Abstract
The aim of this study was to evaluate the treatment and prognosis of gastric gastrointestinal stromal tumors (GISTs) according to the 7th UICC/AJCC tumor-node-metastasis (TNM) system and the modified National Institutes of Health (NIH) risk classification. The study cohort consisted of 1057 patients with gastric GIST who underwent surgery between January 2000 and December 2007 from 13 institutions in Korea and 2 in Japan. Clinicopathologic characteristics, surgical outcomes, recurrence, and 5-year recurrence-free survival were evaluated.
The mean age of the patients was 58.6 years. Thirty patients (2.8%) had distant metastasis preoperatively. Median tumor size was 4.0 cm. Complete resection (R0 resection) was achieved in 1018 patients (96.3%). Eighty-six patients (8.1%) had postoperative complications, and 2 patients (0.2%) died within 30 days after surgery. According to the 7th UICC/AJCC TNM system, 5-year recurrence-free survival rates were 95% to 99% in stage I, 94.1% in stage II, 74.1% in stage IIIA, 48.6% in stage IIIB, and 50.0% in stage IV patients. On survival analysis of high-risk patients according to the TNM system, the 5-year recurrence-free survival rates were 91.6% in stage II, 74.1% in stage IIIA, and 48.6% in stage IIIB patients. Independent factors of recurrence following surgery for gastric GIST were gender, tumor size, mitotic count, and radicality on multivariate analysis.
The treatment outcome and prognosis of gastric GIST in Korea and Japan seem more favorable compared to those in Western countries. Compared to the modified NIH risk classification, the 7th UICC/AJCC TNM system is more reflective of the 5-year recurrence-free survival of patients with gastric GIST.
INTRODUCTION
Gastrointestinal stromal tumors (GIST) originate from the interstitial cells of Cajal, intestinal pacemaker cells in the gastrointestinal tract.1,2 GISTs metastasize mainly to the liver via hematogenous spread and disseminate throughout the peritoneal cavity.3 They are primarily located in the stomach (60–70%) and are also found in the small intestine (20–30%), esophagus (5%), and colon and rectum (5%).4 Surgery is a potentially curative treatment for patients with resectable GIST. The purpose of surgery for resectable GIST is complete resection with tumor-free margins, avoiding tumor rupture,5 because tumor rupture, either spontaneously or at surgery, is associated with a high risk of recurrence. Whether rupture is an independent risk factor remains controversial.6 However, the prognosis of patients with primary GIST is influenced by tumor size and its mitotic count.3,4,6 Imatinib mesylate (Glivec®, Novartis, Basel, Switzerland) was established as the treatment of choice for patients with inoperable or metastatic disease. A recent prospective multicenter phase III trial has shown that 3 years of imatinib therapy improves overall survival compared to 1 year of therapy.7 Imatinib is also being investigated in the neoadjuvant setting to downstage unresectable tumors to resectable stage.8
In 2005, Miettinen et al9 reported on a large retrospective study of 1765 patients with gastric GISTs who were followed over the long term. Their study provides extensive information on gastric GIST, including clinicopathologic, immunohistochemical, and molecular genetic characteristics and corresponding prognosis. According to the new 7th Union for International Cancer Control/American Joint Committee on Cancer (UICC/AJCC) tumor-node-metastasis (TNM) system, progression rates of gastric GIST were 0% to 3.6% in stage I, 12% to 316% in stage II, 55% in stage IIIA, and 86% in stage IIIB.10 Gastric GIST had a more favorable prognosis than other GISTs.11
There have been a number of studies on clinical outcome, risk of recurrence, prognosis, and imatinib therapy for all GISTs including gastric GIST. However, there has been no multicenter study on gastric GIST patients with long-term follow-up that has sufficient information regarding follow-up and surgery. The aim of this study was to evaluate the treatment and prognosis of gastric GISTs according to the 7th UICC/AJCC TNM system and the modified National Institutes of Health (NIH) risk classification.
MATERIALS AND METHODS
Study Cohort and Data Collection
The study cohort consisted of 1057 patients with gastric GIST who were treated with surgery between January 2000 and December 2007 at 1 of 13 institutions in Korea: Ulsan University, Seoul National University, Sungkyunkwan University, Yonsei University, Keimyung University, Dong-A University, National Cancer Center, Ajou University, Seoul National University Bundang Hospital, the Catholic University Yeouido St. Mary's Hospital, Chungnam University, Soonchunhayng University, and the Catholic University Incheon St. Mary's Hospital and 2 institutions in Japan: Osaka University and Kanagawa Cancer Center Hospital. The diagnosis of GIST was confirmed at each institution by positive staining for KIT (CD117) protein and/or CD34 as assessed by immunohistochemical staining, regardless of myogenic and neurogenic markers.
Data were collected by reviewing the medical records and prospectively designed gastric GIST database from each institution. The Institutional Review Board of each participating institution approved this study. All data were collected in the same case report form (CRF) from each institution, and data were collected without revealing any personal information.
Personal Characteristics and Follow-Up
Clinicopathologic characteristics included age, gender, weight (kg), height (m), preoperative metastatic status, preoperative symptoms, tumor location, tumor size (cm), mitotic count (/50 high power field [HPF]), National Institutes of Health (NIH) classification, and whether the patient received pre- or postoperative imatinib mesylate treatment. Surgical outcomes included operation date, operation method, radicality, type of surgery, intraoperative tumor rupture, combined surgery, operation time (minute), postoperative hospital stay (day), complications, and death within 30 days after operation. Fifty-one patients who could not be classified via NIH classification, 10 patients lost to follow-up, and 30 preoperative M1 patients were excluded in the analysis of recurrence and long-term survival.
The follow-up schedule for patients after curative resection in most institutions was as follows: physical examination, endoscopy, and abdominal pelvic computed tomography (CT) scan performed annually for very low-risk or low-risk patients and every 6 months for intermediate or high-risk patients. Recurrence was diagnosed from clinical, radiologic, or endoscopic findings of disease.
Statistics
For statistical analysis, qualitative data are presented as number (%). Continuous variables are expressed as mean with standard deviation (SD). Actuarial recurrence free survival was calculated using the Kaplan–Meier method. Factors associated with recurrence were tested by univariate log-rank analysis. Variables that were significant in univariate analysis were entered into multivariate analysis. Multivariate analysis was performed with the Cox proportional hazard regression model. Odds ratio (OR) for comparison of the 2 groups was summarized with its 95% confidence interval (CI) and P-value using logistic regression. ORs were also adjusted for factors affecting the response variable. All statistical analyses were carried out using PASW ver. 18.0 (IBM Co., Armonk, NY); the level of significance was set at P < 0.05.
RESULTS
Annual Patient Number
The number of patients with gastric GIST increased annually. Laparoscopic surgery was performed in more than half of the patients at 2006 (Figure 1).
FIGURE 1.

Annual number of patients. The number of patients with gastric GIST increased annually. Laparoscopic surgery was performed in more than half of the patients since 2006.
Clinicopathologic Characteristics of 1057 Gastric GISTs
The mean age of the patients was 58.6 years, and there were approximately the same number of male and female patients. Thirty patients (2.8%) had distant metastasis preoperatively. There were 11 cases of metastasis to the liver, 19 to the peritoneum, 2 to the pancreas, 1 to the spleen, and 1 to the lungs and bone. Almost half of the patients had symptoms such as abdominal pain (36.4%), dyspepsia (30.2%), melena (18.5%), and a palpable mass (4.4%). Other symptoms were fatigue, diarrhea, dizziness, dyspnea, shoulder pain, and chest pain. The most common site was the upper body (41.7%) of the stomach. The most common category of tumor size was >2.1 cm and ≤5 cm (51.8%). The median tumor size was 4.0 cm. Only 9 patients (0.9%) were treated by imatinib preoperatively and 94 (8.9%) postoperatively (Table 1).
TABLE 1.
Clinicopathologic Characteristics of 1057 Gastric GISTs

Surgical Outcomes of 1057 Gastric GISTs
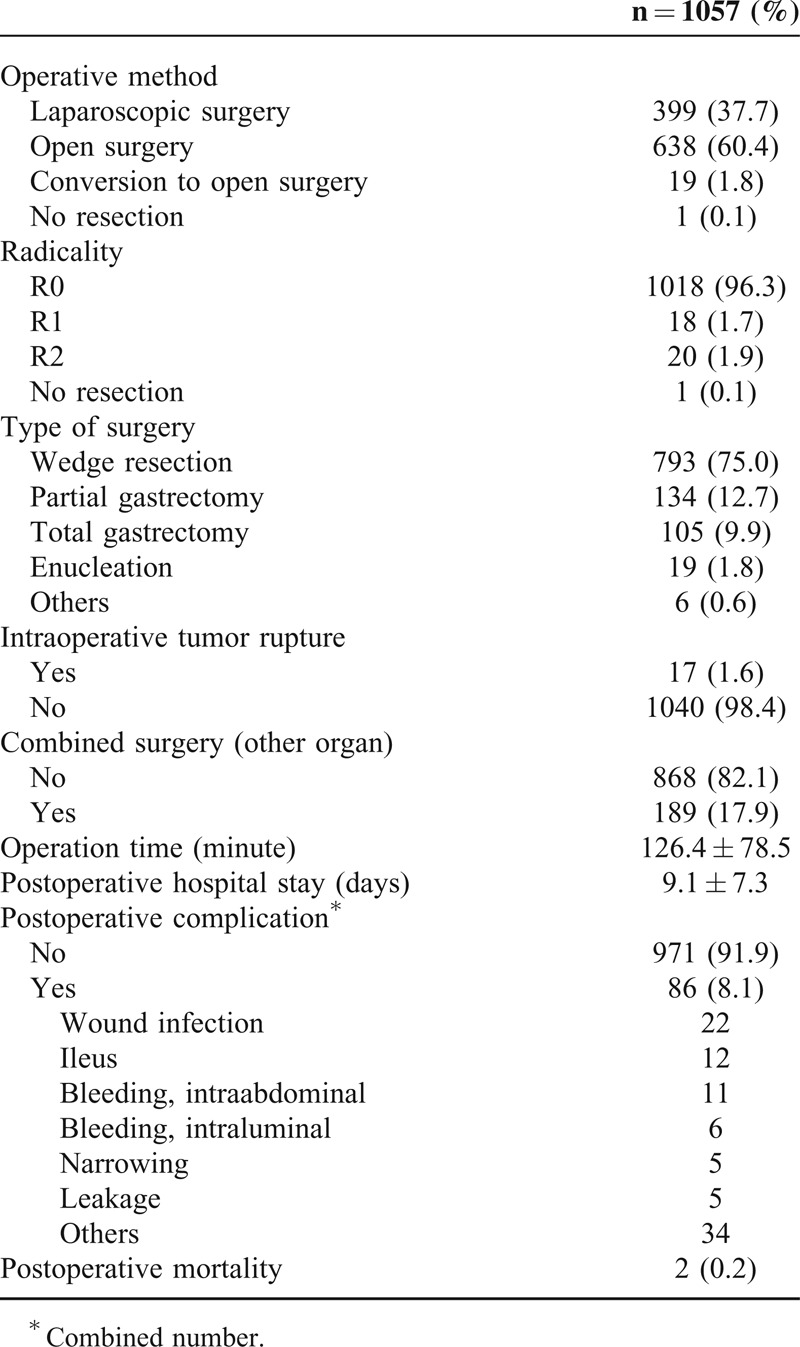
Complete resection (R0 resection) was achieved in 1018 patients (96.3%). Laparoscopic surgery was performed in 399 patients (37.7%), even though more than half the patients underwent open surgery. Various types of surgery were performed and included wedge resection for 793 patients (75.0%), partial gastrectomy for 134 patients (12.7%), total gastrectomy for 105 patients (9.9%), and enucleation for 19 patients (1.8%). Intraoperative tumor rupture occurred in only 17 patients (1.6%). Postoperative complications occurred in 86 (8.1%) patients. Wound infection was found in 22 patients, ileus in 12 patients, intraabdominal bleeding in 11 patients, and intraluminal bleeding in 6 patients. Two patients (0.2%) died within 30 days after surgery (Table 2).
TABLE 2.
Surgical Outcomes of 1057 Gastric GIST
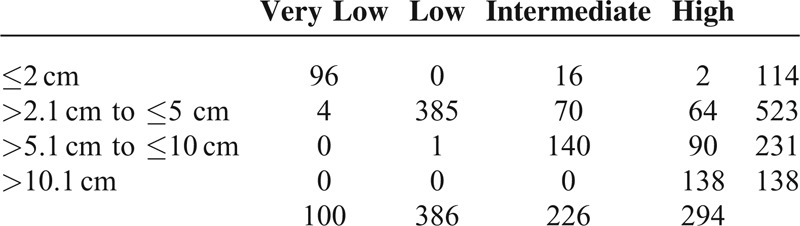
Modified NIH Risk Classification According to Tumor Size
The most common NIH risk was low-risk (38.4%), even though there were 138 (13.7%) patients with tumor >10 cm. In clinical practice, the decision to perform surgery for gastric GIST depends on preoperative tumor size. The percentage of patients who were classified as high-risk gastric GIST stratified by tumor size were 1.8% in ≤2 cm, 12.2% in >2.1 cm and ≤5 cm, 39.0% in >5.1 cm and ≤10 cm, and 100% in >10.1 cm (Table 3).
TABLE 3.
Modified NIH Risk Classification According to Tumor Size (n = 1006)
Five-Year Recurrence-Free Survival Rates by Modified NIH Risk Classification and 7th UICC/AJCC TNM System
According to the NIH classification, the 5-year recurrence-free survival rates were 9% to 99% in very low- or low-risk patients, 96.3% in intermediate-risk, and 74.9% in high-risk patients. Moreover, according to the 7th UICC/AJCC TNM system, the 5-year recurrence-free survival rates were 95% to 99% in stage I, 94.1% in stage II, 74.1% in stage IIIA, 48.6% in stage IIIB, and 50.0% in stage IV patients (Figure 2).
FIGURE 2.
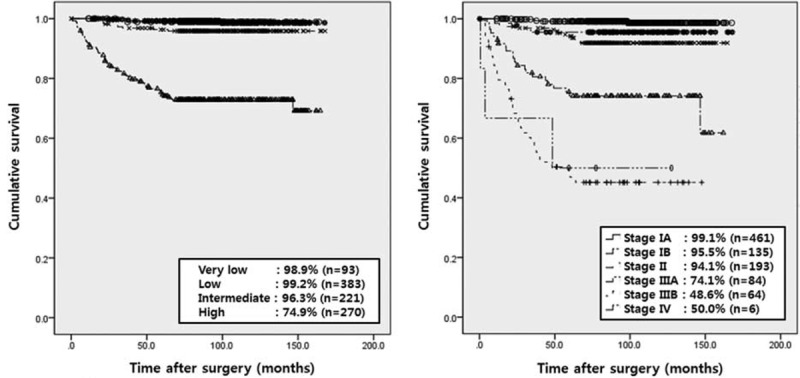
Five-year recurrence-free survival rate by modified NIH risk classification and 7th UICC/AJCC TNM system. According to the NIH classification, the 5-year recurrence-free survival rates were 98% to 99% in very low- or low-risk patients, 96.3% in intermediate-risk, and 74.9% in high-risk patients. Moreover, according to the 7th UICC/AJCC TNM system, the 5-year recurrence-free survival rates were 95% to 99% in stage I patients, 94.1% in stage II, 74.1% in stage IIIA, and 48.6% in stage IIIB patients.
On survival analysis of high-risk patients according to TNM system, the 5-year recurrence-free survival rates were 91.6% in stage II, 74.1% in stage IIIA, and 48.6% in stage IIIB patients (Figure 3).
FIGURE 3.
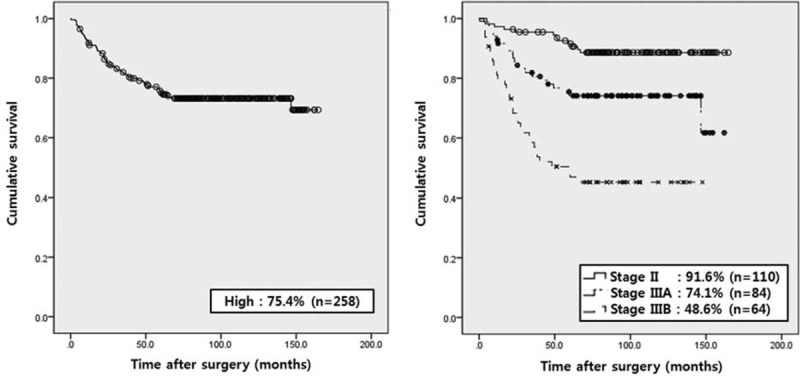
Five-year recurrence-free survival rate of high-risk patients according to NIH classification and TNM systems. The 5-year recurrence-free survival rates were 91.6% in stage II, 74.1% in stage IIIA, and 48.6% in stage IIIB patients.
Recurrence
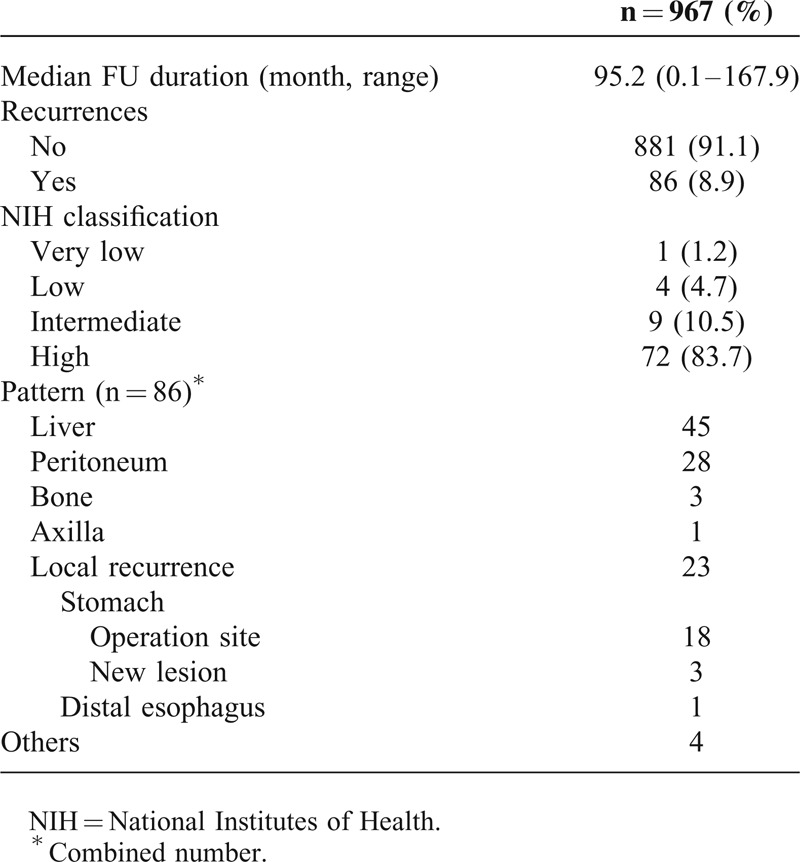
Among 1057 patients, 51 patients who could not be classified using the NIH classification, 10 patients lost to follow-up, and 30 preoperative M1 patients were excluded. The median follow-up duration of 967 patients was 95.2 (0.1–167.9) months. The overall recurrence rate was 8.9%. One hundred three episodes of recurrence occurred in 86 patients: 45 in the liver, 28 in the peritoneum, 3 in the bone, 1 in the axilla, and 4 in undefined sites. In addition, there were 23 incidences of local recurrence; 18 occurred in previous operation sites, 3 in other sites of the stomach, and 1 in the distal esophagus. Most recurrences occurred in patients who were intermediate- or high risk (Table 4).
TABLE 4.
Recurrence in 967 Patients
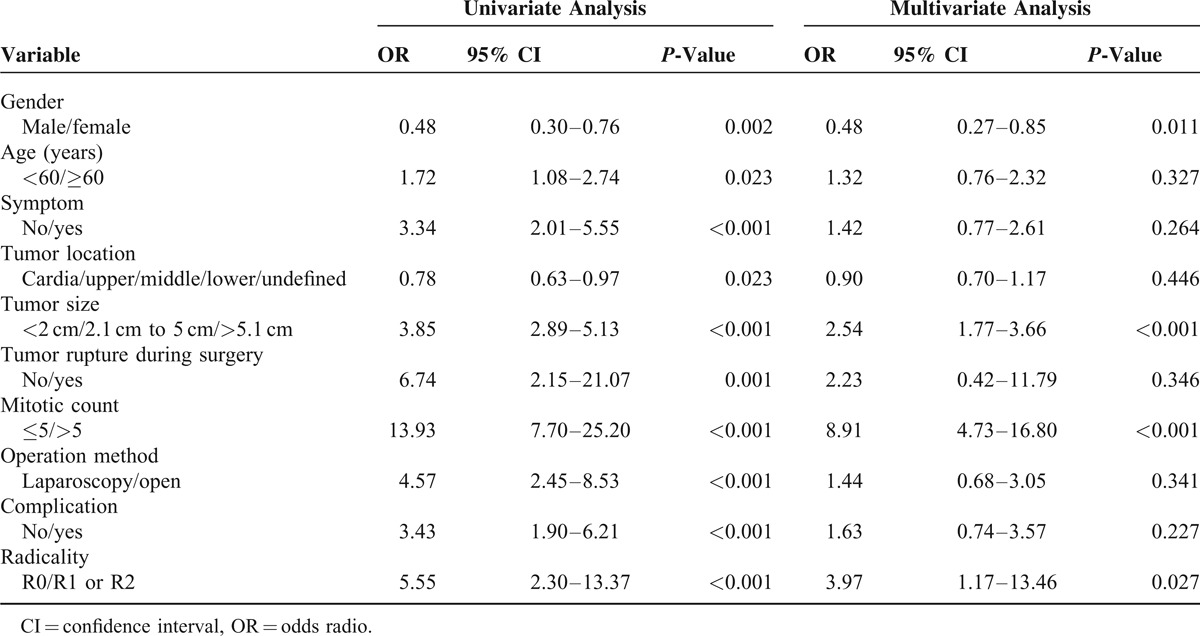
Factors Associated With Recurrence Following Surgical Treatment for Gastric GIST
On univariate analysis, gender, age, preoperative symptom, tumor location, tumor size, tumor rupture during surgery, mitotic count, operation method, postoperative complication, and radicality were associated with recurrence. In contrast, independent factors associated with recurrence following surgical treatment on multivariate analysis for gastric GIST were gender (odds radio [OR] 0.48, 0.27 < 95% confidence interval [CI] < 0.85, P = 0.012), tumor size (OR 3.85, 1.77 < 95% CI < 3.66, P < 0.001), mitotic count (OR 13.93, 4.73 < 95% CI < 16.80, P < 0.001), and radicality (OR 5.55, 1.17 < 95% CI < 13.46, P = 0.027) (Table 5).
TABLE 5.
Factors Associated With Recurrence Following Surgical Treatment for Gastric GIST (n = 967)
DISCUSSION
This large-scale multicenter and retrospective study can be useful for evaluating clinical outcomes of gastric GIST, which is a rare disease. Previous studies using a national registry database had some limitations,9,12 such as a lack of sufficient information regarding surgery and follow-up visits. This information can affect the surgical outcome of patients with gastric GIST, because the gold standard for localized primary gastric GIST is surgical resection. Moreover, although the risk of recurrence of all GISTs after surgery has been analyzed and published,6 the risk of recurrence of gastric GIST should be investigated separately, because gastric GIST has a more favorable prognosis than other GISTs. This study was conducted on patients who had gastric GIST and long-term follow-up using a prospectively designed database in 15 representative institutes in Korea and Japan.
Compared to results in Western countries,9,11 patients in this study were younger and their gastric GIST was detected earlier, likely due to improved nationwide surveillance for gastric cancer in Korea and Japan. Therefore, the patients in this study have distinctive characteristics, such as smaller tumor size, fewer symptoms, earlier stage of tumor, more frequent treatment with laparoscopic surgery, higher rate of R0 resection and local resection (wedge resection or enucleation), and lower postoperative mortality rate. These differences can explain the difference in prognosis of gastric GIST between patients from western and eastern countries. In this study, only 9 and 94 patients were treated with imatinib mesylate preoperatively and postoperatively, respectively. At that time, the use of imatinib mesylate was limited due to national insurance in Korea.
It is well known that gastric GIST usually presents in clinical practice as gastric submucosal tumor (SMT). Almost half of gastric SMT is found to be gastric GIST after surgery.13 As demonstrated in Table 3, preoperative tumor size can predict the incidence of the level of risk of gastric GIST after surgery if the tumor is GIST and thus determine the follow-up plan. Roughly one-third of patients with gastric GIST >5.1 cm to ≤10 cm can be high risk.
One hundred three recurrences in 86 patients occurred, and the most common recurrence site was the liver (43.7%). The 5-year recurrence-free survival rate of very low-, low-, and intermediate-risk patients was above 96%, and the 5-year recurrence-free survival rate of stage I and II patients using the 7th UICC/AJCC TNM system was above 94%. Otherwise, patients who were high risk or stage III had high recurrence rates. High-risk patients can be divided into stage II or IIIA or IIIB according to the TNM staging system. The 5-year recurrence-free survival rate of those stages of high-risk patients was similar to the TNM stages in all risk patients. The 7th UICC/AJCC TNM system is more reflective of the 5-year recurrence-free survival of patients with gastric GIST rather than the modified NIH risk classification. Therefore, adjuvant treatment with imatinib after surgery may be reasonable for over stage IIIA patients rather than all high-risk patients.
Tumor rupture is thought to be associated with a substantially higher risk. Hohenberger et al14 stated that patients with a preoperative spontaneous or intraoperative rupture of GIST into the peritoneal cavity have a risk of recurrence of nearly 100%. However, whether or not rupture is an independent risk factor for recurrence is controversial,6 because there has been no study on intraoperative tumor rupture and long-term prognosis. Tumor rupture during surgery in this study occurred in 17 patients. After surgery, 5 patients had recurrence.
In other studies on all GISTs,5,6 independence adverse prognostic factors were larger tumor, high mitotic count, nongastric location, presence of rupture, and male sex. The mitotic count was a predictor of outcome following surgical resection of 112 gastric GISTs.15 On multivariate analysis of this study, male, larger tumor, high mitotic count, and R1 or R2 resection were associated with recurrence after curative resection of gastric GIST. Operation method (laparoscopy vs. open) was not a factor associated with recurrence. Further comparison study of laparoscopy versus open surgery for gastric GIST according to tumor size is being prepared using our data.
This study has 2 limitations. First, there is lack of central review of pathology results from multicenter data. Pathologic diagnosis for GIST has been well established at each institution in Korea since 2000. Therefore, we enrolled the patients diagnosed with gastric GIST at each institution since 2000. Second, no molecular genetic study was performed. The method and response of imatinib treatment has been shown to be related to these findings. Recently, many studies of imatinib mesylate given in accordance with IHC and molecular genetic results have been published.16–18 However, we focused on only long-term surgical outcomes of gastric GIST regardless of molecular genetic results.
CONCLUSIONS
The treatment outcome and prognosis of gastric GIST in Korea and Japan seem more favorable compared to those in Western countries, likely due to the greater proportion of patients in Korea and Japan who are younger and diagnosed at an early stage as well as the high rate of complete resection. Compared to the modified NIH risk classification, the 7th UICC/AJCC TNM system is more reflective of the 5-year recurrence-free survival of patients with gastric GIST.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, CRF = case report form, GIST = gastrointestinal stromal tumor, HPF = high power field, NIH = National Institutes of Health, TNM = tumor-node-metastasis, UICC = Union for International Cancer Control.
Author contributions—Conception and design: all authors; provision of study materials or patients: Jeong-Hwan Yook and Min-Chan Kim; collection and assembly of data: Min-Chan Kim and Ki-Han Kim; data analysis and interpretation: Min-Chan Kim and Ki-Han Kim; manuscript writing: Min-Chan Kim; and final approval of manuscript: all authors.
The authors have no funding and conflicts of interest to disclose.
This work was supported by Korean GIST study group.
REFERENCES
- 1.Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998; 152:1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 2.Hirota S, Isozaki K, Moriyama Y, et al. Gain-or-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998; 279:577–580. [DOI] [PubMed] [Google Scholar]
- 3.Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol 2005; 100:162–168. [DOI] [PubMed] [Google Scholar]
- 4.Bennett JJ, Rubino MS. Gastrointestinal stromal tumors of the stomach. Surg Oncol Clin N Am 2012; 21:21–33. [DOI] [PubMed] [Google Scholar]
- 5.DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000; 231:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012; 13:265–274. [DOI] [PubMed] [Google Scholar]
- 7.Joensuu H, Eriksson M, Hatrmann J, et al. Twelve vs 36 months of adjuvant imatinib (IM) as treatment of operable GIST with a high risk of recurrence: final results of a randomized trial (SSGXVIII/AIO). 47th Annual Meeting of the American Society of Clinical Oncology. Abstract No. LBA1. Chicago (IL), June 5, 2011. [Google Scholar]
- 8.Raut CP, Posner M, Desai J, et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol 2006; 24:2325–2331. [DOI] [PubMed] [Google Scholar]
- 9.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 2005; 29:52–68. [DOI] [PubMed] [Google Scholar]
- 10.Agaimy A. Gastrointestinal stromal tumors (GIST) from risk stratification systems to the new TNM proposal: more questions than answers? A review emphasizing the need for a standardized GIST reporting. Int J Clin Exp Pathol 2010; 3:461–471. [PMC free article] [PubMed] [Google Scholar]
- 11.Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 2006; 130:1466–1478. [DOI] [PubMed] [Google Scholar]
- 12.Woodall CE, III, Brock GN, Fan J, et al. An evaluation of 2537 gastrointestinal stromal tumors for a proposed clinical staging system. Arch Surg 2009; 144:670–678. [DOI] [PubMed] [Google Scholar]
- 13.Yang HK, Kim MC, Kim YW, et al. Nationwide survey of laparoscopic gastric surgery in Korea. J Korean Gastric Cancer Assoc 2004; 4:196–204. [Google Scholar]
- 14.Hohenberger P, Ronellenfitsch U, Oladeji O, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg 2010; 97:1854–1859. [DOI] [PubMed] [Google Scholar]
- 15.Yang HK, Park do J, Lee HJ, et al. Clinicopathologic characteristics of gastrointestinal stromal tumor of the stomach. Hepatogastroenterology 2008; 55:1925–1930. [PubMed] [Google Scholar]
- 16.Zheng S, Huang KE, Pan YL, et al. KIT and BRAF heterogeneous mutations in gastrointestinal stromal tumors after secondary imatinib resistance. Gastric Cancer 2015; 18:796–802. [DOI] [PubMed] [Google Scholar]
- 17.Tap WD, Schwartz GK. That's the “GIST” of it: use of adjuvant imatinib after resection of a primary GI stromal tumor. J Clin Oncol 2014; 32:1543–1546. [DOI] [PubMed] [Google Scholar]
- 18.Antonescu CR, Romeo S, Zhang L, et al. Dedifferentiation in gastrointestinal stromal tumor to an anaplastic KIT-negative phenotype: a diagnostic pitfall: morphologic and molecular characterization of 8 cases occurring either de novo or after imatinib therapy. Am J Surg Pathol 2013; 37:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]


