Abstract
This study examined the influence of preoperative administration of amiodarone and metoprolol in preventing postoperative atrial fibrillation (AF) after coronary artery bypass grafting (CABG) surgery.
The study comprised 251 patients who underwent CABG surgery at our hospital between January 2012 and May 2014. The patients were randomly divided into 2 groups: amiodarone therapy group (n = 122 patients) and metoprolol therapy group (n = 129 patients).
In the amiodarone group, the patients received amiodarone tablet orally 1 week before coronary bypass surgery and during the postoperative period. In the metoprolol group, the patients received metoprolol tablet orally 1 week before surgery and during the postoperative period. The AF development rate was retrospectively evaluated between the first 3 days and 4 weeks after surgery.
AF developed in 14 patients in the amiodarone group and 16 patients in the metoprolol group 4 weeks after the operation (P = 0.612).
No significant difference was observed between the groups in terms of intensive care unit and hospital stay. Furthermore, hospital charges were similar in both groups (P = 0.741).
The results of the logistic regression analysis showed age, left ventricular ejection fraction, left atrial diameter, and aortic cross-clamping time to be predictors for postoperative AF.
This study demonstrates that amiodarone and metoprolol have similar effects in prevention of AF after cardiac surgery. However, larger-scale studies need to be conducted to substantiate these findings.
INTRODUCTION
Atrial fibrillation (AF) is still among the most common morbidities after cardiac surgery with its incidence being ranged from 10% to 65% despite the advances in both surgical practice and medical management. The incidence is mainly dependent on the type of operation as well as on patient characteristics, definition of the arrhythmia, and study follow-up or design. As the population is aging and number of cardiac surgical operations is increasing, incidence of AF has gradually increased recently. Moreover, AF brings about several problems, including hemodynamic derangement, thromboembolic complications, longer time of hospital stay, and higher costs.1,2
There have been several pharmacological and nonpharmacological strategies suggested for prevention against AF after coronary artery bypass grafting (CABG).3,4 Some studies showed that β-blockers did not achieve any benefit in prevention of postoperative AF in patients undergoing CABG.2–4 Recent evidence demonstrated that use of certain drugs, such as antiplatelet agents, statins, and angiotensin-converting enzyme inhibitors, decreases the risk of postoperative complications. These medications were also shown to inhibit the inflammatory response that occurs because of cardiopulmonary bypass (CPB), thereby preventing early arrhythmias including AF.5–7 The present study investigated the role of amiodarone and metoprolol in prevention of AF following CABG. If not contraindicated, we suggest continuation of these drugs both before and after the operation in patients undergoing open heart surgery.
PATIENTS, MATERIAL, AND METHODS
This was a prospective randomized study and made up of a total of 251 patients (147 male, 104 female) undergoing CABG in our department between January 2012 and May 2014. The study was approved by the institutional review board of Erzurum Regional Training and Research Hospital. A procedure-oriented informed consent form was signed by each patient. Hospital Ethical Committee also approved the study. All procedures were performed in accordance with the Declaration of Helsinki.
The patients were randomly divided into 2 groups: the amiodarone therapy group (group I; n = 122 patients) and the metoprolol therapy group (group II; n = 129 patients). In group I, the patients received amiodarone tablets orally (200 mg/d as 3 × 1) 1 week before coronary bypass surgery and during the postoperative period. In group II, the patients received metoprolol tablets orally (50 mg/d as 2 × 1) 1 week before surgery and during the postoperative period. The mean age in group I was 56.1 ± 4.1 years (45–78 years) and in group II was 59.2 ± 4.2 years (46–75 years). On transthoracic echocardiography, left ventricle ejection fraction (LVEF) was found to be 42.2% ± 4.0% in group I and 43.1% ± 3.9% in group II. Eleven (9%) patients in group I and 13 (10%) patients in group II had the insertion of an intraaortic balloon pulsation (IABP) preoperatively. The criteria for preoperative IABP were as follows: cardiogenic shock or refractory ventricular failure, hemodynamic instability, refractory angina, ventricular arrhythmia, and a critical left main stenosis (>70%).
Inclusion Criteria
All consecutive adult patients undergoing cardiac surgery and without contraindications to β-blockage and amiodarone were included in this study. The criteria for inclusion were that patients needed to be referred for primary elective coronary artery and should be in normal sinus rhythm.
Exclusion Criteria
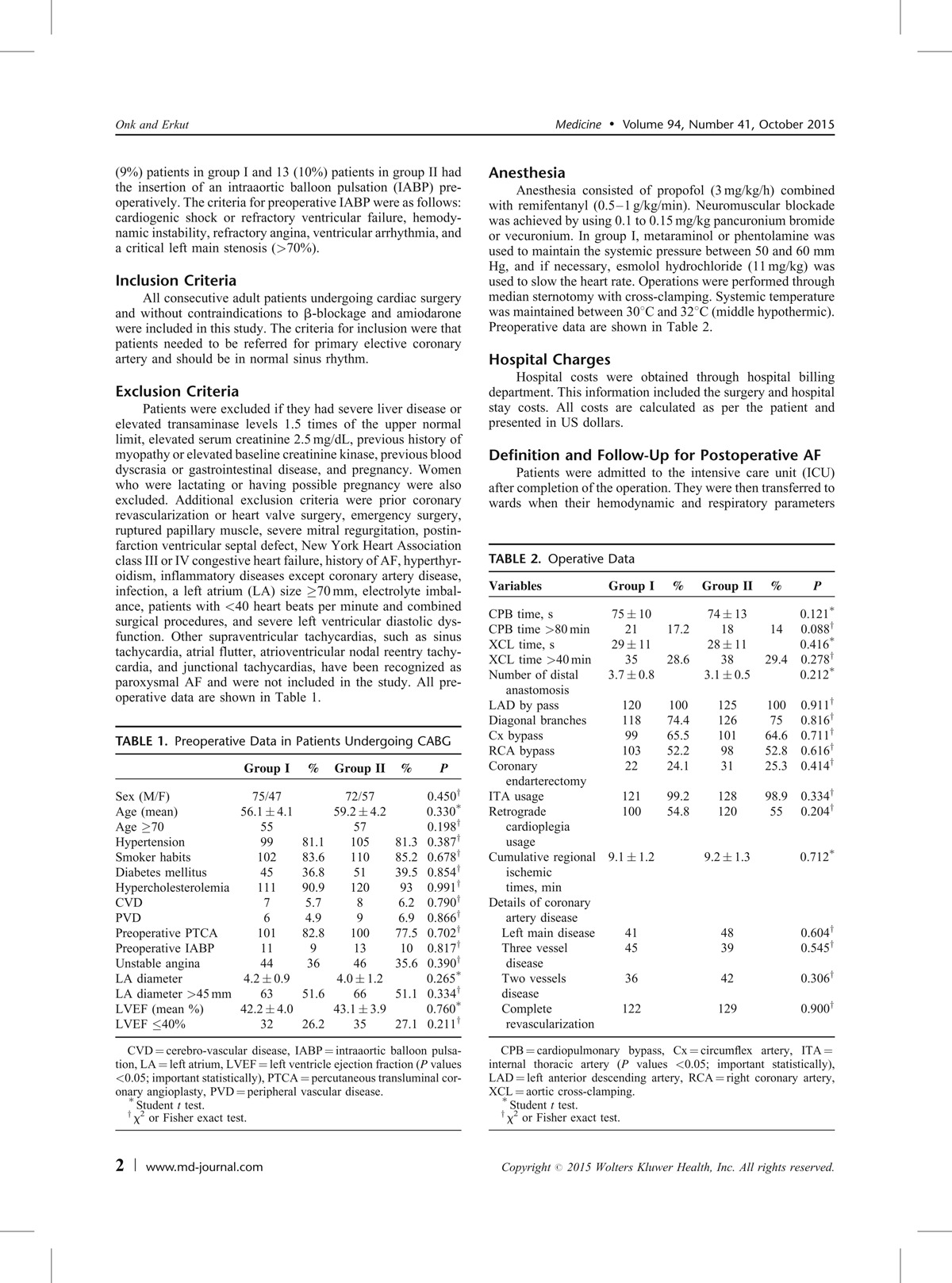
Patients were excluded if they had severe liver disease or elevated transaminase levels 1.5 times of the upper normal limit, elevated serum creatinine 2.5 mg/dL, previous history of myopathy or elevated baseline creatinine kinase, previous blood dyscrasia or gastrointestinal disease, and pregnancy. Women who were lactating or having possible pregnancy were also excluded. Additional exclusion criteria were prior coronary revascularization or heart valve surgery, emergency surgery, ruptured papillary muscle, severe mitral regurgitation, postinfarction ventricular septal defect, New York Heart Association class III or IV congestive heart failure, history of AF, hyperthyroidism, inflammatory diseases except coronary artery disease, infection, a left atrium (LA) size ≥70 mm, electrolyte imbalance, patients with <40 heart beats per minute and combined surgical procedures, and severe left ventricular diastolic dysfunction. Other supraventricular tachycardias, such as sinus tachycardia, atrial flutter, atrioventricular nodal reentry tachycardia, and junctional tachycardias, have been recognized as paroxysmal AF and were not included in the study. All preoperative data are shown in Table 1.
TABLE 1.
Preoperative Data in Patients Undergoing CABG
Anesthesia
Anesthesia consisted of propofol (3 mg/kg/h) combined with remifentanyl (0.5–1 g/kg/min). Neuromuscular blockade was achieved by using 0.1 to 0.15 mg/kg pancuronium bromide or vecuronium. In group I, metaraminol or phentolamine was used to maintain the systemic pressure between 50 and 60 mm Hg, and if necessary, esmolol hydrochloride (11 mg/kg) was used to slow the heart rate. Operations were performed through median sternotomy with cross-clamping. Systemic temperature was maintained between 30°C and 32°C (middle hypothermic). Preoperative data are shown in Table 2.
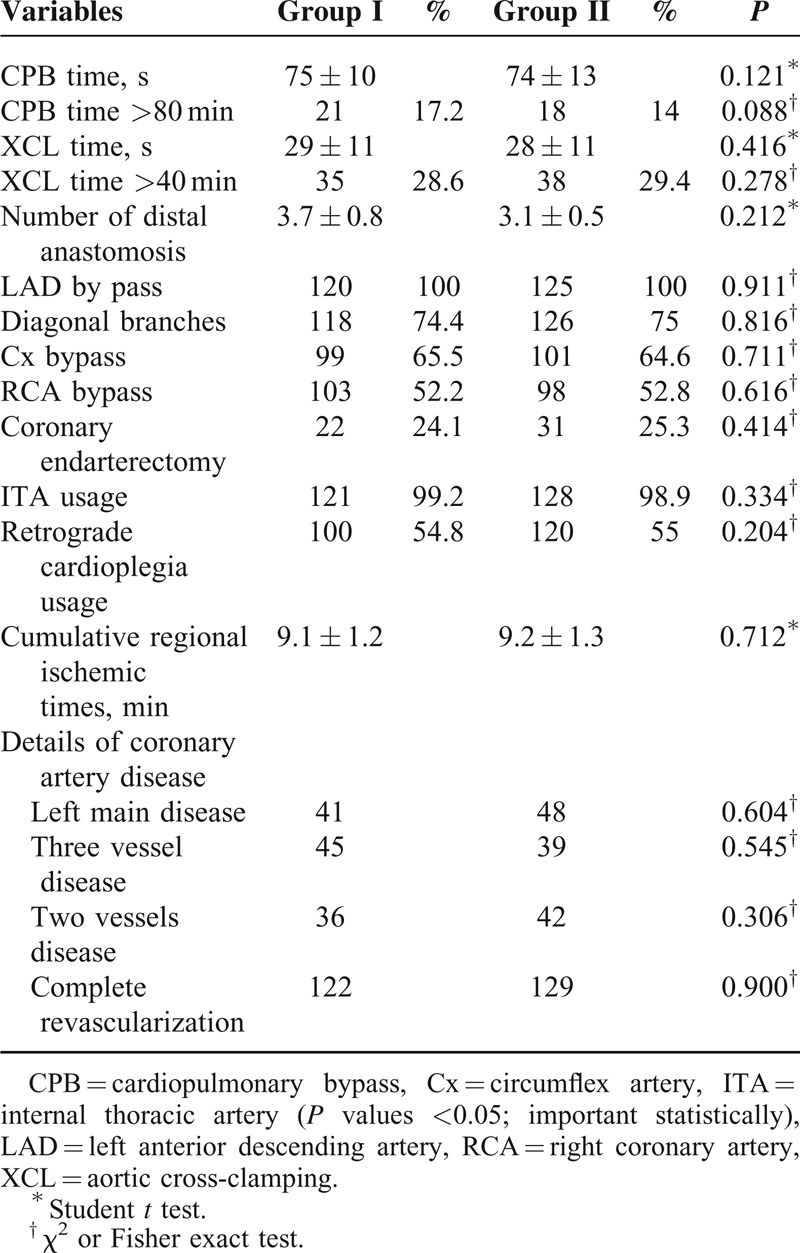
TABLE 2.
Operative Data
Hospital Charges
Hospital costs were obtained through hospital billing department. This information included the surgery and hospital stay costs. All costs are calculated as per the patient and presented in US dollars.
Definition and Follow-Up for Postoperative AF
Patients were admitted to the intensive care unit (ICU) after completion of the operation. They were then transferred to wards when their hemodynamic and respiratory parameters become stable. Routine electrocardiography monitoring was continued during the operation and during 2 days after the operation. Twelve-lead electrocardiography monitoring was performed in the ward. An electrocardiograph was taken at least 2 times daily on a routine basis. All episodes of AF were documented with 12-lead electrocardiographs, and these were assessed by 2 blinded cardiologists. All patients underwent a control echocardiography and electrocardiography within 4 weeks after the operation. Survival information was obtained by phone interview.
Hospital mortality was defined as death for any reason occurring within 30 days after the operation. An increase of ≥1.4% in plasma creatinine indicated the impairment of renal function.8 Neurological complications were defined as any transient or permanent neurological deficit that developed after surgery. Gastrointestinal complications included confirmed diagnosis of upper and lower gastrointestinal hemorrhage, intestinal ischemia, acute cholecystis, and pancreatitis. Generally, mortality, preoperative acute myocardial infarction, IABP usage, incidence of low cardiac output syndrome (LCOS), renal failure, use of inotropic agent, ICU and hospital stay, cardiac hemodynamic changes, bleeding, revision rates, gastrointestinal, pulmonary, and neurological complications, infections, and survive rates were determined.
Statistical Analysis
All statistical calculations were done using the software package SPSS (Statistical Package for the Social Science, version 17; SPSS Inc, Chicago, IL) for Microsoft Windows. Data were statistically expressed in terms of the mean ± standard deviation, a frequency (number of cases), or a percentage when appropriate for all the continuous variables. The difference in continuous variables was analyzed using unpaired Student t test. Categorical variables between the 2 groups were compared using the χ2 test, corrected by the Fisher exact test when appropriate. The relationship between each variable and the development of postoperative AF was evaluated by a logistic regression analysis (univariate as dependent variables and the preoperative factors as independent variables). First, the univariate logistic regression analysis was performed to determine the significant predictors of AF after CABG surgery. Factors with a P value of <0.05 in the univariate analysis were considered as candidates for multivariable analysis, which was performed to determine the independent predictors of AF. The results of the logistic regression analysis were presented as odds ratios (ORs) and 95% confidence intervals (CIs). Statistically significant differences were noted for each analysis, with statistical significance based on a P value of <0.05.
RESULTS
Baseline patients’ characteristics were similar for the 2 study groups (Table 1). No differences were observed in the preoperative patients’ characteristics between the 2 groups, and no statistically significant differences were reported in the preoperative features (P > 0.05).
Table 2 shows intraoperative variables of the patients. The groups were similar with respect to the number of grafts (including the use of internal thoracic vessels), ischemic time and total perfusion time, retrograde cardioplegia usage, the number of endarterectomies conducted, and internal thoracic artery usage; these values were not statistically different (Table 2). The mean overall number of distal anastomoses was 3.7 ± 0.8 versus 3.1 ± 0.5 (P = 0.212).
No difference was reported in the number of bypassed vessels, type of arterial conduits, or sites of surgical anastomoses between the groups. The details on the extent of coronary artery disease are shown in Table 2.
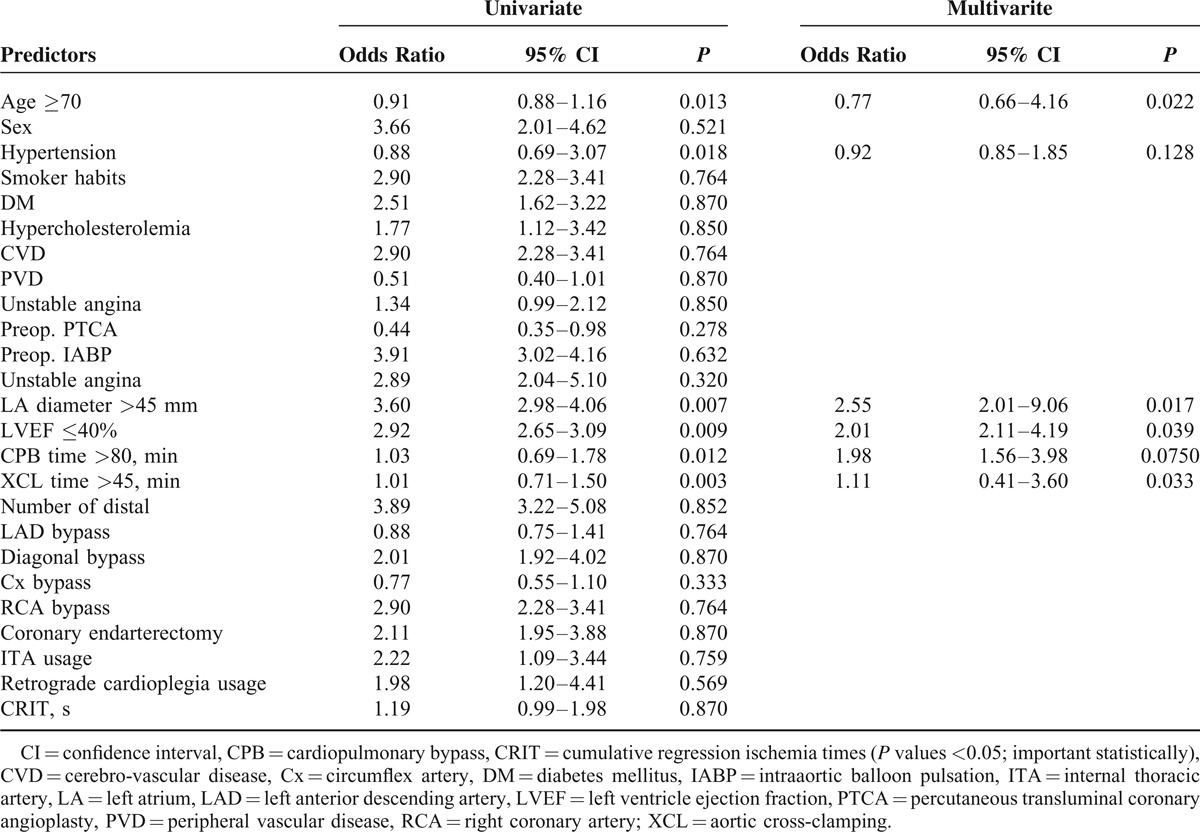
Table 3 shows the results of univariate analysis of factors related with the development of postoperative AF. The unadjusted univariate analysis demonstrated that the risk factors related with AF were age ≥70 (P = 0.013), hypertension (P = 0.018), LA antero-posterior diameter >45 mm (P = 0.007), LVEF ≤40 (P = 0.009), CPB time >80 minutes (P = 0.012), and aortic cross-clamping (XCL) time >45 minutes (P = 0.003). Other variables were not significantly associated with the development of postoperative AF. After eliminating variables that were closely related to others, these independent risk factors for AF were adopted as confounders in the logistic regression model for the multivariate analysis. Four factors were identified as independent predictors of postoperative AF after CABG surgery in a multivariate analysis: age ≥70 (P = 0.022, OR: 0.77; 95% CI: 0.66–4.16), LA diameter >45 mm (P = 0.017, OR: 2.55; 95% CI: 2.01–9.06), LVEF ≤40 (P = 0.039, OR: 2.01, 95% CI: 2.11–4.19), and XCL time >45 minutes (P = 0.033, OR: 1.11; 95% CI: 0.41–3.60).
TABLE 3.
Univariate and Multivariate Logistic Regression Analysis to Identify Predictors for Risk Factors Associated With Postoperative AF
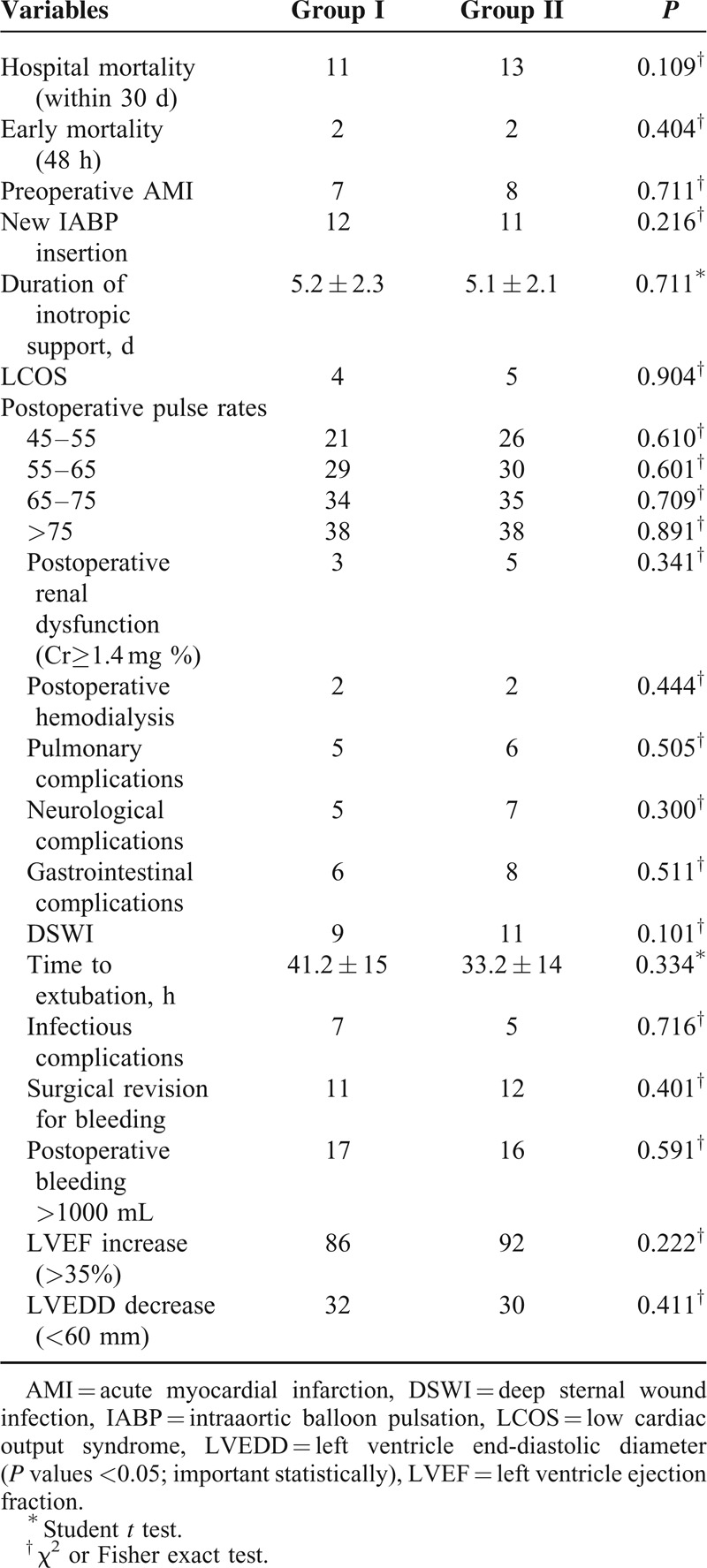
Postoperative survival, complications, and data between the groups are shown in Table 4. There were no statistical differences in the amount of bleeding, amount of blood products’ use, duration of inotropic support, amount of drainage, duration of extubation, revision for bleeding, and sternal dehiscence in the groups. The postoperative use of IABP, preoperative acute myocardial infarction, postoperative renal dysfunction, and LCOS were similar in the groups (P > 0.05). Although pulmonary, neurological, gastrointestinal, and infectious complications were identified postoperatively in both groups, these complications were not statistically different between the groups (Table 4). Hospital mortality was observed in 11 patients (9%) in group I versus 13 patients (10%) in the control group (P = 0.109). Operative mortality was the same for the 2 groups. The cause of death was low cardiac output. Early mortality within 48 hours was observed in 2 patients in group I and 2 patients in group II (P = 0.404). Early and late pericardial effusion was detected by echocardiography. There was no pleural effusion requiring intervention in any group, and we did not encounter pericardial tamponade in the patients. The echocardiographic examination within a month revealed improvement in the left ventricle function. Ejection fraction increase and left ventricle end-diastolic diameter decrease were higher in both groups. However, these differences between the groups were not statistically significant (P = 0.222 and P = 0.411, respectively; Table 4).
TABLE 4.
Postoperative Parameters Between Groups
The mean follow-up time of the survivors was 4 weeks. In the assessment between the first 3 days and a week, the AF rates were statistically similar for the 2 groups. At the end of the fourth week, AF appeared in 22 patients (18.1 %) in group I and 25 patients (19.3%) in group II. No statistically significant difference was observed between the groups with respect to AF (P = 0.612; Table 5).
TABLE 5.
The Comparison of Postoperative AF, Length of Stay in ICU and Hospital, Hospital Fees Between Groups

ICU and hospital length of stay was significantly higher in patients with AF than without AF (Table 5). However, compared to without AF patient, it was observed that the duration of ICU and hospital stay was similar between the treatment groups, and there were no statistically significant differences (P = 0.636 and P = 0.505, respectively; Table 5).
The hospital charges were >$5000 in 21 patients in group I and 24 patients in group II ongoing AF. In the non-AF patients, hospital charges were over expenses $5000, only in 2 patients in the group I and 3 patients in the group II. These results show us the number of patients that costs >$5000 were higher in patients who continue AF (Table 5), but there was no statistically significant difference in patients without AF between treatment groups in terms of hospital charge (P = 0.212).
DISCUSSION
Despite significant progress in terms of heart surgery in the last 50 years, AF after CABG is still the most common complication. It may often cause prolonged ICU and hospital stay after surgical treatment.9 As reported in the literature, AF occurs most frequently in the first week postoperatively, and the incidence ranges between 30% and 60%.10,11 Although it usually does not cause postoperative mortality, AF often can induce hemodynamic impairment and thromboembolic events, and requires antiarrhythmic therapy. Because AF extends the duration of ICU and hospital stay, it accounts for the increased hospital costs.9,12
Many factors can lead to the development of AF postoperatively, and the reentry mechanism is acceptable as the main cause of AF. Operative trauma, increased atrial pressure, autonomic nervous system disproportion, metabolic and electrolyte changes, and myocardial ischemia contribute to arrhythmia. Moreover, old age, hypertension, low ejection fraction, inadequate myocardial preservation, and ICU stress can increase the incidence of postoperative AF. However, the electrophysiological mechanisms of postoperative AF are not fully understood yet. Some of the factors triggering AF are pericardial inflammation combined with autonomic imbalance, extreme catecholamine release, and hemodynamic factors. Systemic and local inflammatory responses can contribute to the pathogenesis of postoperative AF. Despite many etiologic and predisposing factors, it is difficult to demonstrate a single causal factor. It is possible that the main reason for postoperative AF is the interaction between all these factors, as observed with all cardiac surgery patients.
A number of treatments and drugs, such as atrial pacing, oral or intravenous amiodarone and/or metoprolol, and magnesium therapy, have been reported to prevent AF after surgery.11–14 However, this needs to be investigated further because the results concerning the efficacy of these medications and treatments in preventing postoperative AF are conflicting.15 Because the effective treatments remain uncertain, this study compared the effect of metoprolol and amiodarone in preventing AF after CABG.
The efficacy of pharmacologic prophylaxis in reducing the incidence of AF has been investigated in several studies.16,17 Two main groups of drugs have been shown to be effective in preventing postoperative AF: antiarrhythmic and anti-inflammatory agents.18–20 In addition, calcium channel-blocking agents, and recently nonchannel blockers, have been proven as promising candidates.
Some clinical trials using β-adrenergic antagonists have demonstrated prophylactic benefit in postoperative AF21–23; however, other studies showed conflicting conclusions.16,24,25 A possible reason for this difference may be that in some studies, patients randomly assigned to placebo were treated with β-adrenergic blockers until the time of surgery.11 According to the American College of Cardiology/American Heart Association and the European Society of Cardiology Guidelines for AF, the preoperative or early postoperative administration of β-blockers in patients without contraindications is evidence for preventing AF after CABG surgery.26 β-blockers have been demonstrated as strong prophylactic agents and to have a lower risk than other antiarrhythmic agents.26,27 The AF rate observed in this study was similar to the findings of several other studies,17,28,29 and the postoperative AF rate significantly reduced with the prophylactic use of β-blockers. The efficacy and safety of metoprolol have been studied in preventing AF after cardiac surgery.21,22,27,30–32 These studies concluded that both β-blockers were effective in AF prophylaxis when compared with the placebo. Metoprolol has been presumed to protect better than traditional β-blockers because it blocks β1- and β2-receptors. Therefore, this study investigated the efficacy of metoprolol therapy in preventing AF. No potential side effects were observed during the therapy. The postoperative AF ratio is known to be 30% to 60%. In the present study, the AF ratio was found to be 19.3% with metoprolol treatment, a slightly greater reduction than that reported by Janssen et al,21 who also used the same medication with fixed dosages and almost the same type of monitoring. The meta-analyses by Andrews et al33 and Kowey et al34 reported 74% and 51% reduction, respectively, in the risk of arrhythmias with the use of β-blockers. The present study was more effective in AF prevention compared with previous works.
Amiodarone is well tolerated and does not cause major complications postoperatively. With the more widespread use of amiodarone, several side effects have been identified. In the majority of patients, these are well tolerated and are often modified by a reduction in dosage so that the discontinuation of therapy is rarely necessary. Although the mechanisms are unclear, amiodarone can cause thyroid, pulmonary, hepatic, neurological, and ocular function disorders. Patients should be monitored in time because of unwanted side effects. Amiodarone therapy was not associated with pro-arrhythmia or serious adverse reactions, even among patients with severe coronary disease. Among the CABG surgery patients, amiodarone reduced the ventricular rate and AF more significantly than the placebo. The effectiveness of amiodarone in preventing AF has been demonstrated in many studies.15,35–37 According to the double-blind randomized placebo-controlled study of Gu Song et al,36 postoperative AF occurred in 16% of patients receiving amiodarone and in 37.7% of patients receiving placebo. In this study, the AF rate was 18.1% in patients who received amiodarone. In contrast, Mahoney et al38 assessed the cost-effectiveness of intravenous amiodarone therapy, and found that the routine use of intravenous amiodarone after CABG is not cost-effective. The AF frequency significantly reduced in this study with the prophylactic use of amiodarone, and the results were similar to the findings of other studies.35,37,38 Despite opposing views, the effects of this antiarrhythmic agent were compared with those of β-blockers. Each of the 2 agents was found to be superior in preventing AF; no statistical difference was noted between the 2 agents in terms of AF prevention after CABG.
The incidence of AF after cardiac surgery is influenced by various factors.31,36,39,40 Age has been repeatedly shown to be the major risk factor for AF after cardiac surgery.12,39,41 Despite no statistical difference between the groups in terms of age, our results showed that age ≥70 years is a risk factor for postoperative AF. Thus, on the basis of age alone, patients >70 years are considered to be at high risk for developing AF. Researchers found that patients with a history of AF had an increased risk of postoperative AF. Mathew et al40 found that postoperative AF increased by approximately twofold in patients with a history of AF. No preoperative atrial arrhythmias were reported in the 2 groups. LA diameter increase was shown to be a major cause for AF in many studies.42–44 No difference was observed between the groups in terms of preoperative LA diameter in this study. However, LA diameter increase (>45 mm) is found to be a significant risk factor for AF processing. Although some authors have emphasized that a decrease in LVEF rate is insignificant for the development of postoperative AF,45,46 a few others have realized this as a risk factor for postoperative AF.47–49 In this study, no significant difference was observed between the groups in relation to ejection fraction in preoperative and postoperative periods. However, LVEF ≤40% has been identified as a major risk factor for postoperative AF. XCL time was significant in some studies in terms of the occurrence of postoperative AF,50 but some studies found it to be insignificant.51 When it is seen in the comparison between groups, although there is no difference in terms of XCL time, but XCL time > 45 minutes has been identified as a risk factor for postoperative AF. In accordance with our results in previous studies, the history of AF, ejection fraction <50%, left atrial size >50 mm, and XCL time were significantly related to post-open heart AF.30,36 In this study, multivariate analysis revealed that age ≥70 years, LA diameter >45 mm, LVEF ≤40%, and XCL times >45 minutes were associated with an increased risk of AF and identified as predictors for posterative AF.
The lower heart rate observed in patients receiving amiodarone compared with patients receiving metoprolol confirms the beneficial effect of amiodarone in the present study, but this result was not statistically significant. The discontinuation of β-blockers or amiodarone postoperatively has been a point of discussion for a long time because it would leave the patient more exposed to the action of circulating catecholamines, increasing the risk of arrhythmias.30,31,40 In both groups, the patients were followed up for the first weeks. The electrocardiography monitor device was applied only during the first 48 hours postoperatively in both the groups. The later rhythm follow-up was performed with daily electrocardiogram until the day of discharge, and the final follow-up was made in the first month. Side effects, such as bradycardia and heart block, were not observed in any of our patients; hence, the medication was not discontinued in any of the groups. All the patients tolerated amiodarone and metoprolol well with acceptable side effects; no complications were reported pre- and postoperatively, and postoperative thyroid function tests were within normal limits. This study demonstrates the safe initiation of amiodarone and metoprolol treatment after CABG.
Postoperative AF can increase the length of hospital and ICU stay after CABG,52 and hence increasing the costs to US $10,000.53,54 Crystal et al15 conducted meta-analysis on 52 randomized trials, and concluded that β-blockers reduced the percentage of patients with AF from 33% in the control group to 19% in the β-blocker group with no significant effect on hospital stay after open heart surgery. In addition, the percentage of patients with AF was reduced from 37% in the control group to 22.2% in the β-blocker group with significant differences between the length of hospital stay and total hospital costs.53,54 If rhythm problems do not occur, the length of ICU stay is approximately 2 days after CABG. The length of ICU stay was approximately 1.7 and 1.8 days in our groups (without AF), respectively. When rhythm problems such as AF develop, the period can extend up to 3 to 5 days. A prolonged stay in the ICU also extends the length of hospital stay. The duration of hospital stay is 7 to 10 days normally. The length of hospital stay was 8.2 and 8.4 days in our groups (without AF), respectively, which may increase up to 10 to 15 days in patients with AF (Table 5). These results show that the duration of ICU and hospital stay was within the normal limits in patients without AF. In our hospital, the operation cost was $4000 to $5000 for uncomplicated CABG surgery. If rhythm problems develop, the duration of hospitalization increases and the cost exceeds $5000. The hospital costs were >$5000 in patients with AF until the discharge day in this study. There was no difference between the treatment groups in patients with ongoing AF. In this study, the amiodarone and metoprolol groups showed fewer incidences of postoperative AF, shorter ICU and hospital stay, and reduced hospital costs (Table 5). The results were similar to the study of Nayeem et al30 and were consistent with the relevant literature.15,53
Prophylactic amiodarone and metoprolol for 1 week before CABG and after elective CABG were well tolerated and significantly reduced the incidence of postoperative AF and the number of symptomatic episodes of AF occurring after discharge. In addition, this study demonstrated that these agents significantly reduced the length and total cost of hospitalization. Amiodarone and metoprolol have similar efficiency in controlling postoperative AF. However, a larger randomized controlled trial is needed to corroborate the results of this study.
LIMITATIONS
The first limitation of this study is that Holter monitoring could not be performed to detect postoperative AF because of device failure. In addition, asymptomatic AF was improbable to influence patient care or make it unfavorable. However, the primary goal of this study was to address the clinical utility of prophylactic amiodarone and metoprolol.
The second limitation is that the administration of AF was directed by the cardiac surgeon. To avoid disruptions in the follow-up, the patients were followed up in the hospital where they were operated on. In addition, because of socio-cultural trends in our region, the patients wanted to be followed up in the same clinic where the surgery was performed.
Footnotes
Abbreviations: AF = atrial fibrillation, CABG = coronary artery bypass grafting, CI = confidence interval, CPB = cardiopulmonary bypass, IABP = intraaortic balloon pulsation, ICU = intensive care unit, LA = left atrium, LCOS = low cardiac output syndrome, LVEF = left ventricle ejection fraction, ORs = odds ratios, XCL = aortic cross-clamping.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Khan MF, Wendel CS, Movahed MR. Prevention of post-coronary artery bypass grafting (CABG) atrial fibrillation: efficacy of prophylactic beta-blockers in the modern era: a meta-analysis of latest randomized controlled trials. Ann Noninvasive Electrocardiol 2013; 18:58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villareal RP, Hariharan R, Liu BC, et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol 2004; 43:742–748. [DOI] [PubMed] [Google Scholar]
- 3.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 2006; 114:e257–e354. [DOI] [PubMed] [Google Scholar]
- 4.Balcetyte-Harris N, Tamis JE, Homel P, et al. Randomized study of early intravenous esmolol versus oral beta-blockers in preventing post-CABG atrial fibrillation in high risk patients identified by signal-averaged ECG: results of a pilot study. Ann Noninvasive Electrocardiol 2002; 7:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomic V, Russwurm S, Moller E. Transcriptomic and proteomic patterns of systemic inflammation in on-pump and off-pump coronary artery bypass grafting. Circulation 2005; 112:2912–2920. [DOI] [PubMed] [Google Scholar]
- 6.Wijeysundera DN, Beattie WS, Djaiani G. Off pump coronary artery surgery for reducing mortality and morbidity: meta-analysis of randomized and observational studies. J Am Coll Cardiol 2005; 46:872–882. [DOI] [PubMed] [Google Scholar]
- 7.Patti G, Chello M, Candura D. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA-3 (Atorvastatin for Reduction of Myocardial Dysrhythmia After cardiac surgery) study. Circulation 2006; 114:1455–1461. [DOI] [PubMed] [Google Scholar]
- 8.Mistiaen W, Van Cauwelaert P, Muylaert P, et al. A thousand pericardial valves in aortic position: risk factors for postoperative acute renal function impairment in elderly. J Cardiovasc Surg (Torino) 2009; 50:233–237. [PubMed] [Google Scholar]
- 9.Creswell LL, Schuessler RB, Rosenbloom M, et al. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg 1993; 56:539–549. [DOI] [PubMed] [Google Scholar]
- 10.Fuller JA, Adams GG, Buxton B. Atrial fibrillation after coronary artery bypass surgery grafting: is it a disorder of the elderly? J Thorac Cardiovasc Surg 1989; 97:821–825. [PubMed] [Google Scholar]
- 11.Frost L, Molgaard H, Christiansen EH, et al. Atrial fibrillation and flutter after coronary artery bypass surgery: epidemiology, risk factors and preventive trials. Int J Cardiol 1992; 36:253–261. [DOI] [PubMed] [Google Scholar]
- 12.Leitch JW, Thomson D, Baird DK, et al. The importance of age as a predictor of atrial fibrillation and flutter after coronary artery bypass grafting. J Thorac Cardiovasc Surg 1990; 100:338–342. [PubMed] [Google Scholar]
- 13.Miller S, Chrystal E, Garfinkle M, et al. Effects of magnesium on atrial fibrillation after cardiac surgery: a meta-analysis. Heart 2005; 91:618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiga T, Wajima Z, Inove T, et al. magnesium prophylaxis for arrhythmias after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Med 2004; 117:325–333. [DOI] [PubMed] [Google Scholar]
- 15.Crystal E, Connolly SJ, Sleik K, et al. Intervention on prevention of postoperative atrial fibrillation in patients undergoing heart surgery. A meta-analysis. Circulation 2002; 106:75–80. [DOI] [PubMed] [Google Scholar]
- 16.Martinussen HS, Lolk A, Szezepanski C, et al. Supraventricular tachyarrhythmia's after coronary bypass surgery: a double blind randomized trial of prophylactic low dose propranolol. Thorac Cardiovasc Surg 1988; 36:206–207. [DOI] [PubMed] [Google Scholar]
- 17.Imren Y, Benson AA, Zor H. Preoperative beta-blocker use reduces atrial fibrillation in off-pump coronary bypass surgery. ANZ J Surg 2007; 77:429–432. [DOI] [PubMed] [Google Scholar]
- 18.Camm AJ, Kirchhof P, Lip GY, et al. European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010; 31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 19.Imazio M, Brucato A, Ferrazzi P, et al. Colchicine reduces postoperative atrial fibrillation: results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) atrial fibrillation substudy. Circulation 2011; 124:2290–2295. [DOI] [PubMed] [Google Scholar]
- 20.Reinhart K, Baker WL, Ley-Wah Siv M. Beyond the guidelines: new and novel agents for the prevention of atrial fibrillation after cardiothoracic surgery. J Cardiovasc Pharmacol Ther 2011; 16:5–13. [DOI] [PubMed] [Google Scholar]
- 21.Janssen J, Loomans L, Harink J. Prevention and treatment of supraventricular tachycardia shortly after coronary artery bypass grafting: a randomized open trial. Angiology 1986; 37:601–609. [DOI] [PubMed] [Google Scholar]
- 22.Suttorp MJ, Kingma JH, Tjon Joe Gin RM. Efficacy and safety of low- and high-dose sotalol versus propranolol in the prevention of supraventricular tachyarrhythmias early after coronary artery bypass operations. J Thorac Cardiovasc Surg 1990; 100:921–926. [PubMed] [Google Scholar]
- 23.Nystrom U, Edvardsson N, Berggren H, et al. Oral sotalol reduces the incidence of atrial fibrillation after coronary artery bypass surgery. Thorac Cardiovasc Surg 1993; 41:34–37. [DOI] [PubMed] [Google Scholar]
- 24.Ivey MF, Ivey TD, Bailey WW, et al. DW Jr. Influence of propranolol on supraventricular tachycardia early after coronary artery revascularization: a randomized trial. J Thorac Cardiovasc Surg 1983; 85:214–218. [PubMed] [Google Scholar]
- 25.Shafei H, Nashef SA, Turner MA, et al. Does low-dose propranolol reduce the incidence of supraventricular tachyarrhythmias following myocardial revascularization? A clinical study. Thorac Cardiovasc Surg 1988; 36:202–205. [DOI] [PubMed] [Google Scholar]
- 26.Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypasses graft surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). Circulation 2004; 110:e340–437. [PubMed] [Google Scholar]
- 27.Evrard P, Gonzalez M, Jamart J. 2000. Prophylaxis of supraventricular and ventricular arrhythmias after coronary artery bypass grafting with low-dose sotalol. Ann Thorac Surg 2000; 70:151–156. [DOI] [PubMed] [Google Scholar]
- 28.Kerstein J, Soodan A, Qamar M. Giving IV and oral amiodarone perioperatively for the prevention of postoperative atrial fibrillation in patients undergoing coronary artery bypass surgery: the GAP study. Chest 2004; 126:716–724. [DOI] [PubMed] [Google Scholar]
- 29.Martinez EA, Bass EB, Zimetbaum P, et al. Pharmacologic control of rhythm: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest 2005; 128 suppl 2:48S–55S. [DOI] [PubMed] [Google Scholar]
- 30.Nayeem-ul-hassan, Dar AM, Wani ML, et al. A comparative study on the effect of amiodarone and metaprolol for prevention of arrythmias after open heart surgery. Int Cardivasc Res J 2013; 7:1–4. [PMC free article] [PubMed] [Google Scholar]
- 31.Salazar C, Frishman W, Friedman S. Beta-blockade therapy for supraventricular tachyarrhythmias after coronary artery surgery: a propranolol withdrawal syndrome? Angiology 1979; 30:816–819. [DOI] [PubMed] [Google Scholar]
- 32.White HD, Antman GM, Glyn MA. Efficacy and safety of timolol for prevention of supraventricular tachyarrhythmias after coronary bypass surgery. Circulation 1984; 70:479–484. [DOI] [PubMed] [Google Scholar]
- 33.Andrews TC, Reimold CS, Berlin JA. Prevention of supraventricular arrhythmias after coronary artery bypass surgery: a meta-analysis of randomized control trials. Circulation 1991; 84 suppl III:236–244. [PubMed] [Google Scholar]
- 34.Kowey PR, Taylor JE, Rials SL, et al. Meta-analysis of effectiveness of prophylactic drug therapy in preventing supraventricular arrhythmias early after coronary artery bypass grafting. Am J Cardiol 1992; 69:963–965. [DOI] [PubMed] [Google Scholar]
- 35.Daoud EG, Strickberger SA, Ching Man K, et al. Preoperative amiodaroni as prophylaxis against atrial fibrillation after heart surgery. N Engl J Med 1997; 337:1785–1791. [DOI] [PubMed] [Google Scholar]
- 36.Gu S, Su PX, Liu Y, et al. Low-dose amiodarone for the prevention of atrial fibrillation after coronary artery bypass grafting in patients older than 70 years. Chin Med J (Engl) 2009; 122:2928–2932. [PubMed] [Google Scholar]
- 37.Hohnloser SH, Meinertz T, Dammbacher T. Electrocardiographic and antiarrhythmic effects of intravenous amiodarone: results of a prospective, placebo-controlled study. Am Heart J 1991; 121:89–95. [DOI] [PubMed] [Google Scholar]
- 38.Mahoney EM, Thompson TD, Veledar E, et al. Cost-effectiveness of targeting patients undergoing cardiac surgery for therapy with intravenous amiodarone to prevent atrial fibrillation. J Am Coll Cardiol 2002; 40:737–745. [DOI] [PubMed] [Google Scholar]
- 39.Cox JL, Canavan TE, Schuessler RB, et al. The surgical treatment of atrial fibrillation. II. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991; 101:406–426. [PubMed] [Google Scholar]
- 40.Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 2004; 291:1720–1729. [DOI] [PubMed] [Google Scholar]
- 41.De Jong MJ, Morton PG. Predictors of atrial dysrhythmias for patients undergoing coronary artery bypass grafting. Am J Crit Care 2000; 9:388–396. [PubMed] [Google Scholar]
- 42.Rostagno C, Blanzola C, Pinelli F, et al. Atrial fibrillation after isolated coronary surgery. Incidence, long term effects and relation with operative technique. Heart Lung Vessel 2014; 6:171–179. [PMC free article] [PubMed] [Google Scholar]
- 43.Faustino A, Providência R, Barra S, et al. Which method of left atrium size quantification is the most accurate to recognize thromboembolic risk in patients with non-valvular atrial fibrillation? Cardiovasc Ultrasound 2014; 12:28.doi: 10.1186/1476-7120-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhuang J, Wang Y, Tang K, et al. Association between left atrial size and atrial fibrillation recurrence after single circumferential pulmonary vein isolation: a systematic review and meta-analysis of observational studies. Europace 2012; 14:638–645. [DOI] [PubMed] [Google Scholar]
- 45.Jalalian R, Ghafari R, Ghazanfari P. Comparing the therapeutic effects of carvedilol and metoprolol on prevention of atrial fibrillation after coronary artery bypass surgery, a double-blind study. Int Cardiovasc Res J 2014; 8:111–115. [PMC free article] [PubMed] [Google Scholar]
- 46.Haghjoo M, Saravi M, Hashemi MJ, et al. Optimal beta-blocker for prevention of atrial fibrillation after on-pump coronary artery bypass graft surgery: carvedilol versus metoprolol. Heart Rhythm 2007; 4:1170–1174. [DOI] [PubMed] [Google Scholar]
- 47.Acikel S, Bozbas H, Gultekin B, et al. Comparison of the efficacy of metoprolol and carvedilol for preventing atrial fibrillation after coronary bypass surgery. Int J Cardiol 2008; 126:108–113. [DOI] [PubMed] [Google Scholar]
- 48.Shahzamani M, Ghanavati A, Froutagheh AN, et al. Carvedilol compared with metoprolol on left ventricular ejection fraction after coronary artery bypass graft. J Perianesth Nurs 2011; 26:384–387. [DOI] [PubMed] [Google Scholar]
- 49.Dogan SM, Buyukates M, Kandemir O, et al. Predictors of atrial fibrillation after coronary artery bypass surgery. Coron Artery Dis 2007; 18:327–331. [DOI] [PubMed] [Google Scholar]
- 50.Nisanoglu V, Erdil N, Aldemir M, et al. Atrial fibrillation after coronary artery bypass grafting in elderly patients: incidence and risk factor analysis. Thorac Cardiovasc Surg 2007; 55:32–38. [DOI] [PubMed] [Google Scholar]
- 51.Nakai T, Lee RJ, Schiller NB, et al. The relative importance of left atrial function versus dimension in predicting atrial fibrillation after coronary artery bypass graft surgery. Am Heart J 2002; 143:181–186. [DOI] [PubMed] [Google Scholar]
- 52.Ali IM, Sanalla AA, Clark V. Beta-blocker effects on postoperative atrial fibrillation. Eur J Cardiothorac Surg 1997; 11:1154–1157. [DOI] [PubMed] [Google Scholar]
- 53.Bradley D, Creswell LL, Hogue CW, Jr, et al. Pharmacologic prophylaxis: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest 2005; 128 suppl 2:39S–47S. [DOI] [PubMed] [Google Scholar]
- 54.Aranki SF, Shaw DP, Adams DH, et al. Predictors of atrial fibrillation after coronary artery surgery: current trends and impact on hospital resources. Circulation 1996; 94:390–397. [DOI] [PubMed] [Google Scholar]


