary and analysis of the results of previous studies, so the ethical approval was not necessary.
Identification and Selection of Studies
Pubmed and EBSCO were searched for studies evaluating the correlation between NF-κB expression and survival in solid tumors from 2004 to January 2015. The search terms “NF-κB” and “neoplasms” were used and only human studies of solid tumors were included. In addition, the entry “NF-κB” and the name of each specific solid tumor were used to screen additional studies. We identified a total of 9168 and 13,092 entries, respectively. The inclusion criteria for selecting articles were the measurement of NF-κB by immunohistochemistry (IHC), availability of survival data for at least 3 years, and publication in English. Studies evaluating gene expression of NF-κB measured by real-time polymerase chain reaction were excluded. We screened the citation lists of retrieved articles to ensure sensitivity of the search strategy. Interreviewer agreement was evaluated using Cohen kappa coefficient. Disagreement was resolved by discussion and a final consensus was achieved.
Endpoints of Interest
Overall survival (OS) at 3, 5, and 10 years were the primary endpoints. Where not available, data for disease-free survival (DFS) at 3 and 5 years were collected. Tumors were classified by NF-κB expression status using cut-off value as defined by each study.
Data Collection Process and Quality Assessment
Two authors (DW and PW) independently reviewed and extracted data using predefined data abstraction forms from each eligible studies. Extracted information included first author's name, publication year, country, type of cancer, number of patients, median age, gender, Tumor, Lymph Node, Metastasis (TNM) stage, time of follow-up, antibody used for the evaluation, technique used to quantify NF-κB, and cut-off value to determine NF-κB positivity. OS and DFS data were extracted from the text, tables, or Kaplan–Meier curves for both NF-κB negative and NF-κB positive group.
The studies included in this meta-analysis were cohort studies. The quality of each included cohort study was evaluated using Newcastle–Ottawa Scale (NOS) by 2 independent authors.25 The studies with 6 scores or more were classified as high quality studies. A consensus NOS score for each item was achieved.
Data Synthesis
The relative frequency of 3-, 5-, and 10-year OS or DFS between NF-κB negative and NF-κB positive group was expressed as an odds ratio (OR) and its 95% confidence interval (CI). Sensitivity analyses were carried out for different analytical methods and cut-offs for defining NF-κB expression and NOS scores for quality assessment of included studies.
Statistical Analysis
Data were extracted, computed into ORs, and pooled using the Mantel–Haenszel random-effect model by RevMan 5.3 analysis software (Cochrane Collaboration, Copenhagen, Denmark). Heterogeneity was evaluated with the Cochran's Q and I2 statistics. Statistical differences between subgroups were assessed using the methods described in Cochrane Handbook for Systematic Reviews of Interventions.26 Meta-regression analysis was conducted using Stata 12.0 software (StataCorp LP, College Station, TX). All statistical tests were 2-sided, and P values less than.05 were defined statistically significant.
RESULTS
Search Results
Literature searches yield 22,260 records and the results are shown in Figure 1. The potentially relevant articles were screened for eligibility by duplication, language, abstract and article type, and 13,101 records were excluded. Next, 9114 citations were excluded for detailed evaluation and at last 44 studies with survival data were included.
FIGURE 1.
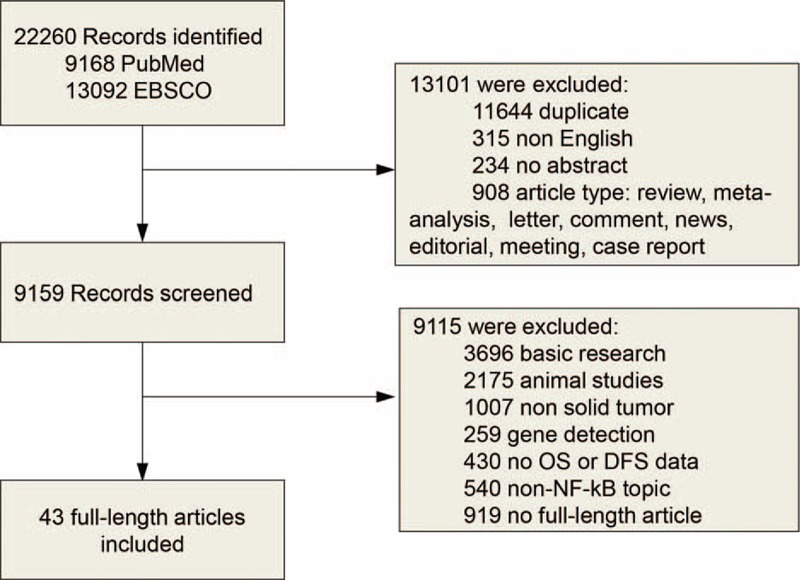
Flow diagram of study selection. NF-κB = nuclear factor-kappaB.
Description of Studies
Characteristics of studies including OS and DFS data are shown in Table 1 . Data were available for OS from 43 studies. Nine evaluated colorectal cancer,27–34 5 evaluated esophageal carcinoma,15,35–38 5 evaluated gastric cancer,14,39–42 5 evaluated ovarian cancer,18,43–46 4 evaluated lung cancer,47–50 2 studies evaluated cervical cancer,19,51 2 evaluated laryngeal squamous cell carcinoma,52,53 2 evaluated nasopharyngeal carcinoma,54,55 and 1 each evaluated salivary glands carcinoma,56 astrocytomas,57 gallbladder carcinoma,58 head and neck squamous cell carcinoma,59 cholangiocarcinoma,60 oral squamous cell carcinoma,61 prostate cancer,62 urothelial carcinoma,63 and pancreatic cancer.64 For DFS, 9 studies provided data at 3 years,15,34,37,38,41,48,55,62,65 and 7 studies provided data at 5 years.37–39,41,48,55,62 A total of 4418 patients were included in those studies.
TABLE 1.
Characteristics of Studies Including OS and DFS in the Meta-Analysis
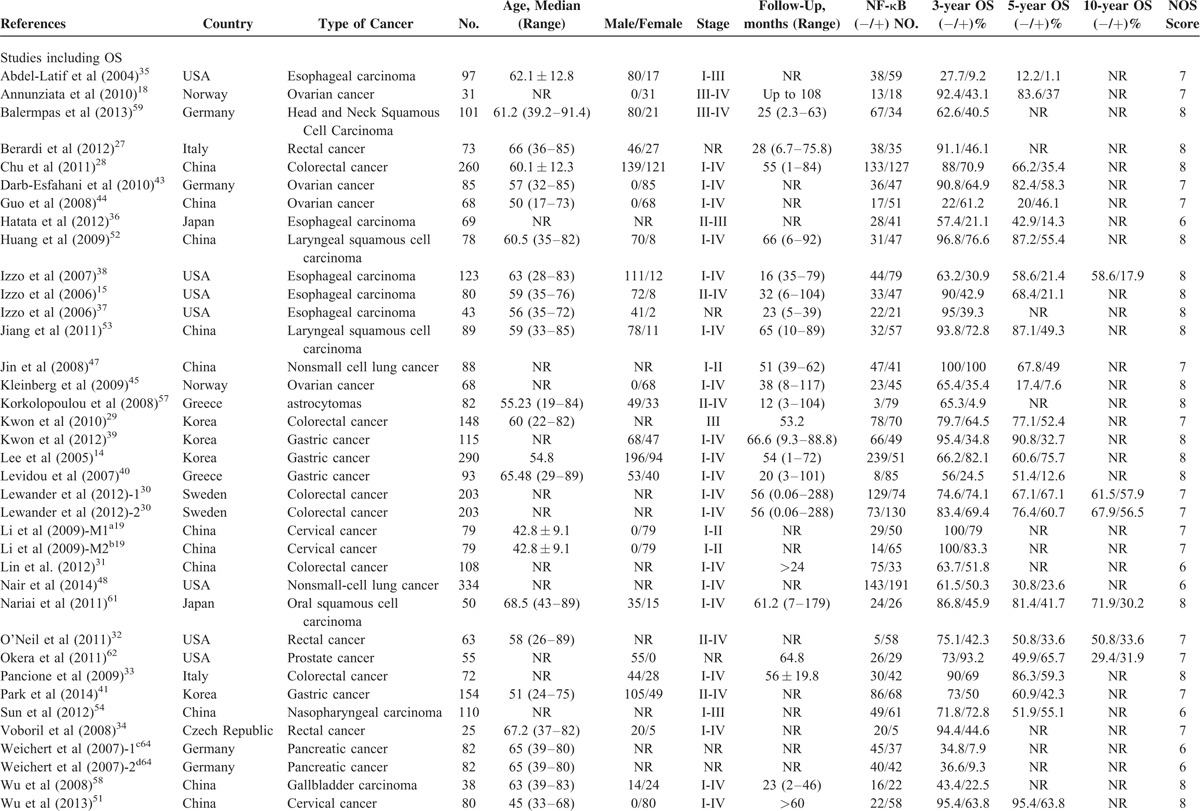
TABLE 1 (Continued).
Characteristics of Studies Including OS and DFS in the Meta-Analysis
Evaluation and Expression of NF-κB
A description of the antibodies, detection, and definition method of NF-κB used in the included studies is shown in Table S1, http://links.lww.com/MD/A445. Diverse antibodies were used for the evaluation of NF-κB expression. For NF-κB p50 antibody, 4 studies used clone sc114, 1 study used clone sc1190, and 1 study did not report the clone. For NF-κB p65 antibody, 4 studies used clone sc8008, 3 studies used clone G96-337, 6 studies used clone sc109, 1 study each used clone sc372, sc502, D14E12, and 24 studies did not report the clone. The cut-off for NF-κB positivity depended on the staining score and the detection method used. Among the groups determined as NF-κB positive, the median expression of NF-κB staining was 55.33%. NF-κB expression in solid tumors ranged 17.59% to 96.34%.
Association of NF-κB With OS
There were 41 studies reporting 3-year OS data. Results showed that NF-κB overexpression in tumor tissue was associated with unfavorable 3-year OS of solid tumors (OR = 3.40, 95% CI = 2.41–4.79, P < 0.00001) (Fig. 2). Significant heterogeneity among studies (Cochran Q P < 0.00001, I2 = 74%) was observed, so we conducted meta-regression analysis and subgroup meta-analysis to explore the possible sources of heterogeneity.
FIGURE 2.
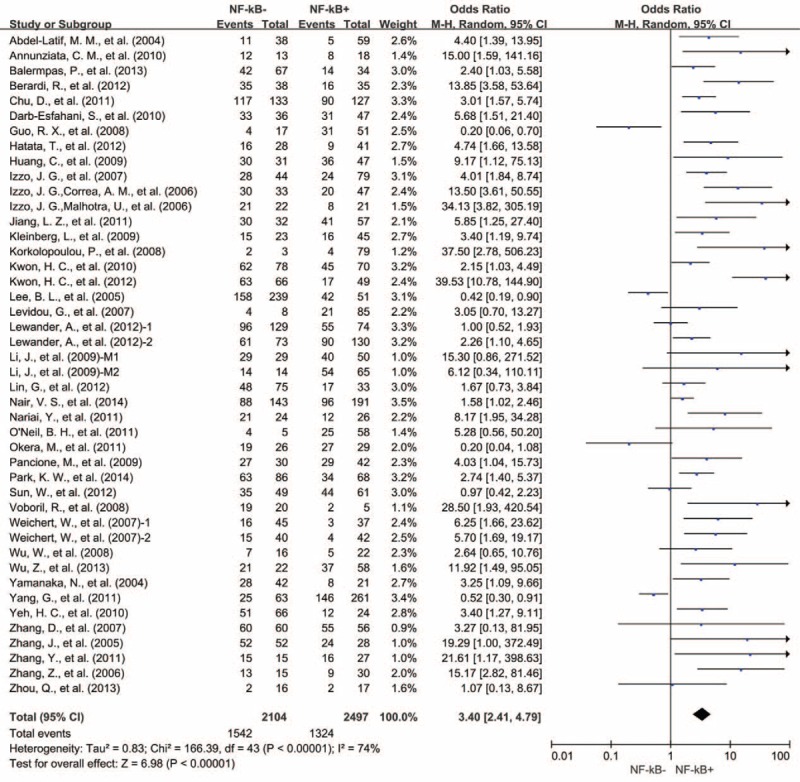
Three-year OS by NF-κB expression. M1 = Marker 1, NF-kB p65; M2 = Marker 2, NF-kB p50, NF-κB = nuclear factor-kappaB, OS = overall survival, 1 = nuclear expression; 2 = cytoplasmic expression.
Eight studies provided 3-year OS for colorectal cancer, 5 studies for esophageal carcinoma, 5 studies for gastric cancer, 4 studies for lung cancer, and 5 studies for ovarian cancer. In the stratified analysis by type of cancer, NF-κB overexpression was associated with worse 3-year OS of colorectal cancer (OR = 2.70, 95% CI = 1.64–4.46, P < 0.0001) and esophageal carcinoma (OR = 6.00, 95% CI = 3.29–10.94, P < 0.00001) (Fig. 3). However, there was no significant association between NF-κB overexpression and 3-year OS of gastric cancer (OR = 3.21, 95% CI = 0.83–12.45, P = 0.09), nonsmall cell lung cancer (OR = 3.84, 95% CI = 0.72–20.49, P = 0.12), and ovarian cancer (OR = 1.69, 95% CI = 0.43–6.55, P = 0.45) (Figure S1, http://links.lww.com/MD/A445).
FIGURE 3.
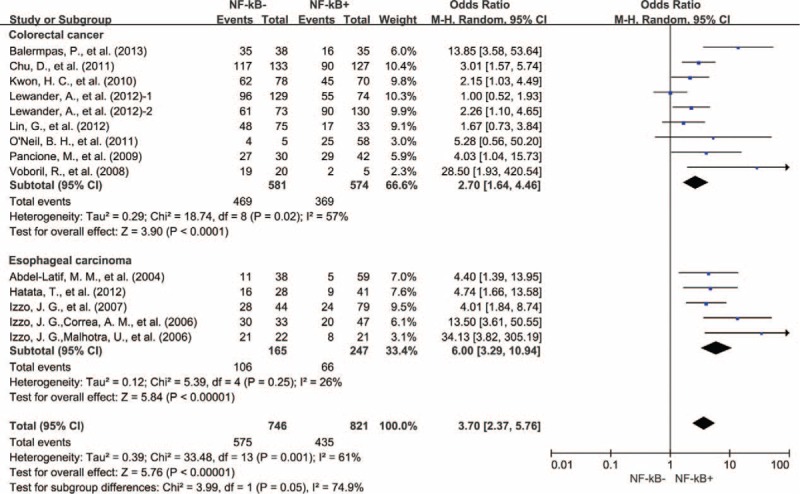
Subgroup analysis of 3-year OS by NF-κB expression in different tumor types. NF-κB = nuclear factor-kappaB, OS = overall survival, 1 = nuclear expression; 2 = cytoplasmic expression.
We also evaluated the correlation between NF-κB overexpression and the TNM stage of tumor. High expression level of NF-κB was significantly associated with TNM stage III-IV (OR = 0.5, 95% CI = 0.33–0.77, P = 0.001) (Figure S2, http://links.lww.com/MD/A445). Next, we conducted subgroup meta-analysis according to TNM stage. NF-κB expression was associated with poor 3-year OS in both TNM stage I-II (OR = 9.11, 95% CI = 2.90–28.68, P = 0.0002) and III-IV (OR = 2.59, 95% CI = 1.61–4.15, P < 0.0001) (Fig. 4).
FIGURE 4.
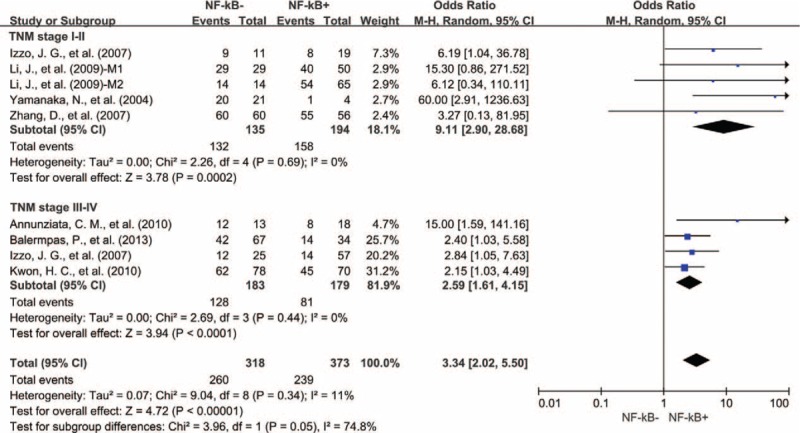
Subgroup analysis of 3-year OS by NF-κB expression in different TNM stages of tumor. NF-κB = nuclear factor-kappaB, OS = overall survival, M1 = Marker 1, NF-kB p65; M2 = Marker 2, NF-kB p50.
The association of NF-κB expression and decreased 3-year OS seemed independent of NF-κB localization, with similar result for studies including only nuclear expression (OR = 3.75, 95% CI = 1.90–7.42, P < 0.0001), those including only cytoplasmic expression (OR = 3.97, 95% CI = 2.15–6.95, P < 0.0001) and those with unselected NF-κB expression (OR = 3.15, 95% CI = 1.82–5.45, P < 0.0001) (Figure S3, http://links.lww.com/MD/A445).
Meta-regression analysis showed that publication year, country, age, gender, and NOS score did not contribute to the heterogeneity (data not shown).
Thirty-two studies reported 5-year OS data. Analogous result was observed with 3-year OS data that NF-κB overexpression was significantly correlated with poor 5-year OS of solid tumors (OR = 2.72, 95% CI = 1.92–3.85, P < 0.00001) (Fig. 5). Heterogeneity among studies was also very high (Cochran Q P < 0.00001, I2 = 77%); therefore, we conducted subgroup meta-analysis according to type of cancer for heterogeneity exploration.
FIGURE 5.
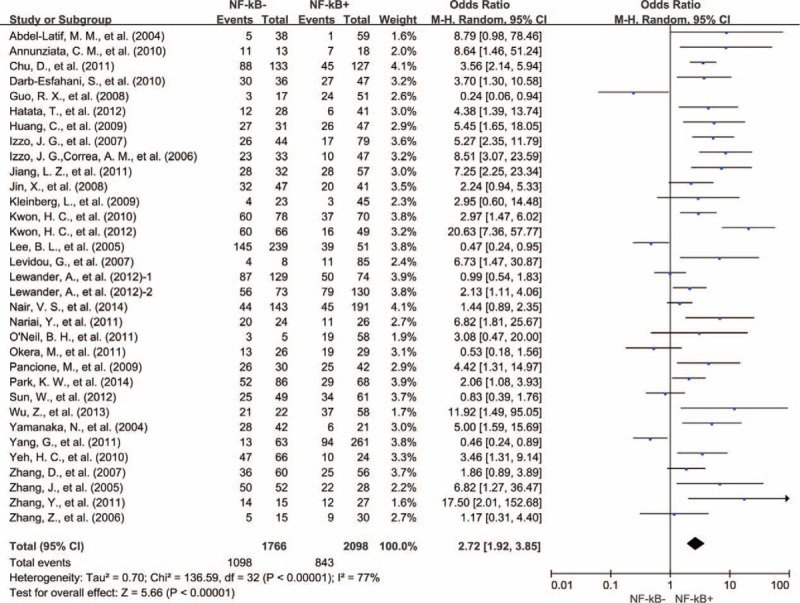
Five-year OS by NF-κB expression. NF-κB = nuclear factor-kappaB, OS = overall survival, 1 = nuclear expression; 2 = cytoplasmic expression.
Five studies provided 5-year OS for colorectal cancer, 4 studies for esophageal carcinoma, 5 studies for gastric cancer, 4 studies for lung cancer, and 5 studies for ovarian cancer. NF-κB overexpression was associated with worse 5-year OS of colorectal cancer (OR = 2.40, 95% CI = 1.48–3.90, P < 0.00001), esophageal carcinoma (OR = 5.96, 95% CI = 3.48–10.18, P < 0.00001), and nonsmall cell lung cancer (OR = 1.69, 95% CI = 1.20–2.38, P = 0.002) (Fig. 6). However, there was no significant association between NF-κB overexpression and the 5-year OS of gastric cancer (OR = 3.48, 95% CI = 0.93–13.03, P = 0.06) and ovarian cancer (OR = 1.46, 95% CI = 0.41–5.21, P = 0.56) (Figure S4, http://links.lww.com/MD/A445).
FIGURE 6.
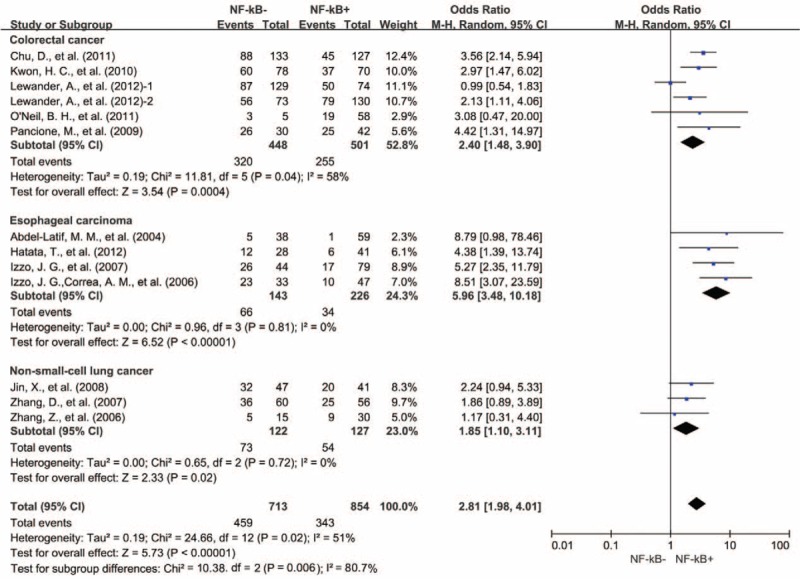
Subgroup analysis of 5-year OS by NF-κB expression in different tumor types. NF-κB = nuclear factor-kappaB, OS = overall survival, 1 = nuclear expression, 2 = cytoplasmic expression.
Similar to 3-year OS results, the association of NF-κB expression and decreased 5-year OS seemed independent of NF-κB localization, with similar result for studies including only nuclear expression (OR = 2.61, 95% CI = 1.18–5.77, P = 0.02), those including only cytoplasmic expression (OR = 3.27, 95% CI = 1.82–5.89, P < 0.0001) and those with unselected NF-κB expression (OR = 2.58, 95% CI = 1.59–4.19, P = 0.0001) (Figure S5, http://links.lww.com/MD/A445).
Results from 8 studies showed that NF-κB overexpression was significantly associated with worse 10-year OS of solid tumors (OR = 2.63, 95% CI = 1.34–5.16, P = 0.005) (Fig. 7).
FIGURE 7.
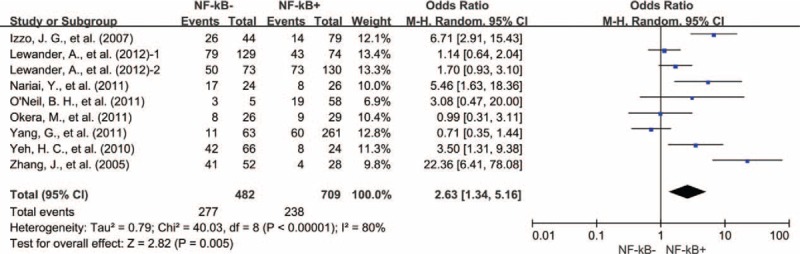
Ten-year OS by NF-κB expression. NF-κB = nuclear factor-kappaB, OS = overall survival, 1 = nuclear expression, 2 = cytoplasmic expression.
Association of NF-κB With DFS
A total of 9 studies reported results for 3-year DFS. There was no statistically significant heterogeneity between studies (Cochran Q P = 0.05, I2 = 49%). NF-κB expression was associated with a statistically significant worse 3-year DFS (OR = 3.17, 95% CI = 1.87–5.38, P < 0.0001) (Fig. 8). Seven studies reported results for 5-year DFS. NF-κB expression was associated with an unfavorable 5-year DFS (OR = 4.65, 95% CI = 2.39–9.06, P < 0.00001) (Fig. 6). However, there was significant heterogeneity among studies (Cochran Q P = 0.01, I2 = 64%).
FIGURE 8.
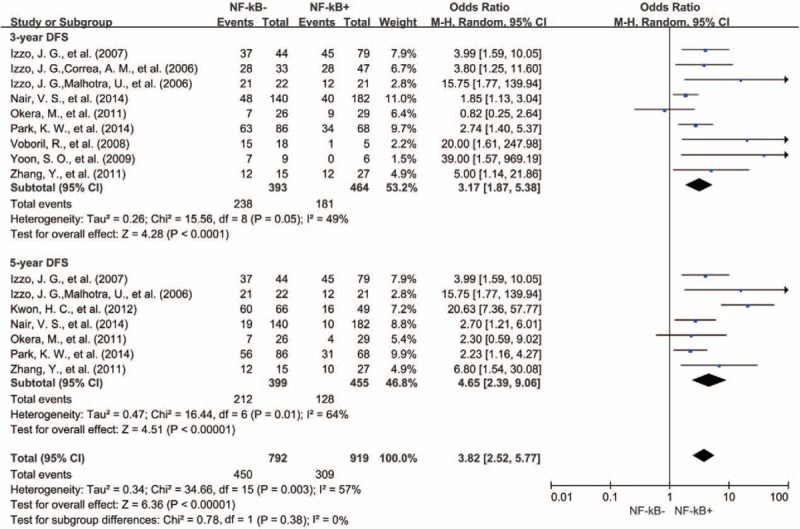
Forest plots showing odds ratios of human NF-κB overexpression versus normal NF-κB expression for DFS at 3 and 5 years. DFS = disease free survival, NF-κB = nuclear factor-kappaB.
Sensitivity Analyses
Removal of the studies that was an outlier (score, IRS, >50% vs 1%–25% for other studies) or no report (NR) with regard to the cut-off of NF-κB overexpression by IHC did not influence results for 3- or 5-year OS (OR = 4.33, 95% CI = 2.84–6.60, P < 0.00001; OR = 2.81, 95% CI = 1.83–4.31, P < 0.00001, respectively), but changed the result for 10-year OS (OR = 1.69, 95% CI = 0.75–3.83, P = 0.21). Exclusion of these studies did not reduce heterogeneity for 3- or 5-year OS (Cochran Q P < 0.00001, I2 = 72%; Cochran Q P < 0.00001, I2 = 79%, respectively).
Removal of studies using marker p50 or no report to assess the expression of NF-κB by IHC (p50, NR vs p65 for other studies) did not substantially affect the association between NF-κB expression and worse 3-, 5-, or 10-year OS compared with no NF-κB expression (OR = 3.08, 95% CI = 2.14–4.43, P < 0.00001; OR = 2.65, 95% CI = 1.84–3.83, P < 0.00001, OR = 2.61, 95% CI = 1.28–5.33, P = 0.008, respectively). Exclusion of these studies did not reduce heterogeneity for 3-, 5-, or 10-year OS (Cochran Q P < 0.00001, I2 = 77%; Cochran Q P < 0.00001, I2 = 79%, Cochran Q P < 0.00001, I2 = 82%, respectively).
Removal of studies with NOS score 6 did not influence results for 3-, 5-, or 10-year OS (OR = 2.94, 95% CI = 2.47–3.49, P < 0.00001; OR = 3.23, 95% CI = 2.23–4.67, P < 0.00001; OR = 3.17, 95% CI = 1.59–6.34, P = 0.001, respectively). Exclusion of these studies did not reduce heterogeneity for 3-, 5-, or 10-year OS (Cochran Q P < 0.00001, I2 = 70%; Cochran Q P < 0.00001, I2 = 73%, Cochran Q P < 0.00001, I2 = 73%, respectively).
Publication Bias
Funnel plot analysis and Egger test showed that there was no statistical evidence of publication bias in our meta-analysis (data not shown).
DISCUSSION
Multiple approaches for targeting NF-κB to treat cancer have been put forward. However, there remain unanswered questions about the effect of NF-κB on outcome and whether the outcome is consistent among different types of solid tumor. Our comprehensive meta-analysis of 4418 patients included in 44 different studies demonstrates that the expression of NF-κB is a marker of poor prognosis. This effect appeared independent of the TNM stage of tumor and NF-κB localization.
Experimental data from laboratories have established strong support for the critical role of NF-κB in tumor development and progression.66 Therefore, targeting NF-κB is thought to be a potent node of pharmacological interference against tumors and acquires clinical benefit response. However, there is no evidence on the basis of the evidence-based medicine with regard to the association between NF-κB expression and worse survival of tumors to date. Our meta-analysis shows that NF-κB overexpression is associated with worse 3-, 5-, 10-year OS and 3-, 5-year DFS of solid tumors. Moreover, NF-κB expression is associated with poor survival in both TNM stage I-II and III-IV. These results suggest that NF-κB is a valuable biomarker for prognostic prediction for solid tumors.
Among the tumor types evaluated, the expression of NF-κB is associated with worse 3- and 5-year OS for colorectal cancer and esophageal carcinoma, while NF-κB is not significantly associated with either 3- or 5-year OS for gastric cancer and ovarian cancer. This suggests that the prognostic significance of NF-κB depends on the types of tumor. Our study may provide strong supporting evidence for blocking NF-κB as a target for clinical intervention of different solid tumors in future.
From a mechanistic perspective, nuclear expression is considered an active marker of NF-κB, whereas cytoplasmic localization of NF-κB is generally thought to indicate inactivation of the pathway.67 However, 1 interesting finding of our study was that the expression of NF-κB, either nuclear or cytoplasmic, was correlated with unfavorable prognosis of solid tumors. Studies on kinetics of nuclear translocation of NF-κB proteins following activation demonstrated that only −10% of the total Rel and p50 proteins, which were freed from IκB, was detected in the nucleus and most of the NF-κB proteins remained within the cytoplasm.68 Moreover, Tam et al69 found that cytoplasmic localization of p65 required the nuclear export receptor CRM1, indicating that cytoplasmic NF-κB p65 may be a secondary event due to coactivation of other biological factors. These experimental researches might explain that cytoplasmic localization of NF-κB also has its clinical significance in tumor outcome.
This study has several important implications. First, it shows that NF-κB expression is associated with worse outcome of solid tumors, which suggests that NF-κB may be a potential therapeutic target. Second, it identifies a subgroup of tumors with unfavorable outcome potentially in colorectal cancer and esophageal carcinoma. Third, it highlights the relevance of NF-κB expression with adverse outcome of solid tumors is independent of cellular localization. Finally, it emphasizes the importance of the development of a valuable biomarker for prognostic assessment.
Some limitations also exist in this meta-analysis. First, the method and cut-off values for assessing NF-κB expression are inconsistent. Second, significant heterogeneity observed across studies cannot be completely accounted despite the use of appropriate meta-analytic techniques with random-effect models. Finally, small studies with negative results may not be published, which can cause publication bias.
In conclusion, our analyses show that overexpression of NF-κB in solid tumor tissues, as measured by IHC, is associated with a worse prognosis in different types of tumor, which suggests that targeting NF-κB could be a promising therapeutic approach for solid tumors.
Acknowledgments
The authors thank the members of the laboratory for helpful discussions. This work was supported by grants from the National Natural Science Foundation of China (81572800, PW), the Science and Technology Department of Zhejiang Province (2011c13034∼1, JH), and Natural Science Foundation of Zhejiang Province (Z2100366, JH and LY15H160041, PW).
Footnotes
Abbreviations: CI = confidence interval, DFS = disease-free survival, IHC = immunohistochemistry, NF-κB = nuclear factor-kappaB, NOS = Newcastle–Ottawa Scale, ORs = odds ratios, OS = overall survival.
Supplemental Digital Content is available for this article.
DW and PW contributed equally to this work.
This work was supported by grants from the National Natural Science Foundation of China (81572800, PW), the Science and Technology Department of Zhejiang Province (2011c13034∼1, JH), and Natural Science Foundation of Zhejiang Province (Z2100366, JH and LY15H160041, PW)
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646–674. [DOI] [PubMed] [Google Scholar]
- 2.Yu H, Lee H, Herrmann A, et al. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer 2014; 14:736–746. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol 2011; 12:715–723. [DOI] [PubMed] [Google Scholar]
- 4.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev 2010; 21:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell 1986; 47:921–928. [DOI] [PubMed] [Google Scholar]
- 6.Nabel GJ, Verma IM. Proposed NF-kappa B/I kappa B family nomenclature. Genes Dev 1993; 7:2063. [DOI] [PubMed] [Google Scholar]
- 7.Karin M, Cao Y, Greten FR, et al. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2002; 2:301–310. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell 2002; 109 (Suppl):S81–S96. [DOI] [PubMed] [Google Scholar]
- 9.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol 2002; 3:221–227. [DOI] [PubMed] [Google Scholar]
- 10.Papa S, Bubici C, Zazzeroni F, et al. The NF-kappaB-mediated control of the JNK cascade in the antagonism of programmed cell death in health and disease. Cell Death Differ 2006; 13:712–729. [DOI] [PubMed] [Google Scholar]
- 11.Nolan GP, Ghosh S, Liou HC, et al. DNA binding and I kappa B inhibition of the cloned p65 subunit of NF-kappa B, a rel-related polypeptide. Cell 1991; 64:961–969. [DOI] [PubMed] [Google Scholar]
- 12.Davis RE, Brown KD, Siebenlist U, et al. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med 2001; 194:1861–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu HG, Yu LL, Yang Y, et al. Increased expression of RelA/nuclear factor-kappa B protein correlates with colorectal tumorigenesis. Oncology 2003; 65:37–45. [DOI] [PubMed] [Google Scholar]
- 14.Lee BL, Lee HS, Jung J, et al. Nuclear factor-kappaB activation correlates with better prognosis and Akt activation in human gastric cancer. Clin Cancer Res 2005; 11:2518–2525. [DOI] [PubMed] [Google Scholar]
- 15.Izzo JG, Correa AM, Wu TT, et al. Pretherapy nuclear factor-kappaB status, chemoradiation resistance, and metastatic progression in esophageal carcinoma. Mol Cancer Ther 2006; 5:2844–2850. [DOI] [PubMed] [Google Scholar]
- 16.Shukla S, Maclennan GT, Marengo SR, et al. Constitutive activation of P I3 K-Akt and NF-kappaB during prostate cancer progression in autochthonous transgenic mouse model. Prostate 2005; 64:224–239. [DOI] [PubMed] [Google Scholar]
- 17.Oya M, Ohtsubo M, Takayanagi A, et al. Constitutive activation of nuclear factor-kappaB prevents TRAIL-induced apoptosis in renal cancer cells. Oncogene 2001; 20:3888–3896. [DOI] [PubMed] [Google Scholar]
- 18.Annunziata CM, Stavnes HT, Kleinberg L, et al. Nuclear factor kappaB transcription factors are coexpressed and convey a poor outcome in ovarian cancer. Cancer 2010; 116:3276–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Jia H, Xie L, et al. Association of constitutive nuclear factor-kappaB activation with aggressive aspects and poor prognosis in cervical cancer. Int J Gynecol Cancer 2009; 19:1421–1426. [DOI] [PubMed] [Google Scholar]
- 20.Baby J, Pickering BF, Vashisht Gopal YN, et al. Constitutive and inducible nuclear factor-kappaB in immortalized normal human bronchial epithelial and non-small cell lung cancer cell lines. Cancer Lett 2007; 255:85–94. [DOI] [PubMed] [Google Scholar]
- 21.Jackson-Bernitsas DG, Ichikawa H, Takada Y, et al. Evidence that TNF-TNFR1-TRADD-TRAF2-RIP-TAK1-IKK pathway mediates constitutive NF-kappaB activation and proliferation in human head and neck squamous cell carcinoma. Oncogene 2007; 26:1385–1397. [DOI] [PubMed] [Google Scholar]
- 22.Pacifico F, Mauro C, Barone C, et al. Oncogenic and anti-apoptotic activity of NF-kappa B in human thyroid carcinomas. J Biol Chem 2004; 279:54610–54619. [DOI] [PubMed] [Google Scholar]
- 23.Chaturvedi MM, Sung B, Yadav VR, et al. NF-kappaB addiction and its role in cancer: ’one size does not fit all’. Oncogene 2011; 30:1615–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25:603–605. [DOI] [PubMed] [Google Scholar]
- 26.Deeks JJHJ, Altman DG. Higgins JPT, Green S. Analysing and presenting results. John Wiley & Sons, Cochrane Handbook for Systematic Reviews of Interventions 425 Chichester. UK:2006. [Google Scholar]
- 27.Berardi R, Maccaroni E, Mandolesi A, et al. Nuclear factor-kappaB predicts outcome in locally advanced rectal cancer patients receiving neoadjuvant radio-chemotherapy. Dig Liver Dis 2012; 44:617–622. [DOI] [PubMed] [Google Scholar]
- 28.Chu D, Zhou Y, Zhang Z, et al. Notch1 expression, which is related to p65 Status, is an independent predictor of prognosis in colorectal cancer. Clin Cancer Res 2011; 17:5686–5694. [DOI] [PubMed] [Google Scholar]
- 29.Kwon HC, Kim SH, Oh SY, et al. Clinicopathological significance of nuclear factor-kappa B, HIF-1 alpha, and vascular endothelial growth factor expression in stage III colorectal cancer. Cancer Sci 2010; 101:1557–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewander A, Gao J, Carstensen J, et al. NF-kappaB p65 phosphorylated at serine-536 is an independent prognostic factor in Swedish colorectal cancer patients. Int J Colorectal Dis 2012; 27:447–452. [DOI] [PubMed] [Google Scholar]
- 31.Lin G, Tang Z, Ye YB, et al. NF-kappaB activity is downregulated by KRAS knockdown in SW620 cells via the RAS-ERK-IkappaBalpha pathway. Oncol Rep 2012; 27:1527–1534. [DOI] [PubMed] [Google Scholar]
- 32.O’Neil BH, Funkhouser WK, Calvo BF, et al. Nuclear factor kappa-light chain-enhancer of activated B cells is activated by radiotherapy and is prognostic for overall survival in patients with rectal cancer treated with preoperative fluorouracil-based chemoradiotheraphy. Int J Radiat Oncol Biol Phys 2011; 80:705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pancione M, Forte N, Sabatino L, et al. Reduced beta-catenin and peroxisome proliferator-activated receptor-gamma expression levels are associated with colorectal cancer metastatic progression: correlation with tumor-associated macrophages, cyclooxygenase 2, and patient outcome. Hum Pathol 2009; 40:714–725. [DOI] [PubMed] [Google Scholar]
- 34.Voboril R, Voborilova J, Rychterova V, et al. Dissociated invasively growing cancer cells with NF-kappaB/p65 positivity after radiotherapy: a new marker for worse clinical outcome in rectal cancer? Preliminary data. Clin Exp Metastasis 2008; 25:491–496. [DOI] [PubMed] [Google Scholar]
- 35.Abdel-Latif MM, O’Riordan J, Windle HJ, et al. NF-kappaB activation in esophageal adenocarcinoma: relationship to Barrett's metaplasia, survival, and response to neoadjuvant chemoradiotherapy. Ann Surg 2004; 239:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hatata T, Higaki K, Tatebe S, et al. Immunohistochemical study of nuclear factor-kappaB expression in esophageal squamous cell carcinoma: prognostic significance and sensitivity to treatment with 5-FU. Dis Esophagus 2012; 25:716–722. [DOI] [PubMed] [Google Scholar]
- 37.Izzo JG, Malhotra U, Wu TT, et al. Association of activated transcription factor nuclear factor kappab with chemoradiation resistance and poor outcome in esophageal carcinoma. J Clin Oncol 2006; 24:748–754. [DOI] [PubMed] [Google Scholar]
- 38.Izzo JG, Malhotra U, Wu TT, et al. Clinical biology of esophageal adenocarcinoma after surgery is influenced by nuclear factor-kappaB expression. Cancer Epidemiol Biomarkers Prev 2007; 16:1200–1205. [DOI] [PubMed] [Google Scholar]
- 39.Kwon HC, Kim SH, Oh SY, et al. Clinicopathologic significance of expression of nuclear factor-kappaB RelA and its target gene products in gastric cancer patients. World J Gastroenterol 2012; 18:4744–4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levidou G, Korkolopoulou P, Nikiteas N, et al. Expression of nuclear factor kappaB in human gastric carcinoma: relationship with I kappaB a and prognostic significance. Virchows Arch 2007; 450:519–527. [DOI] [PubMed] [Google Scholar]
- 41.Park KW, Kim SJ, Oh SY. Clinicopathologic significance of nuclear factor-kappaB and vascular endothelial growth factor expression in advanced gastric cancer patients. Oncol Res Treat 2014; 37:183–190. [DOI] [PubMed] [Google Scholar]
- 42.Yamanaka N, Sasaki N, Tasaki A, et al. Nuclear factor-kappaB p65 is a prognostic indicator in gastric carcinoma. Anticancer Res 2004; 24:1071–1075. [PubMed] [Google Scholar]
- 43.Darb-Esfahani S, Sinn BV, Weichert W, et al. Expression of classical NF-kappaB pathway effectors in human ovarian carcinoma. Histopathology 2010; 56:727–739. [DOI] [PubMed] [Google Scholar]
- 44.Guo RX, Qiao YH, Zhou Y, et al. Increased staining for phosphorylated AKT and nuclear factor-kappaB p65 and their relationship with prognosis in epithelial ovarian cancer. Pathol Int 2008; 58:749–756. [DOI] [PubMed] [Google Scholar]
- 45.Kleinberg L, Dong HP, Holth A, et al. Cleaved caspase-3 and nuclear factor-kappaB p65 are prognostic factors in metastatic serous ovarian carcinoma. Hum Pathol 2009; 40:795–806. [DOI] [PubMed] [Google Scholar]
- 46.Yang G, Xiao X, Rosen DG, et al. The biphasic role of NF-kappaB in progression and chemoresistance of ovarian cancer. Clin Cancer Res 2011; 17:2181–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin X, Wang Z, Qiu L, et al. Potential biomarkers involving IKK/RelA signal in early stage non-small cell lung cancer. Cancer Sci 2008; 99:582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nair VS, Gevaert O, Davidzon G, et al. NF-kappaB protein expression associates with (18)F-FDG PET tumor uptake in non-small cell lung cancer: a radiogenomics validation study to understand tumor metabolism. Lung Cancer 2014; 83:189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang D, Jin X, Wang F, et al. Combined prognostic value of both RelA and IkappaB-alpha expression in human non-small cell lung cancer. Ann Surg Oncol 2007; 14:3581–3592. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z, Ma J, Li N, et al. Expression of nuclear factor-kappaB and its clinical significance in nonsmall-cell lung cancer. Ann Thorac Surg 2006; 82:243–248. [DOI] [PubMed] [Google Scholar]
- 51.Wu Z, Peng X, Li J, et al. Constitutive activation of nuclear factor kappaB contributes to cystic fibrosis transmembrane conductance regulator expression and promotes human cervical cancer progression and poor prognosis. Int J Gynecol Cancer 2013; 23:906–915. [DOI] [PubMed] [Google Scholar]
- 52.Huang C, Huang K, Wang C, et al. Overexpression of mitogen-activated protein kinase kinase 4 and nuclear factor-kappaB in laryngeal squamous cell carcinoma: a potential indicator for poor prognosis. Oncol Rep 2009; 22:89–95. [PubMed] [Google Scholar]
- 53.Jiang LZ, Wang P, Deng B, et al. Overexpression of Forkhead Box M1 transcription factor and nuclear factor-kappaB in laryngeal squamous cell carcinoma: a potential indicator for poor prognosis. Hum Pathol 2011; 42:1185–1193. [DOI] [PubMed] [Google Scholar]
- 54.Sun W, Guo MM, Han P, et al. Id-1 and the p65 subunit of NF-kappaB promote migration of nasopharyngeal carcinoma cells and are correlated with poor prognosis. Carcinogenesis 2012; 33:810–817. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Lang JY, Liu L, et al. Association of nuclear factor kappaB expression with a poor outcome in nasopharyngeal carcinoma. Med Oncol 2011; 28:1338–1342. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Peng B, Chen X. Expressions of nuclear factor kappaB, inducible nitric oxide synthase, and vascular endothelial growth factor in adenoid cystic carcinoma of salivary glands: correlations with the angiogenesis and clinical outcome. Clin Cancer Res 2005; 11:7334–7343. [DOI] [PubMed] [Google Scholar]
- 57.Korkolopoulou P, Levidou G, Saetta AA, et al. Expression of nuclear factor-kappaB in human astrocytomas: relation to pI kappa Ba, vascular endothelial growth factor, Cox-2, microvascular characteristics, and survival. Hum Pathol 2008; 39:1143–1152. [DOI] [PubMed] [Google Scholar]
- 58.Wu W, Pan C, Yu H, et al. Heparanase expression in gallbladder carcinoma and its correlation to prognosis. J Gastroenterol Hepatol 2008; 23:491–497. [DOI] [PubMed] [Google Scholar]
- 59.Balermpas P, Michel Y, Wagenblast J, et al. Nuclear NF-kappaB expression correlates with outcome among patients with head and neck squamous cell carcinoma treated with primary chemoradiation therapy. Int J Radiat Oncol Biol Phys 2013; 86:785–790. [DOI] [PubMed] [Google Scholar]
- 60.Zhou Q, Gong Y, Huang F, et al. Expression levels and significance of nuclear factor-kappaB and epidermal growth factor receptor in hepatolithiasis associated with intrahepatic cholangiocarcinoma. Dig Surg 2013; 30:309–316. [DOI] [PubMed] [Google Scholar]
- 61.Nariai Y, Mishima K, Yoshimura Y, et al. FAP-1 and NF-kappaB expressions in oral squamous cell carcinoma as potential markers for chemo-radio sensitivity and prognosis. Int J Oral Maxillofac Surg 2011; 40:419–426. [DOI] [PubMed] [Google Scholar]
- 62.Okera M, Bae K, Bernstein E, et al. Evaluation of nuclear factor kappaB and chemokine receptor CXCR4 co-expression in patients with prostate cancer in the Radiation Therapy Oncology Group (RTOG) 8610. BJU Int 2011; 108:E51–E58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yeh HC, Huang CH, Yang SF, et al. Nuclear factor-kappaB activation predicts an unfavourable outcome in human upper urinary tract urothelial carcinoma. BJU Int 2010; 106:1223–1229. [DOI] [PubMed] [Google Scholar]
- 64.Weichert W, Boehm M, Gekeler V, et al. High expression of RelA/p65 is associated with activation of nuclear factor-kappaB-dependent signaling in pancreatic cancer and marks a patient population with poor prognosis. Br J Cancer 2007; 97:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoon SO, Kim BH, Lee HS, et al. Differential protein immunoexpression profiles in appendiceal mucinous neoplasms: a special reference to classification and predictive factors. Mod Pathol 2009; 22:1102–1112. [DOI] [PubMed] [Google Scholar]
- 66.DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev 2012; 246:379–400. [DOI] [PubMed] [Google Scholar]
- 67.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol 1996; 14:649–683. [DOI] [PubMed] [Google Scholar]
- 68.Miyamoto S, Chiao PJ, Verma IM. Enhanced I kappa B alpha degradation is responsible for constitutive NF-kappa B activity in mature murine B-cell lines. Mol Cell Biol 1994; 14:3276–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tam WF, Lee LH, Davis L, et al. Cytoplasmic sequestration of rel proteins by IkappaBalpha requires CRM1-dependent nuclear export. Mol Cell Biol 2000; 20:2269–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]


