Abstract
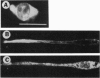
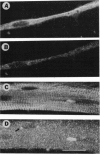
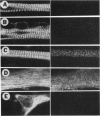
The mRNAs for some cytoskeletal proteins are localized, suggesting that mRNA for these proteins may concentrate at sites appropriate for assembly. To test this hypothesis, we observed vimentin mRNA in developing chicken muscle cultures by in situ hybridization with a digoxigenin-labeled DNA probe to vimentin, detected by confocal microscopy using fluorescent anti-digoxigenin antibody. This method has submicrometer resolution. In developing muscle, vimentin mRNA was bipolar in young myoblasts, somewhat perinuclear in elongated myoblasts and spread fibroblasts, and diffuse in young and developing myotubes. In mature myotubes, vimentin mRNA occurred at costameres with vimentin protein. Localization of mRNA may prove important for assembling and maintaining differentiated cytoskeletal structures, as it is for organizing the embryo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach R. L., Jeffery W. R. Temporal and spatial expression of a cytoskeletal actin gene in the ascidian Styela clava. Dev Genet. 1990;11(1):2–14. doi: 10.1002/dvg.1020110103. [DOI] [PubMed] [Google Scholar]
- Bresser J., Evinger-Hodges M. J. Comparison and optimization of in situ hybridization procedures yielding rapid, sensitive mRNA detections. Gene Anal Tech. 1987 Sep-Oct;4(5):89–104. doi: 10.1016/0735-0651(87)90002-1. [DOI] [PubMed] [Google Scholar]
- Cheng H., Bjerknes M. Asymmetric distribution of actin mRNA and cytoskeletal pattern generation in polarized epithelial cells. J Mol Biol. 1989 Dec 5;210(3):541–549. doi: 10.1016/0022-2836(89)90130-7. [DOI] [PubMed] [Google Scholar]
- Craig S. W., Pardo J. V. Gamma actin, spectrin, and intermediate filament proteins colocalize with vinculin at costameres, myofibril-to-sarcolemma attachment sites. Cell Motil. 1983;3(5-6):449–462. doi: 10.1002/cm.970030513. [DOI] [PubMed] [Google Scholar]
- Danowski B. A., Imanaka-Yoshida K., Sanger J. M., Sanger J. W. Costameres are sites of force transmission to the substratum in adult rat cardiomyocytes. J Cell Biol. 1992 Sep;118(6):1411–1420. doi: 10.1083/jcb.118.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning G. M., Kim I. S., Fulton A. B. Shedding of cytoplasmic actins by developing muscle cells. J Cell Sci. 1988 Feb;89(Pt 2):273–282. doi: 10.1242/jcs.89.2.273. [DOI] [PubMed] [Google Scholar]
- Dix D. J., Eisenberg B. R. Myosin mRNA accumulation and myofibrillogenesis at the myotendinous junction of stretched muscle fibers. J Cell Biol. 1990 Nov;111(5 Pt 1):1885–1894. doi: 10.1083/jcb.111.5.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner C. C., Tucker R. P., Matus A. Selective localization of messenger RNA for cytoskeletal protein MAP2 in dendrites. Nature. 1988 Dec 15;336(6200):674–677. doi: 10.1038/336674a0. [DOI] [PubMed] [Google Scholar]
- Gottlieb E. Messenger RNA transport and localization. Curr Opin Cell Biol. 1990 Dec;2(6):1080–1086. doi: 10.1016/0955-0674(90)90159-c. [DOI] [PubMed] [Google Scholar]
- Holmes E., Hermanson G., Cole R., de Vellis J. Developmental expression of glial-specific mRNAs in primary cultures of rat brain visualized by in situ hybridization. J Neurosci Res. 1988 Apr;19(4):389-96, 458-65. doi: 10.1002/jnr.490190402. [DOI] [PubMed] [Google Scholar]
- Hoock T. C., Newcomb P. M., Herman I. M. Beta actin and its mRNA are localized at the plasma membrane and the regions of moving cytoplasm during the cellular response to injury. J Cell Biol. 1991 Feb;112(4):653–664. doi: 10.1083/jcb.112.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs W. B., Cook R. K., Van Atta J. C., Redmond C. M., Fulton A. B. Assembly of vimentin in cultured cells varies with cell type. J Biol Chem. 1989 Oct 25;264(30):17953–17960. [PubMed] [Google Scholar]
- Kleiman R., Banker G., Steward O. Differential subcellular localization of particular mRNAs in hippocampal neurons in culture. Neuron. 1990 Dec;5(6):821–830. doi: 10.1016/0896-6273(90)90341-c. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Intracellular localization of messenger RNAs for cytoskeletal proteins. Cell. 1986 May 9;45(3):407–415. doi: 10.1016/0092-8674(86)90326-0. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Quantitative analysis of in situ hybridization methods for the detection of actin gene expression. Nucleic Acids Res. 1985 Mar 11;13(5):1777–1799. doi: 10.1093/nar/13.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P., Julien J. P., Vilja P., Grosveld F., Rechardt L. Specific detection of neuronal cell bodies: in situ hybridization with a biotin-labeled neurofilament cDNA probe. J Histochem Cytochem. 1986 Jul;34(7):923–926. doi: 10.1177/34.7.3458811. [DOI] [PubMed] [Google Scholar]
- Moon R. T., Lazarides E. Synthesis and post-translational assembly of intermediate filaments in avian erythroid cells: vimentin assembly limits the rate of synemin assembly. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5495–5499. doi: 10.1073/pnas.80.18.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo J. V., Siliciano J. D., Craig S. W. A vinculin-containing cortical lattice in skeletal muscle: transverse lattice elements ("costameres") mark sites of attachment between myofibrils and sarcolemma. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1008–1012. doi: 10.1073/pnas.80.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry B. A., Capco D. G. Spatial reorganization of actin, tubulin and histone mRNAs during meiotic maturation and fertilization in Xenopus oocytes. Cell Differ Dev. 1988 Nov;25(2):99–108. doi: 10.1016/0922-3371(88)90003-2. [DOI] [PubMed] [Google Scholar]
- Pomeroy M. E., Lawrence J. B., Singer R. H., Billings-Gagliardi S. Distribution of myosin heavy chain mRNA in embryonic muscle tissue visualized by ultrastructural in situ hybridization. Dev Biol. 1991 Jan;143(1):58–67. doi: 10.1016/0012-1606(91)90054-7. [DOI] [PubMed] [Google Scholar]
- Sundell C. L., Singer R. H. Actin mRNA localizes in the absence of protein synthesis. J Cell Biol. 1990 Dec;111(6 Pt 1):2397–2403. doi: 10.1083/jcb.111.6.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R. P., Garner C. C., Matus A. In situ localization of microtubule-associated protein mRNA in the developing and adult rat brain. Neuron. 1989 Mar;2(3):1245–1256. doi: 10.1016/0896-6273(89)90309-7. [DOI] [PubMed] [Google Scholar]
- Vikstrom K. L., Borisy G. G., Goldman R. D. Dynamic aspects of intermediate filament networks in BHK-21 cells. Proc Natl Acad Sci U S A. 1989 Jan;86(2):549–553. doi: 10.1073/pnas.86.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehner Z. E., Paterson B. M. The chicken vimentin gene: aspects of organization and transcription during myogenesis. Ann N Y Acad Sci. 1985;455:79–94. doi: 10.1111/j.1749-6632.1985.tb50405.x. [DOI] [PubMed] [Google Scholar]