Supplemental Digital Content is available in the text
Abstract
Metabolic health and obesity are not stable conditions, and changes in the status of these conditions might lead to different clinical outcomes. We aimed to determine whether changes in metabolic health status or obesity over time have any effect on the risk of future diabetes.
Nondiabetic individuals (n = 2692) from a population-based prospective cohort study with baseline and 2 follow-up examinations at 4-year intervals were included. Being “metabolically obese” (MO) was defined as being in the highest quartile of the TyG index (ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2]), whereas falling into the lower 3 quartiles was regarded as being “metabolically healthy” (MH). Individuals were classified as “obese” (O) or “nonobese” (NO) using a body mass index of 25 kg/m2 as a cut-off. The risk of diabetes at year 8 was assessed according to changes of metabolic health status between year 0 and 4.
Multivariate-adjusted relative risks (RRs) (95% confidence interval [CI]) of diabetes were significantly higher in individuals who retained the MONO phenotype (RR 3.72, 95% CI 2.10, 6.60) or who had progressed to MONO from the MHNO phenotype (RR 1.96, 95% CI 1.06, 3.61), whereas it was not significant in individuals who had improved to MHNO from the MONO phenotype (RR 0.67, 95% CI 0.26, 1.74) compared with individuals who retained the MHNO phenotype. In contrast, obese individuals had significantly higher RRs for diabetes, independent of changes in metabolic health status, whereas weight reduction resulted in a decreased risk of diabetes. Sensitivity analysis using the presence or absence of the metabolic syndrome as a definition of metabolic health revealed similar results.
Changes in metabolic health status were an independent risk factor for future diabetes in nonobese individuals, whereas general obesity had a greater contribution to the risk of obese individuals developing diabetes. These observations might imply a different intervention strategy for diabetes prevention according to obesity status.
INTRODUCTION
The importance of metabolic health status, apart from obesity, has recently gained much interest. This concept arose from observations that some people show metabolic characteristics that deviate from the expected dose–response relationship between body mass index (BMI) and metabolic disturbances.1–5 It is now becoming evident that these deviant subpopulations also demonstrate different clinical outcomes, such as the incidence of type 2 diabetes, cardiovascular diseases, and mortality.6–13
Although many different definitions are used for metabolic health, insulin resistance is regarded as the core pathophysiology. Individuals with higher levels of insulin resistance and adiposity, despite having a normal BMI, are classified as metabolically obese but normal-weight (MONW). Among obese individuals, those with a lower degree of insulin resistance and favorable metabolic profiles are classified as metabolically healthy obese (MHO). However, these are not stable conditions, and changes in body weight or metabolic health status might shift an individual to a different category and lead to different health consequences.14–17 In the 11-year prospective Pizarra study, the risk of diabetes in MHO individuals was significantly higher than that in metabolically healthy nonobese (MHNO) individuals. However, this association disappeared in participants who lost weight during the study period.18 In the North West Adelaide Health Study, the MHO group was approximately twice as likely to develop diabetes compared with metabolically healthy normal-weight (MHNW) individuals. This was mainly a consequence of the individuals who acquired the metabolically obese phenotype, because the risk of diabetes did not increase in the two-thirds of the individuals who maintained metabolic health.14 Another study performed in Japan also reached a similar conclusion, suggesting that the transitory nature of metabolic health influences the risk of future diabetes.15
The main problem regarding the implication of metabolic health concept in clinical practice is the absence of unified criteria. The prevalence and metabolic phenotypes of MONW and MHO largely differ depending on the definition of “metabolically obese”; the phenotypes have usually been identified using surrogate markers of insulin resistance, the number of metabolic syndrome components, the amount of visceral fat, or the composition of cardiovascular risk factors. Furthermore, comparisons of various criteria for predicting important clinical outcomes are scarce. Recently, we reported a simple index using a product of fasting glucose and triglycerides (TyG index), which could be used to classify metabolic health status.19 The MONW individuals, as defined using the TyG index, showed a significantly higher risk of developing diabetes compared to MHNW individuals.20
In this prospective cohort study, we aimed to investigate whether changes of metabolic health status or obesity between baseline examination (year 0) and first follow-up (year 4) have any effect on the risk of future (year 8) diabetes. The TyG index was used to define metabolic health, and the results were validated against the presence of metabolic syndrome.
METHODS
Participants
The Chungju Metabolic Disease Cohort (CMC) study is a community-based longitudinal study in a population aged 40 years and over living in the rural area of Chungju City, Korea.21,22 The baseline study was performed during 2003 to 2006, and the participants are being followed up at 4-year intervals. Three hundred thirty-four districts were selected using stratified random cluster sampling, and 11,718 individuals participated in the baseline (first phase) study. In this analysis, we selected individuals who had completed their follow-up visit at both the second phase (2007–2010) and third phase (2011–2014) of the study. Individuals missing anthropometric or laboratory data and those with known or newly diagnosed diabetes in the first or second phase of the study were excluded. Finally, 2692 (1047 men and 1645 women) individuals were included in the present study (Supplemental Figure 1, http://links.lww.com/MD/A458). The institutional review board at The Catholic University of Korea approved this study (No. KCMC070T076, KC13SISI0796), and written informed consent was obtained from all participants.
Study Protocols and Biochemical Assays
Health interview surveys and physical examinations were conducted by trained investigators. Detailed data on lifestyle behaviors, social status, and medical history were obtained via interview on the day of the investigation, including data regarding alcohol consumption, cigarette smoking, and education level. Regular exercise was defined as performing exercise three or more times per week for at least 30 minutes per session. Anthropometric measurements were performed with the participants being barefoot and wearing light clothing, and their height, weight, and waist circumference (WC) were measured to the nearest 0.1 cm and 0.1 kg. WC was measured at the narrowest level between the iliac crest and the lower margin of the ribcage. Blood pressure (BP) was measured twice on the right upper arm using a mercury sphygmomanometer while the participants were in a seated position after 5 minutes of rest, and the average was recorded. Hypertension was defined on the basis of the Joint National Committee 7 report as at least 140 (systolic BP)/90 (diastolic BP) mm Hg, or when the participants reported using antihypertensive medications.
Participants fasted overnight for at least 12 hours, after which blood samples were collected and subsequently analyzed at Samkwang Medical Laboratories (Seoul, Korea). Samples were subjected to various tests using the following methods21,22: total cholesterol (TC), enzymatic colorimetric test; triglyceride (TG), enzymatic colorimetric test; high-density lipoprotein (HDL)-cholesterol, selective inhibition method; low-density lipoprotein (LDL)-cholesterol, Friedewald formula; glucose, hexokinase method; insulin, radioimmunoassay kit (Dainabot, Tokyo, Japan); and creatinine, enzymatic method. All variables were measured in serum except for fasting glucose, which was measured in plasma (fasting plasma glucose [FPG]), and all tests had intra and interassay coefficients of variance <5%. Determination of the Homeostasis Model Assessment Estimate of Beta-Cell Function (HOMA-β) was based on the formula: 20 × fasting insulin (mIU/L)/(FPG [mmol/L] − 3.5). Determination of insulin resistance (HOMA-IR) was based on the formula: FPG (mmol/L) × fasting insulin (mIU/L)/22.5.23 Determination of the TyG index was based on the formula: ln (fasting TG [mg/dL] × FPG [mg/dL]/2).24,25
Definition of Metabolic Health Status
Being “metabolically obese” was defined as being in the highest quartile (Q4) of the TyG index. Being “metabolically healthy” was defined as falling into the lower 3 quartiles (Q1–Q3) of the TyG index. Individuals with BMI ≥25 kg/m2 were classified as “obese,” whereas others were classified as “nonobese.” In this study, we used the terms MHNO or metabolically obese nonobese (MONO) instead of MHNW or MONW, because both normal-weight and overweight individuals were included in the “nonobese” group. For sensitivity analysis, having metabolic syndrome was used as another definition of “metabolically obese.” Individuals were considered to have metabolic syndrome when they had at least 3 of the following criteria: FPG ≥5.6 mmol/L; BP ≥130/85 mm Hg, or current use of antihypertensive medication; men, HDL-cholesterol <1.03 mmol/L or women, HDL-cholesterol <1.29 mmol/L, or taking antihyperlipidemic agents; fasting TG ≥1.69 mmol/L or taking antihyperlipidemic agents; men, WC ≥90 cm or women, WC ≥80 cm. This definition is based on modified American Heart Association/National Heart, Lung and Blood Institute criteria.26 WC criteria were adjusted based on abdominal obesity criteria for the World Health Organization–Asian Pacific region.
Definition of Diabetes Mellitus
To examine the incidence of diabetes according to changes in metabolic health status between the phase 1 and phase 2 studies, all participants underwent a 75-g oral glucose tolerance test (OGTT) at the phase 3 study. Diabetes was defined according to the American Diabetes Association criteria.27
Statistical Analysis
Data are presented as the mean ± standard deviation (SD), as medians (25th–75th percentiles), or as proportions. A logarithmic transformation was performed for parameters showing non-normal distributions (insulin, HOMA-IR, HOMA-β, TG). The comparison of baseline and changes of characteristics among the subgroups were analyzed using Student t tests or chi-square tests as appropriate. A log-binomial regression model using the GENMOD procedure was performed, and the relative risk (RR) and 95% confidence interval (CI) values were calculated for testing the role of the baseline TyG index, changes in the TyG index over time, or changes of metabolic health status over time in predicting the development of future diabetes, and are presented as RR (95% CI). All statistical analyses were performed using SAS 9.3 software (SAS Institute Inc., Cary, NC), and a P value <0.05 was considered significant.
RESULTS
Baseline Characteristics of Participants
The mean age, BMI, and TyG index were 61.7 ± 8.8 years, 23.6 ± 2.9 kg/m2, and 8.67 ± 0.59 for men; and 61.2 ± 8.8 years, 24.6 ± 3.2 kg/m2, and 8.63 ± 0.53 for women. During the 8-year follow-up period, 214 (7.95%) individuals had progressed to diabetes. When the baseline characteristics were compared according to the diabetes status at the last follow-up, the individuals who had progressed to diabetes were older, had significantly higher body weight, BMI, WC, waist-to-hip ratio, systolic BP, diastolic BP, FPG levels, fasting insulin levels, HOMA-IR, and TC and TG levels; in addition, they were more likely to have hypertension, metabolic syndrome, and family history of diabetes than those who remained in the nondiabetic group. The TyG index at baseline (year 0) and first follow-up (year 4) were also significantly higher in those who progressed to diabetes (Supplemental Table 1, http://links.lww.com/MD/A458).
TyG Index and the Risk of Developing Diabetes
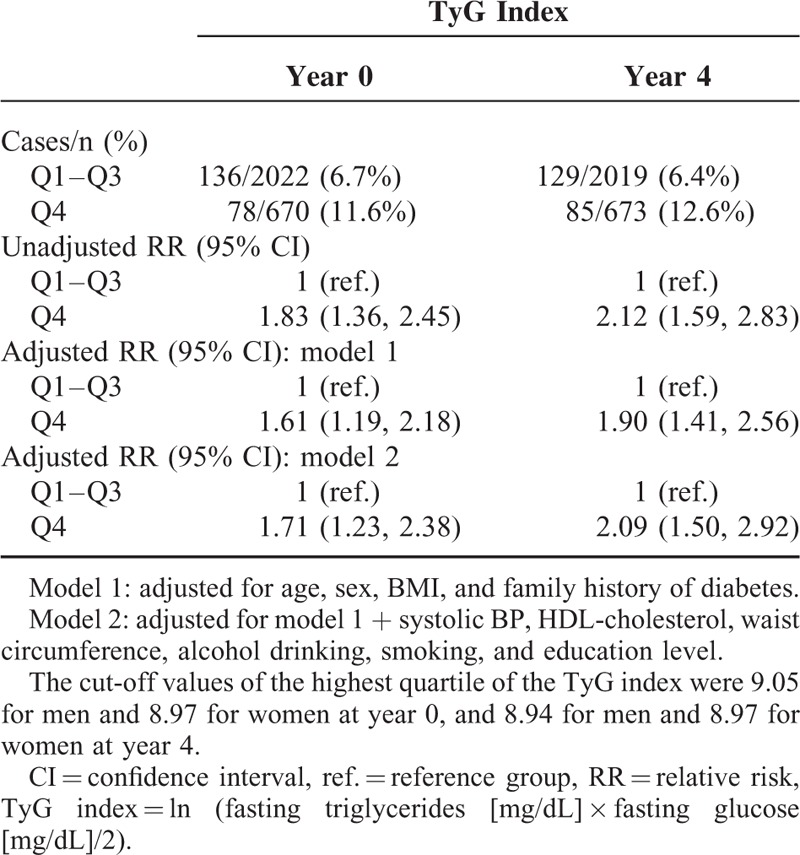
We examined the role of the TyG index at single time points in predicting the development of future diabetes. Individuals in the TyG index Q4 group showed significantly higher incidence (11.6% vs 6.7% at year 0, P < .001; 12.6% vs 6.4% at year 4, P < .001) and multivariate-adjusted RR (1.71 [1.23, 2.38] at year 0; 2.09 [1.50, 2.92] at year 4) of diabetes compared with individuals in Q1 to Q3 group of the TyG index at both time points (Table 1). This suggests that a single measurement of the TyG index during the nondiabetic stage could be a meaningful indicator for identifying individuals at risk of future diabetes.
TABLE 1.
TyG Index at Year 0 and 4 and the Risk of Developing Diabetes at Year 8
Changes in the TyG Index Over Time and the Risk of Developing Diabetes
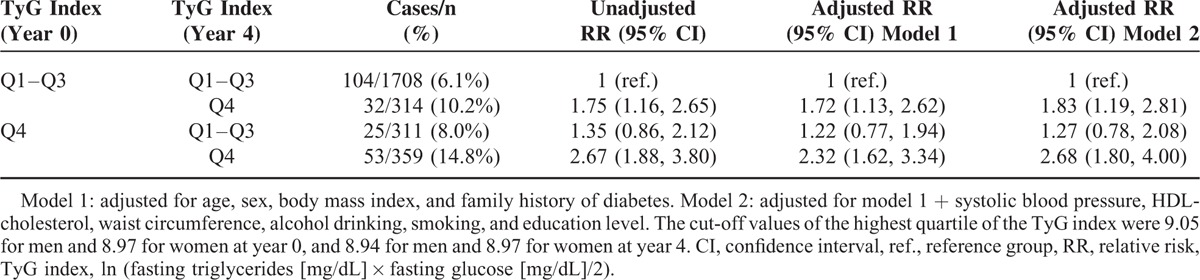
We classified the participants into 4 groups according to changes in the TyG index quartile groups between year 0 and 4. Table 2 shows the comparison of baseline characteristics of these 4 groups. Among those with a low TyG index (Q1–Q3) at baseline, individuals with higher body weight, BMI, WC, waist-to-hip ratio, systolic and diastolic BP, FPG, fasting insulin, HOMA-IR, dyslipidemic profile, or metabolic syndrome were more likely to transit to the TyG Q4 group at year 4. In contrast, among those with a high TyG index (Q4) at baseline, individuals with lower body weight, BMI, WC, FPG, fasting insulin, HOMA-IR, HOMA-β, or TG were more likely to transit to the TyG Q1 to Q3 group at year 4. Individuals who were in the TyG Q4 group at both year 0 and 4 had the highest incidence of diabetes (14.8%), followed by the individuals whose TyG index had increased from the Q1 to Q3 group to the Q4 group (10.2%). The risk of developing diabetes was significantly higher in individuals in the TyG Q4 group at both year 0 and 4 (2.67 [1.88, 3.80]) when compared to the individuals in the TyG Q1 to Q3 group at both time points. Individuals who transited from the TyG index Q1 to Q3 group to the Q4 group also demonstrated a significantly higher risk (1.75 [1.16, 2.65]), whereas the risk of individuals who transited from the TyG index Q4 group to the Q1 to Q3 group was not increased (1.35 [0.86, 2.12]). These associations were not attenuated after adjustment for several confounding factors, including age, sex, BMI, family history of diabetes, systolic BP, HDL-cholesterol, WC, alcohol drinking, smoking status, and education level (Table 3). This serial observation suggests that worsening of the TyG index value increases the risk of diabetes in adults, whereas an improvement decreases the risk.
TABLE 2.
Baseline Characteristics of the Study Participants According to Changes in TyG Index Quartile Groups at Year 0 and 4
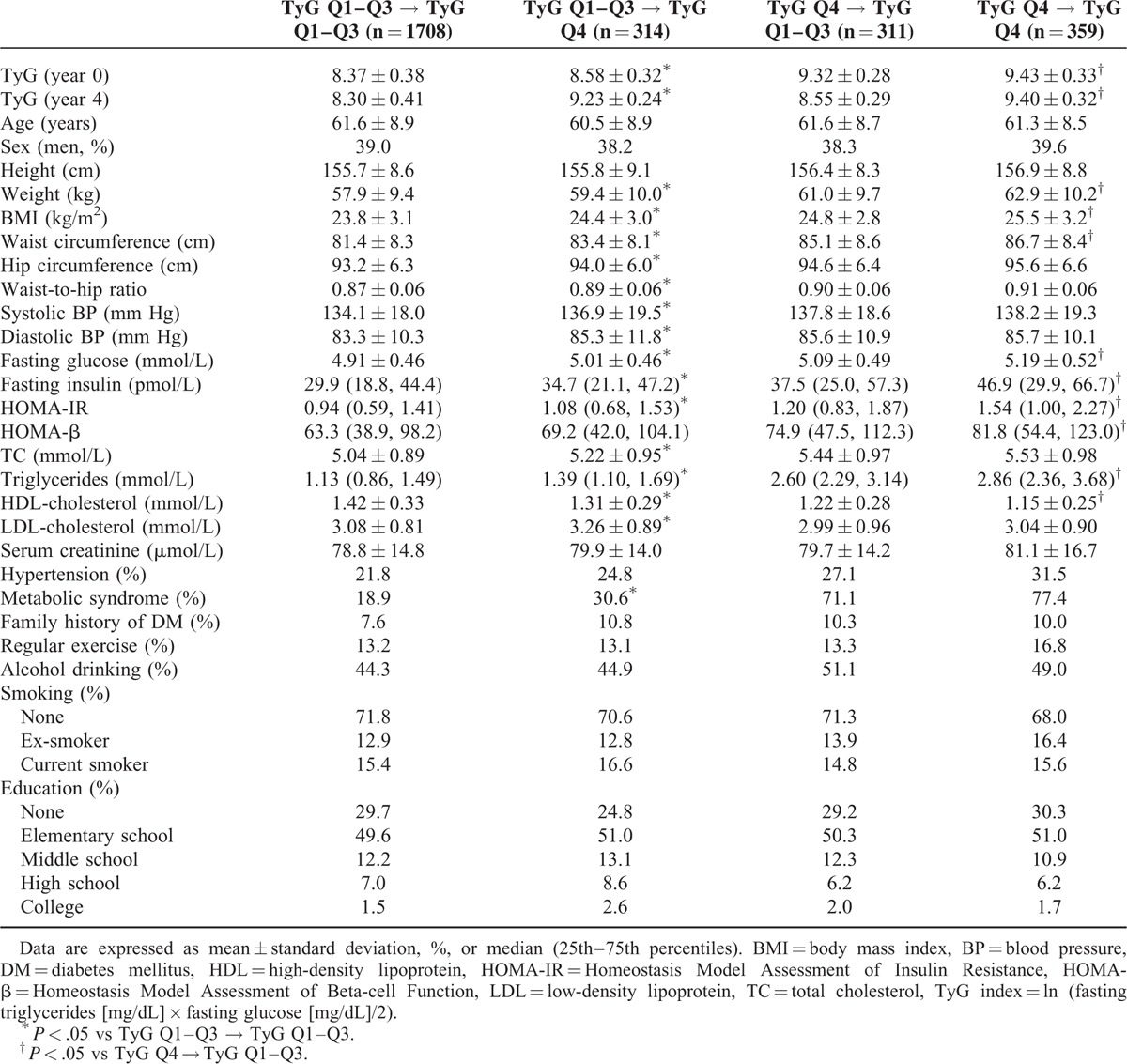
TABLE 3.
Changes in the TyG Index Over 4 Years and the Risk of Developing Diabetes at Year 8
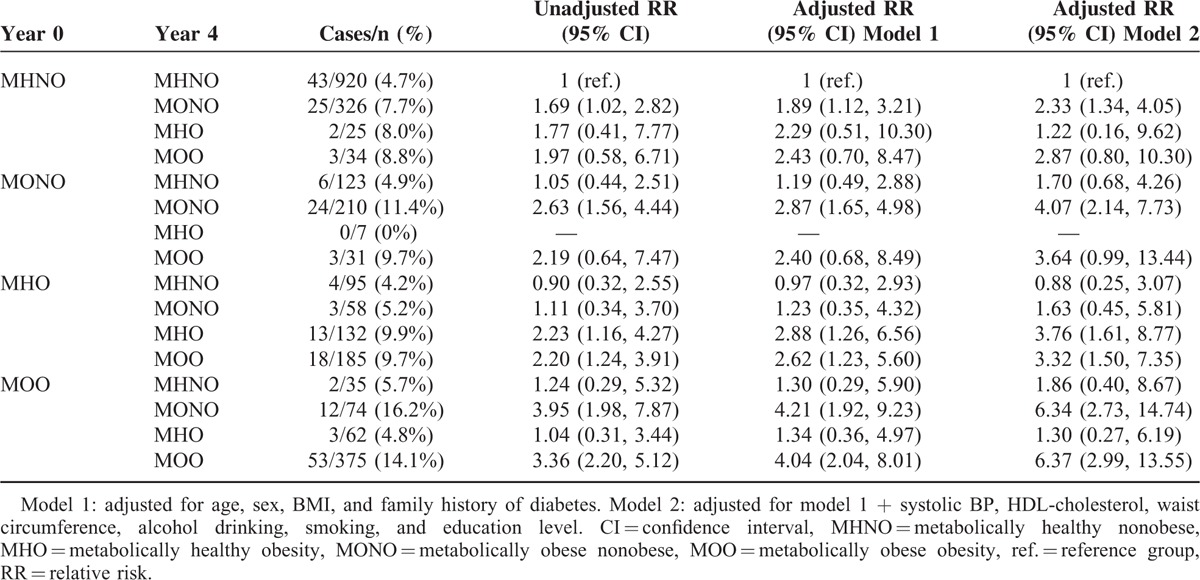
Changes in Metabolic Health Status Over Time and the Risk of Developing Diabetes
To explore the association between changes in metabolic health status and incidence of diabetes, we defined metabolic health phenotypes using BMI and TyG index quartile categories. The main factors contributing to changes in metabolic health phenotypes over time were changes in body weight, FPG, and lipid profiles (Supplemental Tables 2 and 3, http://links.lww.com/MD/A458). Individuals with the MONO phenotype at both year 0 and year 4 showed the highest incidence of diabetes (15.2%), which was similar to that of individuals who retained a metabolically obese obesity (MOO) phenotype at both time points (15.0%). Among nonobese individuals, those who transited from the MHNO to the MONO phenotype had an 8.5% risk of diabetes, whereas those who transited from the MONO to the MHNO phenotype only had a 3.8% risk of diabetes. In contrast, changes in metabolic phenotype among obese individuals had relatively little influence on the incidence of diabetes. Similarly, the RR (95% CI) of developing diabetes at year 8 (using the individuals who remained in the MHNO group as a reference) were significantly higher in those who retained the MONO phenotype (3.72 [2.10, 6.60]) or who had progressed to MONO from the MHNO phenotype (1.96 [1.06, 3.61]), whereas it was not significant in those who had improved to MHNO from the MONO phenotype (0.67 [0.26, 1.74]) after adjustment for confounding variables. Moreover, obese individuals had significantly higher RRs of diabetes, independent of changes in their metabolic health status (Table 4). To further confirm these data, we performed sensitivity analysis using presence or absence of the metabolic syndrome as a different definition of metabolic health and obtained similar data, except that obese individuals who had gained metabolic health (transited from metabolically obese to metabolically healthy) did not show an increased risk of developing diabetes (Table 5). These results suggest that changes in metabolic health status significantly affect the risk of future diabetes in nonobese individuals, but to a milder degree in obese individuals.
TABLE 4.
Changes in Metabolic Health Status Over 4 Years and the Risk of Developing Diabetes at Year 8
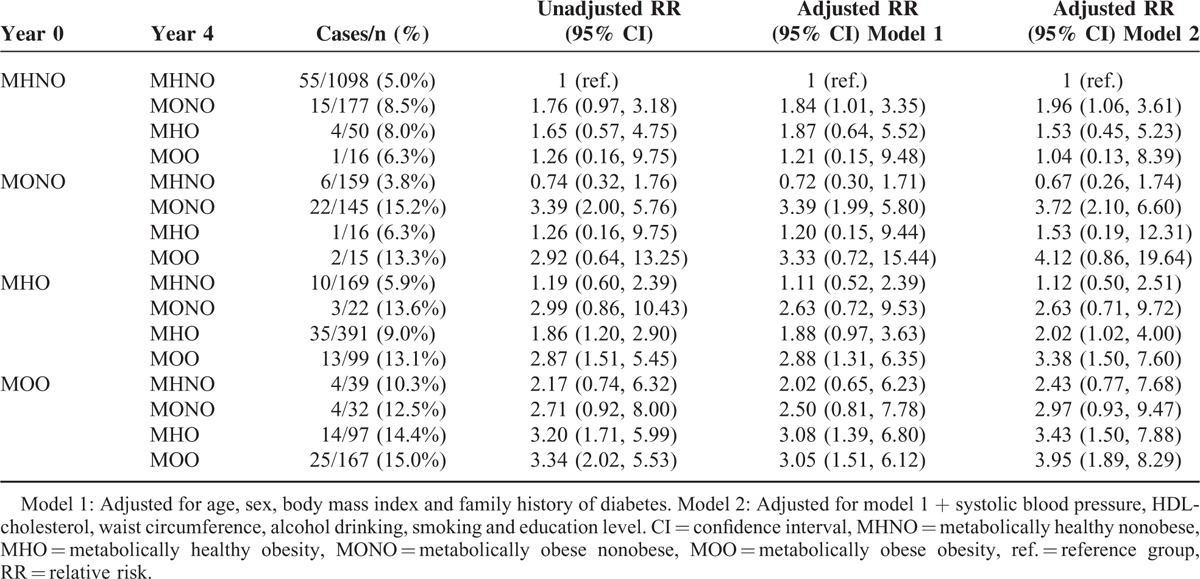
TABLE 5.
Changes in Metabolic Health Status (Defined by Metabolic Syndrome Criteria) Over 4 Years and the Risk of Developing Diabetes at Year 8
DISCUSSION
From a community-based prospective cohort study, our data show that the TyG index measured at a single time point may be an indicator of the risk for developing diabetes; changes in the TyG index level over time modify the risk of developing diabetes; and changes in metabolic health status also serve as an independent risk factor for future diabetes, especially in nonobese individuals.
Fasting TG levels, which are closely correlated with the degree of insulin resistance, have been suggested as an independent risk factor for diabetes in several studies.28,29 Because triglyceride levels are greatly influenced by diet and serve as a sensitive marker for lifestyle factors, sequential measurements might better represent one's risk status than a single measurement. For example, Tirosh et al29,30 showed that changes in TG levels measured 5 years apart modified the risk of diabetes and coronary heart disease in young men. Furthermore, when TG values were combined with those of FPG, a better predictive value for diabetes was observed.31,32 In this context, we tested the role of changes in the TyG index, which is a product of TG and FPG values, in predicting the risk of developing diabetes. As expected, the incidence and RR of diabetes were altered by changes in the TyG index over time. Because the TyG index has been well validated as a surrogate marker of insulin resistance,24,25,33 this might work as a simple and effective measure for identifying individuals at risk for diabetes.
Our previous studies highlighted the value of the TyG index in reflecting metabolic health status; furthermore, these studies proposed a novel criterion for identifying MONW individuals using this index.19,20 There was a stepwise increase in the odds of being categorized into the MONW group and a stepwise decrease in the odds of being categorized into the MHO group across the TyG index quartiles.19 By defining metabolic health status using the TyG index or metabolic syndrome, the current study suggests the importance of improving metabolic health for the prevention of future diabetes. In nonobese individuals, transition to the MONO phenotype from MHNO over a 4-year interval significantly increased the risk of diabetes. However, when MONO individuals gained metabolic health and improved to the MHNO phenotype, the risk of diabetes was not increased compared with individuals who retained the MHNO phenotype. In contrast, obese individuals were predisposed to an increased risk of diabetes by obesity per se. Individuals with the MHO phenotype at both baseline and in the follow-up period already had an increased risk of diabetes, and this was further increased when moving to the MOO phenotype. Of note, weight reduction in MHO individuals that resulted in transition to the MHNO category resulted in a decreased risk of diabetes. Whether an improvement in metabolic health status reduces the risk of diabetes in obese individuals is not clear, because the results were inconsistent according to the definition of metabolic health used. Individuals with the MOO phenotype at baseline had a 3 to 4-fold higher risk of diabetes independent of their changes in metabolic health status when the TyG index criteria were used. Analysis using the metabolic syndrome criteria showed a possible regression of diabetes risk when MOO individuals transited to the MHO group, although this interpretation is limited by the small number of participants in this group. Altogether, our data suggest that metabolic health might be more important in nonobese individuals, whereas general obesity might have a greater contribution in obese individuals for the risk of developing diabetes. These results align with previous studies that show an increased risk of diabetes in MHNW individuals who attain a metabolically unhealthy phenotype or increased body weight,14 and a decreased risk in obese individuals after weight loss.14,18 A 30-year follow-up study also found that obese middle-aged men, even without metabolic syndrome, had an increased risk for cardiovascular disease and mortality, suggesting that general obesity per se compromises the long-term outcome.34 Another study emphasized the independent association of metabolic health status, obesity, and body weight changes with the incidence of diabetes, although the differential effect of changes in metabolic health according to obesity groups was not reported.9
Chronic nutrient overload induces molecular changes in the white adipose tissue. In association with adipocyte hypertrophy, the extracellular matrix undergoes remodeling and eventually leads to immune cell infiltration by proinflammatory cytokines and tissue hypoxia owing to impaired angiogenesis.35 Although many differences in circulating biomarkers and molecular pathways are noted according to metabolic health status, reversal of fundamental obesity-induced changes might be needed to improve long-term outcomes.
It has been debated whether individuals with the MHO phenotype are prone to cardiometabolic diseases and increased mortality.8–11,13,36–38 Similarly, conflicting results of a weight loss program in MHO individuals have been demonstrated. In a few studies, diet and exercise intervention in obese individuals showed a comparable effect on body weight, fat mass, insulin sensitivity, and other cardiovascular risk factors, regardless of initial metabolic health status.39–41 However, a 12-week energy intake restriction in Korean women did not improve lipid profiles, C-reactive protein, or oxidized LDL in MHO individuals despite a similar degree of weight loss compared with MOO individuals.42 Another study also exhibited a different response to a 6-month diet intervention with deterioration of insulin sensitivity in MHO individuals.43 These findings raise an important question: is weight reduction needed to improve the clinical outcome of those with the MHO phenotype? Our data support a positive effect of weight loss for the prevention of incident diabetes in MHO individuals, which is in line with previous observations.14,18 In MONO individuals, lifestyle interventions for risk factor management, such as lowering glucose and BP, and improving lipid profiles, should be the main strategy.
The strengths of our study are that it was from a large-scale cohort with a long follow-up period and that the diagnosis of diabetes was based on an OGTT in every participant to avoid any overlooked cases of isolated post-load hyperglycemia. Limitations also exist. First, the number of participants who moved to a different obesity category was relatively small, making an accurate interpretation difficult in these subgroups. Second, we did not have data on the development of cardiovascular disease or mortality, which also needs to be elucidated. Third, because the participants consisted of middle-aged or elderly Koreans with a relatively low degree of obesity compared with other countries, our data need to be confirmed in other populations of different ages and ethnicities. Fourth, glycated hemoglobin levels were not measured in this cohort, which might have led to a misclassification of individuals in diabetic status. Lastly, the possibility of selection bias due to individuals who were not followed up needs to be considered, as this may limit interpretation of the conclusions drawn from this study.
In conclusion, this study identified changes in metabolic health status as a modifiable risk factor for developing diabetes, especially in nonobese individuals. Individuals with the MONO phenotype who improved metabolic health over time had a decreased risk of diabetes. In addition, as the risk of diabetes was significantly decreased in obese individuals who transited to the nonobese category, obese individuals stand to benefit from weight loss intervention, although it appears to be independent of improvements in metabolic health. In other words, metabolic health was important in nonobese individuals, whereas general obesity had a greater contribution to the risk of obese individuals developing diabetes. These observations might imply a different intervention strategy for diabetes prevention. Further studies in other populations with a consensus definition of metabolic health are warranted for a better understanding of this concept and the establishment of appropriate strategies to lessen the increasing burden of diabetes.
Acknowledgments
The authors wish to thank all the team members and survey personnel of the CMC study and the officers in Chungju Health Center for continued support.
Footnotes
Abbreviations: BMI = body mass index, BP = blood pressure, CI = confidence interval, CMC = Chungju Metabolic Disease Cohort, FPG = fasting plasma glucose, HDL = high-density lipoprotein, HOMA-IR = homeostasis Model Assessment Estimate of Insulin Resistance, HOMA-β = homeostasis Model Assessment Estimate of Beta-cell Function, LDL = low-density lipoprotein, MHNO = metabolically healthy nonobese, MHNW = metabolically healthy normal-weight, MHO = metabolically healthy obese, MONO = metabolically obese nonobese, MONW = metabolically obese but normal-weight, MOO = metabolically obese obesity, OGTT = oral glucose tolerance test, RR = relative risk, SD = standard deviation, TC = total cholesterol, TG = triglyceride, WC = waist circumference.
Funding: This study was supported by the grants from The Catholic Medical Center Research Foundation made in the program year of 2014 (to SHL) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP, 2013M3C8A2075675) (to KHY).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Conus F, Allison DB, Rabasa-Lhoret R, et al. Metabolic and behavioral characteristics of metabolically obese but normal-weight women. J Clin Endocrinol Metab 2004; 89:5013–5020. [DOI] [PubMed] [Google Scholar]
- 2.Dvorak RV, DeNino WF, Ades PA, et al. Phenotypic characteristics associated with insulin resistance in metabolically obese but normal-weight young women. Diabetes 1999; 48:2210–2214. [DOI] [PubMed] [Google Scholar]
- 3.Karelis AD, St-Pierre DH, Conus F, et al. Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab 2004; 89:2569–2575. [DOI] [PubMed] [Google Scholar]
- 4.Ruderman N, Chisholm D, Pi-Sunyer X, et al. The metabolically obese, normal-weight individual revisited. Diabetes 1998; 47:699–713. [DOI] [PubMed] [Google Scholar]
- 5.Seo MH, Rhee EJ. Metabolic and cardiovascular implications of a metabolically healthy obesity phenotype. Endocrinol Metab (Seoul) 2014; 29:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batsis JA, Sahakyan KR, Rodriguez-Escudero JP, et al. Normal weight obesity and mortality in United States subjects >/=60 years of age (from the Third National Health and Nutrition Examination Survey). Am J Cardiol 2013; 112:1592–1598. [DOI] [PubMed] [Google Scholar]
- 7.Han KJ, Lee SY, Kim NH, et al. Increased risk of diabetes development in subjects with the hypertriglyceridemic waist phenotype: a 4-year longitudinal study. Endocrinol Metab (Seoul) 2014; 29:514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinnouho GM, Czernichow S, Dugravot A, et al. Metabolically healthy obesity and risk of mortality: does the definition of metabolic health matter? Diabetes Care 2013; 36:2294–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung HS, Chang Y, Eun Yun K, et al. Impact of body mass index, metabolic health and weight change on incident diabetes in a Korean population. Obesity (Silver Spring) 2014; 22:1880–1887. [DOI] [PubMed] [Google Scholar]
- 10.Kuk JL, Ardern CI. Are metabolically normal but obese individuals at lower risk for all-cause mortality? Diabetes Care 2009; 32:2297–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meigs JB, Wilson PW, Fox CS, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab 2006; 91:2906–2912. [DOI] [PubMed] [Google Scholar]
- 12.Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J 2010; 31:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinnouho GM, Czernichow S, Dugravot A, et al. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: the Whitehall II cohort study. Eur Heart J 2015; 36:551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appleton SL, Seaborn CJ, Visvanathan R, et al. Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype: a cohort study. Diabetes Care 2013; 36:2388–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heianza Y, Kato K, Kodama S, et al. Stability and changes in metabolically healthy overweight or obesity and risk of future diabetes: Niigata wellness study. Obesity (Silver Spring) 2014; 22:2420–2425. [DOI] [PubMed] [Google Scholar]
- 16.Achilike I, Hazuda HP, Fowler SP, et al. Predicting the development of the metabolically healthy obese phenotype. Int J Obesity (2005) 2015; 39:228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eshtiaghi R, Keihani S, Hosseinpanah F, et al. Natural course of metabolically healthy abdominal obese adults after 10 years of follow-up: the Tehran Lipid and Glucose Study. Int J Obesity (2005) 2015; 39:514–519. [DOI] [PubMed] [Google Scholar]
- 18.Soriguer F, Gutierrez-Repiso C, Rubio-Martin E, et al. Metabolically healthy but obese, a matter of time? Findings from the prospective Pizarra study. J Clin Endocrinol Metab 2013; 98:2318–2325. [DOI] [PubMed] [Google Scholar]
- 19.Lee SH, Han K, Yang HK, et al. Identifying subgroups of obesity using the product of triglycerides and glucose: the Korea National Health and Nutrition Examination Survey, 2008–2010. Clin Endocrinol (Oxf) 2015; 82:213–220. [DOI] [PubMed] [Google Scholar]
- 20.Lee SH, Han K, Yang HK, et al. A novel criterion for identifying metabolically obese but normal weight individuals using the product of triglycerides and glucose. Nutr Diabetes 2015; 5:e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SH, Ha HS, Park YJ, et al. Identifying metabolically obese but normal-weight (MONW) individuals in a nondiabetic Korean population: the Chungju Metabolic disease Cohort (CMC) study. Clin Endocrinol (Oxf) 2011; 75:475–481. [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, Kwon HS, Park YM, et al. Predicting the development of diabetes using the product of triglycerides and glucose: The Chungju Metabolic Disease Cohort (CMC) Study. PLoS One 2014; 9:e90430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28:412–419. [DOI] [PubMed] [Google Scholar]
- 24.Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab 2010; 95:3347–3351. [DOI] [PubMed] [Google Scholar]
- 25.Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord 2008; 6:299–304. [DOI] [PubMed] [Google Scholar]
- 26.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 27.Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37 (Suppl 1):S81–S90. [DOI] [PubMed] [Google Scholar]
- 28.Dotevall A, Johansson S, Wilhelmsen L, et al. Increased levels of triglycerides, BMI and blood pressure and low physical activity increase the risk of diabetes in Swedish women. A prospective 18-year follow-up of the BEDA study. Diabet Med 2004; 21:615–622. [DOI] [PubMed] [Google Scholar]
- 29.Tirosh A, Shai I, Bitzur R, et al. Changes in triglyceride levels over time and risk of type 2 diabetes in young men. Diabetes Care 2008; 31:2032–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tirosh A, Rudich A, Shochat T, et al. Changes in triglyceride levels and risk for coronary heart disease in young men. Ann Intern Med 2007; 147:377–385. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt MI, Duncan BB, Bang H, et al. Identifying individuals at high risk for diabetes: The Atherosclerosis Risk in Communities study. Diabetes Care 2005; 28:2013–2018. [DOI] [PubMed] [Google Scholar]
- 32.Tirosh A, Shai I, Tekes-Manova D, et al. Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med 2005; 353:1454–1462. [DOI] [PubMed] [Google Scholar]
- 33.Du T, Yuan G, Zhang M, et al. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol 2014; 13:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnlov J, Ingelsson E, Sundstrom J, et al. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation 2010; 121:230–236. [DOI] [PubMed] [Google Scholar]
- 35.Badoud F, Perreault M, Zulyniak MA, et al. Molecular insights into the role of white adipose tissue in metabolically unhealthy normal weight and metabolically healthy obese individuals. FASEB J 2015; 29:748–758. [DOI] [PubMed] [Google Scholar]
- 36.Aung K, Lorenzo C, Hinojosa MA, et al. Risk of developing diabetes and cardiovascular disease in metabolically unhealthy normal-weight and metabolically healthy obese individuals. J Clin Endocrinol Metab 2014; 99:462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamer M, Stamatakis E. Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J Clin Endocrinol Metab 2012; 97:2482–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morkedal B, Vatten LJ, Romundstad PR, et al. Risk of myocardial infarction and heart failure among metabolically healthy but obese individuals: HUNT (Nord-Trondelag Health Study), Norway. J Am Coll Cardiol 2014; 63:1071–1078. [DOI] [PubMed] [Google Scholar]
- 39.Janiszewski PM, Ross R. Effects of weight loss among metabolically healthy obese men and women. Diabetes Care 2010; 33:1957–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu RH, Wharton S, Sharma AM, et al. Influence of a clinical lifestyle-based weight loss program on the metabolic risk profile of metabolically normal and abnormal obese adults. Obesity (Silver Spring) 2013; 21:1533–1539. [DOI] [PubMed] [Google Scholar]
- 41.Ruiz JR, Ortega FB, Labayen I. A weight loss diet intervention has a similar beneficial effect on both metabolically abnormal obese and metabolically healthy but obese premenopausal women. Ann Nutr Metab 2013; 62:223–230. [DOI] [PubMed] [Google Scholar]
- 42.Shin MJ, Hyun YJ, Kim OY, et al. Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. Int J Obesity (2005) 2006; 30:1529–1534. [DOI] [PubMed] [Google Scholar]
- 43.Karelis AD, Messier V, Brochu M, et al. Metabolically healthy but obese women: effect of an energy-restricted diet. Diabetologia 2008; 51:1752–1754. [DOI] [PubMed] [Google Scholar]


