Abstract
Anisakiasis is a global disease caused by consumption of raw or lightly cooked fish contaminated with L3 Anisakis spp. larvae. High rates of parasitization of fish worldwide make Anisakis a serious health hazard. In fact, anisakiasis is a growing disease in countries such as Spain, Italy, and Japan, where consumption of raw/marinated fish is high.
Some parasitic infections have been recognized as a causative factor for human cancer. Suggested mechanisms include chronic inflammation elicited by the parasite, and a possible tumorigenic effect from certain parasitic secretions.
Anisakis can produce persistent local inflammation and granuloma, and larvae have been incidentally found in gastrointestinal (GI) tumors. Our aim was to discover possible differences in the prevalence of unnoticed or asymptomatic previous Anisakis infection in GI cancer patients compared with healthy individuals. Serum levels of specific antibodies against Anisakis antigens were used as a reliable marker of previous contact with their larvae.
Ninety-four participants without a previous history of Anisakis infection were prospectively allocated into 1 of 2 groups: 47 patients with GI cancer and 47 controls. Specific IgE, IgA1, and IgG1 against the Anisakis recombinant antigens Ani s 1, Ani s 5, Ani s 9, and Ani s 10 were determined by an ELISA assay.
The ratio of positivity to sIgA1, rAni s 1, or rAni s 5 was significantly higher in the cancer patients than in the controls (38.30% vs 6.38%, P < 0.001) and (42.55% vs 10.64%, P < 0.001, respectively). When disaggregated by type of tumor, the patients with gastric cancer showed a higher proportion of positive results for sIgA1 to rAni s 1 (P < 0.001), whereas a higher proportion of colon cancer patients were shown to be positive for sIgA1 to both rAni s 1 (P < 0.05) and rAni s 5 (P < 0.01).
Earlier Anisakis infection might be a risk factor for the development of stomach or colon cancer.
INTRODUCTION
Anisakis spp. is a nematode parasite located worldwide whose infective third-stage larvae are frequently found within the flesh of a great diversity of fish and cephalopod species commonly consumed by humans. The high worldwide rates of fish parasitization1 make infections by the parasitic nematode Anisakis a serious health hazard. In fact, the number of cases of Anisakiasis is increasing in countries such as Spain, Italy, and Japan, where consumption of raw or lightly cooked fish is high.2–4 However, the frequency of the disease could be underestimated in other countries where the consumption of these dishes is less frequent because it can be easily misdiagnosed as appendicitis, gastric ulcer, or other food allergies.4
The accidental ingestion of third-stage larvae present in raw or undercooked fish causes acute gastric infection.4Anisakis larvae anchor to the stomach mucosa, releasing excretory-secretory (ES) products that contain the main parasite antigens responsible for the allergic symptoms and potent proteolytic enzymes that penetrate into the gastrointestinal (GI) mucosa.5 The invasive capacity of the larvae explains the multiple, well-defined, erosive lesions typically detected near the main lesion within the patient's gastric mucosa.6 One of the primary features of the local inflammatory lesions produced by Anisakis larvae is the presence of conspicuous eosinophilic infiltration in the tissues surrounding the parasite. These cells adhere to the nematode's epicuticle in the presence of antibodies (particularly in the oral region, where the ES products are localized) releasing cytotoxic factors that are probably responsible for a great deal of the tissue damage surrounding the parasite observed in both acute and chronic infections.5
The link between inflammation and cancer is well established. Inflammation involves an interaction between various immune cells, chemokines, cytokines, and other mediators that can lead to signaling toward tumor cell proliferation, growth, and invasion.7 In addition to the inflammatory reaction they elicit, some parasites could contribute to preneoplastic changes through the direct effect of their antigens.8 Regarding Anisakis, there are cases in the literature that relate anisakiasis with GI cancer and describe the incidental finding of Anisakis larvae at the tumor site.9–13
Our aim was to discover possible differences in the prevalence of unnoticed or asymptomatic previous Anisakis infection in GI cancer patients compared with healthy controls. Serum levels of specific antibodies against Anisakis antigens were used as a reliable marker of previous contact with their larvae.
MATERIALS AND METHODS
Patient Sera
From 2010 to 2013, 94 participants without a previous history of Anisakis simplex or Helicobacter pylori infections were prospectively allocated into 1 of 2 groups: 47 patients with GI cancer and 47 healthy controls (Tables 1 and 2 ). The study included only those who answered negatively on a questionnaire on previous diagnoses with Anisakis or Helicobacter infections, any symptom after the ingestion of fish, or previous episodes of stomach pain, vomiting, diarrhea, nausea, or intestinal obstruction. The patient group included consecutive individuals with a diagnosis of GI cancer confirmed by biopsy. The healthy controls were recruited by simple random sampling from the list of adults with a health card from the same geographical area as the patients. They were completed a questionnaire and had a blood draw and an interview to rule out any disease.
TABLE 1.
Clinical Data of the 47 Patients Studied
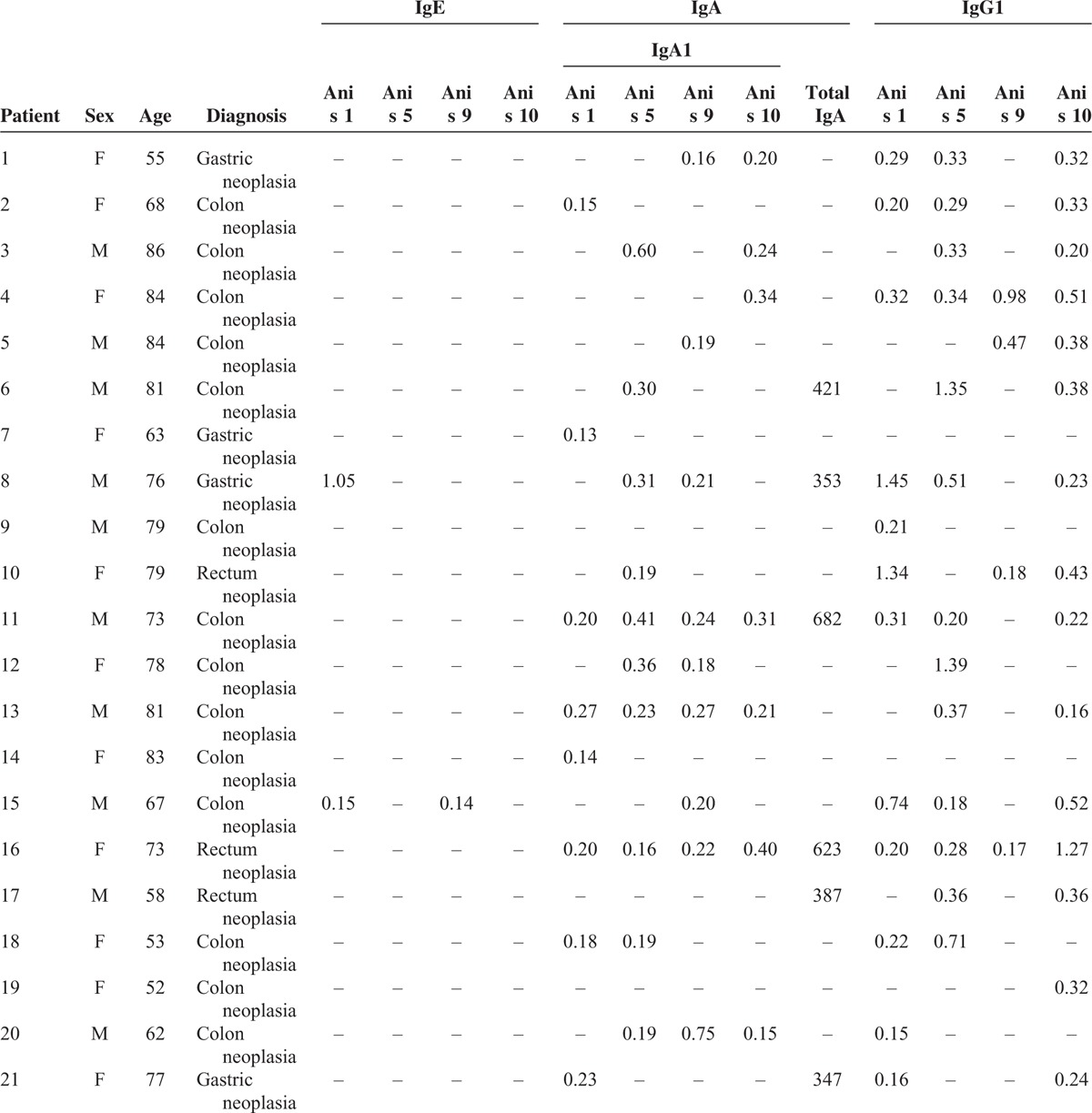
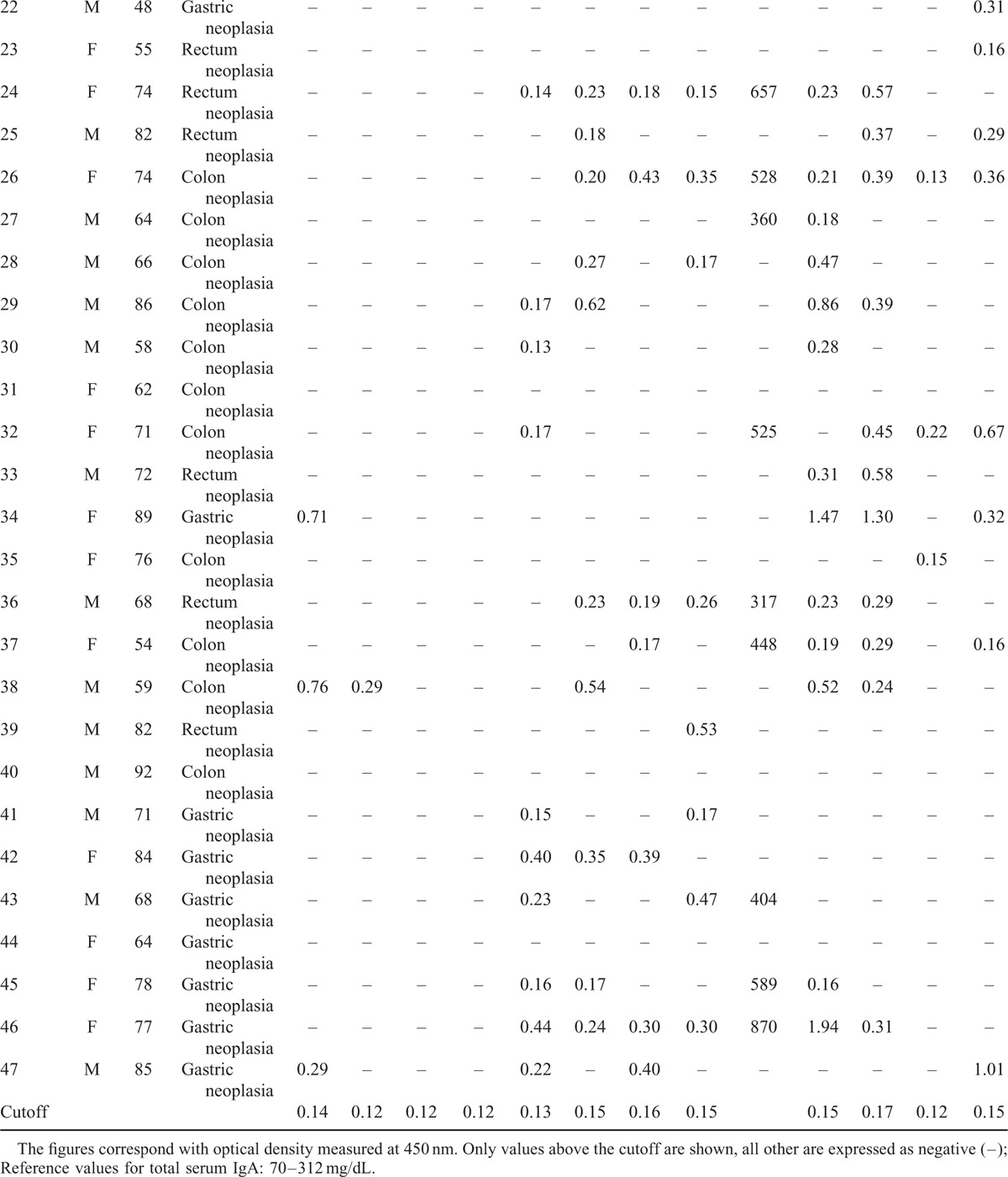
TABLE 1 (Continued).
Clinical Data of the 47 Patients Studied
The study was approved by the ethics committee of the Hospital La Paz-Carlos III and has therefore been performed in accordance with the ethical standards in the 1964 Declaration of Helsinki and its later amendments. Written informed consent for inclusion was obtained from all the participants.
All sera were tested for the specific antibodies IgE, IgA1, and IgG1 against Anisakis antigens with the recombinant allergens or antigens Ani s 1, Ani s 5, Ani s 9, and Ani s 10, by an enzyme-linked immunosorbent assay (ELISA).
Anisakis simplex Recombinant Antigens
Recombinant Ani s 1 was cloned in Pichia pastoris and purified by ion exchange chromatography and RP-HPLC.14 The His-tagged antigens rAni s 5, rAni s 9, and rAni s 10 were expressed in Escherichia coli and purified by affinity chromatography.15–17
Determination of Specific IgE, IgA1, and IgG1 Against Anisakis simplex Recombinant Antigens by ELISA
Specific IgE, IgA1, and IgG1 against the antigens rAni s 1, rAni s 5, rAni s 9, and rAni s 10 were determined by ELISA. Polystyrene 96-well plates (Costar 3590, Corning, NY) were coated for 2 hours at 37 °C with 100 μL of recombinant antigen at 10 μg/mL in carbonate buffer pH 9.6. The coated wells were blocked with 1% bovine serum albumin in phosphate-buffered saline (PBS) for 30 minutes at 37 °C, then incubated overnight at room temperature with 100 μL of the patient's serum (1/4 dilution in 1% bovine serum albumin, 0.05% Tween-20 in PBS). After washing with 0.05% Tween-20 in PBS, the wells were incubated for 2 hours at room temperature with peroxidase-labeled antihuman IgE (SouthernBiotech, Birmingham, AL; 1/2000 dilution), peroxidase-labeled antihuman IgA1 (SouthernBiotech; 1/10,000 dilution), or peroxidase-labeled antihuman IgG1 (SouthernBiotech; 1/8000 dilution). The plates were washed again and then developed with 100 μL of TMB-turbo ELISA substrate (Thermo Scientific, Rockford, IL). The reaction was stopped after 30 minutes with 100 μL of 2 M H2SO4, and the optical density was measured at 450 nm. Assays were performed in duplicate. A blocking buffer was used as a negative control. To establish the cutoff for each antibody with each antigen, we randomly selected 25 sera from the healthy control individuals and calculated the mean of the optical density values and the standard deviation. The cutoff was fixed as the mean plus 4 standard deviations, as described.18
Determination of Total Serum IgA
The determination of total IgA in serum samples from both the patients and the controls was performed by nephelometry using the IMMAGE Immunochemistry System (Beckman Coulter).
Statistics
The results are expressed as percentages of positive results for each variable in the respective group.
For comparisons between the cancer and control groups, a stratification approach was used, and Fisher exact test of independence was applied; P-values < 0.05 were considered to be indicative of statistically significant differences.
RESULTS
The average age of the patient group was 70 years (range 48–92 years; 23 men and 24 women), and the average age of the healthy control group was 65 years (range 46–83 years; 26 men and 21 women).
Determination of Specific IgE, IgA1, and IgG1 Against Anisakis simplex Recombinant Antigens
The results of specific IgE, IgA1, and IgG1 against the antigens rAni s 1, rAni s 5, rAni s 9, and rAni s10 determined from the sera of the patients with GI cancer and the sera of the healthy controls are shown in Tables 1 and 2 , respectively.
The study showed the presence of the specific antibodies IgE, IgA1, and IgG1 against the Anisakis antigens in the sera from both studied groups, with the exceptions of sIgE to rAni s 10 in the patients and sIgE to rAni s 5 in the controls (Table 3). Moreover, the study showed the presence of sIgE, sIgA1, and sIgG1 against at least 1 of the antigens tested in both studied groups.
TABLE 2.
Clinical Data of the 47 Healthy Controls
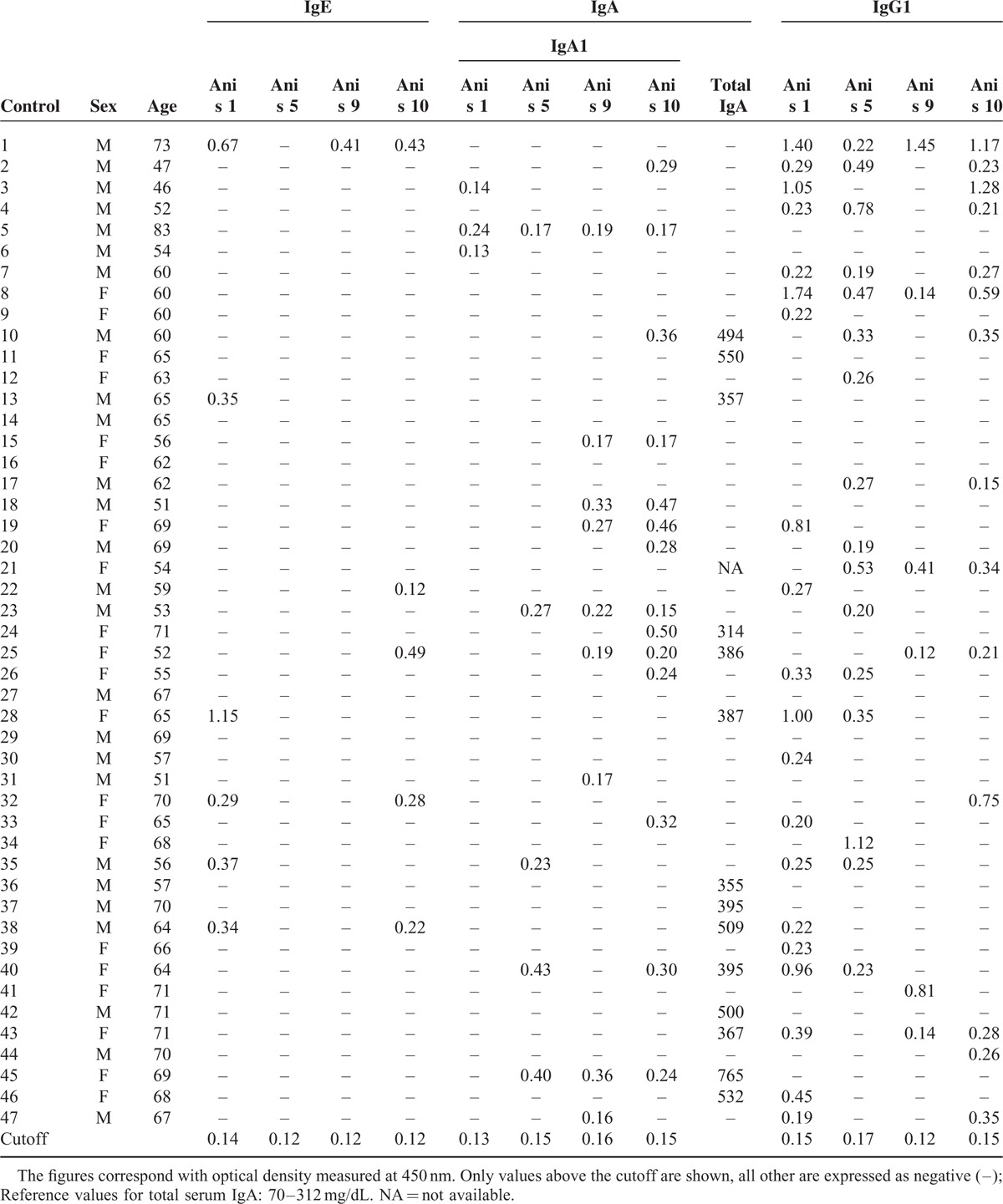
TABLE 3.
Rates of Positive Results for the Specific Antibodies IgE, IgA1, and IgG1 Against 4 Recombinant Antigens of Anisakis simplex and the Total IgA Values Among Patients With Gastrointestinal Cancer (Global and by Tumor Type), Compared With Those of Control Healthy Individuals
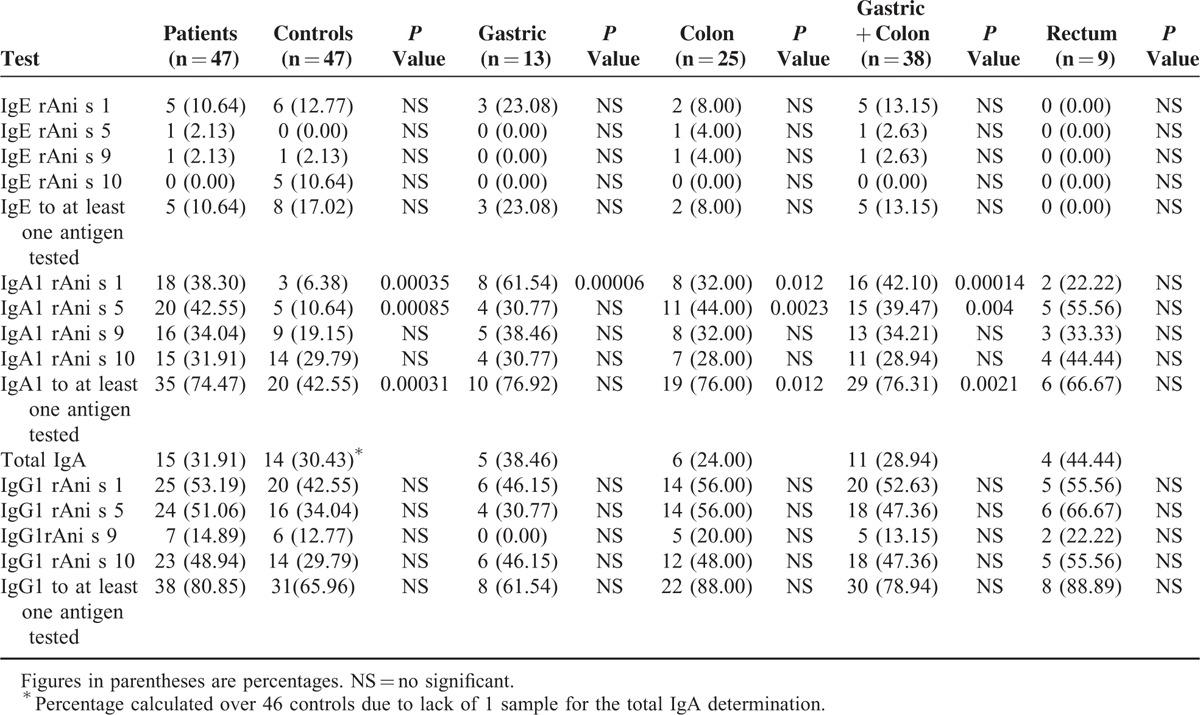
Regarding IgE, no significant differences were found for any of the 4 antigens tested (Table 3). This finding concurs with the fact that none of the patients or the control individuals had been diagnosed with allergy to Anisakis.
With reference to IgA1, the study showed statistically significant differences between the patients and the controls (Table 3). Thus, positive results of specific IgA1 to at least 1 of the antigens tested were significantly more frequent among the patients with GI cancer than the healthy controls (74.47% vs 42.55%; odds ratio [OR] 3.9; 95% confidence interval [CI]: 1.6–9.4; P < 0.001). This result remained true for both gastric (76.92% vs 42.55%; OR 4.5; 95% CI: 1.1–18.5; P < 0.05) and colon cancer patients (76.00% vs 42.55%; OR 4.3; 95% CI: 1.4–12.6; P < 0.001), when analyzed separately. Concerning the panel of antigens studied, the antigens rAni s 1 and rAni s 5 showed significant differences between patients and controls. Sera from the patients showed higher frequencies of specific IgA1 to rAni s 1 or rAni s 5 than the healthy controls (38.30% vs 6.38%, P < 0.001) and (42.55% vs 10.64%, P < 0.001), respectively.
When the results of specific IgA1 were analyzed by type of tumor, the differences found between the patients and the controls remained. Sera from the patients diagnosed with gastric cancer showed significantly high frequencies of specific IgA1 to rAni s 1 (P < 0.001), and sera from the patients diagnosed with colon cancer showed significantly high frequencies of specific IgA1 both to rAni s 1 (P < 0.05) and rAni s 5 (P < 0.01). Furthermore, when combining the data for these 2 types of cancer, significantly high frequencies were found of specific IgA1 both to rAni s 1 (P < 0.001) and rAni s 5 (P < 0.01). No significant differences were found between the patients diagnosed with rectal cancer and the healthy controls.
Finally, regarding IgG1, no significant differences were found for any of the 4 antigens tested between the patients with GI cancer and the healthy controls (Table 3).
Determination of Total Serum IgA
The study of total serum IgA did not show significant differences between the patients diagnosed with GI cancer and the healthy controls (Table 3).
DISCUSSION
We report the analysis of the humoral immune response against A. simplex in patients with GI cancer. For this purpose, we used a panel of 4 recombinant antigens tested for diagnostic specificity with patients from Spain and Italy.19,20 We were interested in studying the immunoglobulins produced after several encounters with Anisakis; therefore, we studied specific IgE, IgA, and IgG, whose levels are highest after 1 month of infection.21 We excluded IgM because it reaches its highest levels within 24 hours and progressively decays over the 6 months following the first parasitization. With reference to the immunoglobulins IgA and IgG, we determined IgA1 and IgG1 to be the predominant subclasses found in human serum.
Our first finding was that there were individuals with specific immunoglobulins against Anisakis in both the patients with GI cancer and the healthy controls. Regarding IgE, 17% of the controls showed specific IgE to at least 1 of the Anisakis antigens tested. This percentage of asymptomatic sensitization agrees with previous studies performed in the same geographical area, in which 15% of the patients who attended the consultation for suspected allergy not related to Anisakis had specific IgE against these Anisakis recombinant antigens.19
Focusing on IgA, the detection of specific IgA1 showed clear differences between the patients with gastric and colon cancers versus the controls, the frequencies of specific IgA1 directed to rAni s 1 and rAni s 5 were significantly high among these patients. However, total IgA was not different between the patients and the controls.
Similarly, the increase in specific serum IgA has been reported in the immune response to H. pylori, whose chronic infection has been associated with gastric cancer.22,23 Thus, the prevalence of specific IgA antibodies was significantly higher in the patients with gastric cancer than in the controls.24,25 Moreover, an increased serum IgA/IgG titer ratio against H. pylori antigens was more frequent in the patients with gastric cancer than in the patients with superficial gastritis or in the controls.26–28
Although care should be taken to avoid making causality assumptions solely based on case–control studies, our results suggest that the group of patients with gastric and colon cancers had greater exposure to or contact with Anisakis than had the healthy controls, as shown by comparing the frequencies of specific IgA1 antibodies. Nevertheless, the patients studied had not had a previous clinical history related to Anisakis, suggesting that they could have had contact with Anisakis larvae without having shown the typical clinical manifestations of the infection (severe epigastric pain or allergic reactions such as angioedema, urticaria, or anaphylaxis a few hours after the ingestion of fish).29,30
The carcinogenesis associated with helminthic infections such as Schistosoma haematobium, Clonorchis sinensis, and Opisthorchis viverrini has been described as a complex process that could involve several different mechanisms, but chronic inflammation is a key feature.31,32 In this sense, regarding anisakiasis, the pathological effects of the infection could be the combined result of the mechanical action of the larva during tissue invasion, the direct tissue effects of the ES products released by the parasite, and the complex interaction between the host immune system and the Anisakis antigens that lead to persistent inflammation or granuloma.5
On the other hand, serum IgA has been associated with immunomodulatory properties related to downregulation of proinflammatory cytokines and upregulation of antiinflammatory cytokines involved in infection-inflammation processes.33,34 This fact could explain the high frequency of specific serum IgA1 found in our study among the patients with gastric and colon cancers. However, we cannot rule out that changes in immune responses during cancer or its treatment could have influenced the results of the serological tests.
To the best of our knowledge, this is the first study to analyze specific antibodies to Anisakis antigens in patients with GI cancer. As a general conclusion, the results of the study point to Anisakis as a possible risk factor for the development of GI tumors and suggest that earlier Anisakis infection might be a risk factor for the development of stomach or colon cancer.
Footnotes
Abbreviations: CI = confidence interval, ELISA = enzyme-linked immunosorbent assay, ES = excretory-secretory, GI = gastrointestinal, OR = odds ratio, PBS = phosphate-buffered saline.
JCG-P and RR-P contributed equally to this work.
This study was co-financed by Fondo de Investigación Sanitario (FIS) and FEDER (grant No. FIS-PI12/00888).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Kuhn T, Garcıa-Marquez J, Klimpel S. Adaptive radiation within marine Anisakid nematodes: a zoogeographical modelling of cosmopolitan, zoonotic parasites. PLoS One 2011; 6:e28642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daschner A, Pascual CY. Anisakis simplex: sensitization and clinical allergy. Curr Opin Allergy Clin Immunol 2005; 5:281–285. [DOI] [PubMed] [Google Scholar]
- 3.AAITO-IFIACI Anisakis Consortium Anisakis hypersensitivity in Italy: prevalence and clinical features: a multicenter study. Allergy 2011; 66:1563–1569. [DOI] [PubMed] [Google Scholar]
- 4.Ishikura H, Kikuchi K, Nagasawa K, et al. Anisakidae and anisakidosis. Prog Clin Parasitol 1993; 3:43–102. [DOI] [PubMed] [Google Scholar]
- 5.Audicana MT, Kennedy MW. Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin Microbiol Rev 2008; 21:360–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Namiki N, Yazaki Y. Ishikura H, Namiki M. Endoscopic findings of gastric anisakiasis with acute symptoms. Gastric Anisakiasis in Japan. Epidemiology, Diagnosis, Treatment. Tokyo, Japan:Springer-Verlag; 1989. 47–51. [Google Scholar]
- 7.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet 2001; 357:539–545. [DOI] [PubMed] [Google Scholar]
- 8.Botelho MC, Oliveira PA, Lopes C, et al. Urothelial dysplasia and inflammation induced by Schistosoma haematobium total antigen instillation in mice normal urothelium. Urol Oncol 2011; 29:809–814. [DOI] [PubMed] [Google Scholar]
- 9.Yokogawa M, Yoshimura H. Clinicopathologic studies on larval anisakiasis in Japan. Am J Trop Med Hyg 1967; 16:723–728. [PubMed] [Google Scholar]
- 10.Tsutsumi Y, Fujimoto Y. Early cancer superimposed on infestation of an Anisakis-like larva: a case report. Tokai J Exp Clin Med 1983; 8:265–273. [PubMed] [Google Scholar]
- 11.Maggi P, Caputi-Iambrenghi O, Scardigno A, et al. Gastrointestinal infection due to Anisakis simplex in southern Italy. Eur J Epidemiol 2000; 16:75–78. [DOI] [PubMed] [Google Scholar]
- 12.Pampiglione S, Rivasi F, Criscuolo M, et al. Human Anisakiasis in Italy: a report of eleven new cases. Pathol Res Pract 2002; 198:429–434. [DOI] [PubMed] [Google Scholar]
- 13.Mineta S, Shimanuki K, Sugiura A, et al. Chronic anisakiasis of the ascending colon associated with carcinoma. J Nippon Med Sch 2006; 73:169–174. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Perez R, Caballero ML, Gonzalez-Munoz M, et al. Cloning and expression of a biologically active Anisakis simplex allergen Ani s 1 in the yeast Pichia pastoris. Mol Biochem Parasitol 2007; 154:115–118. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez-Perez R, Moneo I, Rodriguez-Mahillo A, et al. Cloning and expresión of Ani s 9, a new Anisakis simplex allergen. Mol Biochem Parasitol 2008; 159:92–97. [DOI] [PubMed] [Google Scholar]
- 16.Caballero ML, Umpierrez A, Moneo I, et al. Ani s 10, a new Anisakis simplex allergen: cloning and heterologous expression. Parasitol Int 2011; 60:209–212. [DOI] [PubMed] [Google Scholar]
- 17.García-Mayoral MF, Treviño MA, Pérez-Piñar T, et al. Relationships between IgE/IgG4 epitopes, structure and function in Anisakis simplex Ani s 5, a member of the SXP/RAL-2 protein family. PloS Negl Trop Dis 2014; 8:e2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenzo S, Iglesias R, Leiro J, et al. Usefulness of currently available methods for the diagnosis of Anisakis simplex allergy. Allergy 2000; 55:627–633. [DOI] [PubMed] [Google Scholar]
- 19.Caballero ML, Umpierrez A, Perez-Piñar T, et al. Anisakis simplex recombinant allergens increase diagnosis specificity preserving high sensitivity. Int Arch Allergy Immunol 2012; 158:232–240. [DOI] [PubMed] [Google Scholar]
- 20.Caballero ML, Asero R, Antonicelli L, et al. Anisakis allergy component-resolved diagnosis: clinical and immunologic differences between patients from Italy and Spain. Int Arch Allergy Immunol 2013; 162:39–44. [DOI] [PubMed] [Google Scholar]
- 21.Daschner A, Cuellar C, Sanchez-Pastor S, et al. Gastro-allergic anisakiasis as a consequence of simultaneous primary and secondary immune response. Parasite Immunol 2002; 24:243–251. [DOI] [PubMed] [Google Scholar]
- 22.IARC Infection with Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum 1994; 61:177–240. [PMC free article] [PubMed] [Google Scholar]
- 23.Helicobacter and Cancer Collaborative Group Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut 2001; 49:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atalay C, Atalay G, Altinok M. Serum Helicobacter pylori IgG and IgA levels in patients with gastric cancer. Neoplasma 2003; 50:185–190. [PubMed] [Google Scholar]
- 25.Kosunen TU, Seppala K, Sarna S, et al. Association of Helicobacter pylori IgA antibodies with the risk of peptic ulcer disease and gastric cancer. World J Gastroenterol 2005; 11:6871–6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knekt P, Teppo L, Aromaa A, et al. Helicobacter pylori IgA and IgG antibodies, serum pepsinogen I and the risk of gastric cancer: changes in the risk with extended follow-up period. Int J Cancer 2006; 119:702–705. [DOI] [PubMed] [Google Scholar]
- 27.Manojlovic N, Babic D, Filipovic-Ljeshovic I, et al. Anti Helicobacter pylori IgG and IgA response in patients with gastric cancer and chronic gastritis. Hepatogastroenterology 2008; 55:807–813. [PubMed] [Google Scholar]
- 28.Adamsson J, Lundin SB, Hansson LE, et al. Immune responses against Helicobacter pylori in gastric cancer patients and in risk groups for gastric cancer. Helicobacter 2013; 18:73–82. [DOI] [PubMed] [Google Scholar]
- 29.Audicana MT, Fernandez de Corres L, Munoz D, et al. Recurrent anaphylaxis caused by Anisakis simplex parasitizing fish. J Allergy Clin Immunol 1995; 96:558–560. [DOI] [PubMed] [Google Scholar]
- 30.Del Pozo MD, Audicana M, Diez JM, et al. Anisakis simplex, a relevant etiologic factor in acute urticaria. Allergy 1997; 52:576–579. [DOI] [PubMed] [Google Scholar]
- 31.Vennervald BJ, Polman K. Helminths and malignancy. Parasite Immunol 2009; 31:686–696. [DOI] [PubMed] [Google Scholar]
- 32.Fried B, Reddy A, Mayer D. Helminths in human carcinogenesis. Cancer Lett 2011; 305:239–249. [DOI] [PubMed] [Google Scholar]
- 33.Olas K, Butterweck H, Teschner W, et al. Immunomodulatory properties of human serum immunoglobulin A: anti-inflammatory and pro-inflammatory activities in human monocytes and peripheral blood mononuclear cells. Clin Exp Immunol 2005; 140:478–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leong KW, Ding JL. The unexplored roles of human serum IgA. DNA Cell Biol 2014; 33:823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]


