Abstract
This cross-sectional, exploratory study aimed to compare neuromuscular performance, balance and motor skills proficiencies of typically developing children and those with developmental coordination disorder (DCD) and to determine associations of these neuromuscular factors with balance and motor skills performances in children with DCD.
One hundred thirty children with DCD and 117 typically developing children participated in the study. Medial hamstring and gastrocnemius muscle activation onset latencies in response to an unexpected posterior-to-anterior trunk perturbation were assessed by electromyography and accelerometer. Hamstring and gastrocnemius muscle peak force and time to peak force were quantified by dynamometer, and balance and motor skills performances were evaluated with the Movement Assessment Battery for Children (MABC).
Independent t tests revealed that children with DCD had longer hamstring and gastrocnemius muscle activation onset latencies (P < 0.001) and lower isometric peak forces (P < 0.001), but not times to peak forces (P > 0.025), than the controls. Multiple regression analysis accounting for basic demographics showed that gastrocnemius peak force was independently associated with the MABC balance subscore and ball skills subscore, accounting for 5.7% (P = 0.003) and 8.5% (P = 0.001) of the variance, respectively. Gastrocnemius muscle activation onset latency also explained 11.4% (P < 0.001) of the variance in the MABC ball skills subscore.
Children with DCD had delayed leg muscle activation onset times and lower isometric peak forces. Gastrocnemius peak force was associated with balance and ball skills performances, whereas timing of gastrocnemius muscle activation was a determinant of ball skill performance in the DCD population.
INTRODUCTION
Developmental coordination disorder (DCD) is one of the most common motor disorders in childhood. About 6% of typically developing children are diagnosed with this disorder during their primary school years.1 The main characteristic of DCD in the gross motor domain is poor motor control, including poor postural control, which is the foundation of gross motor skill performance and development.2–4 To date, the majority of relevant studies have focused on the sensory contributions of suboptimal postural control (balance) performance,4–7 and fewer studies have examined the motor contributions to balance disorders in this group of children.8–10 Only 2 studies have assessed the neuromuscular (electromyographic [EMG]) responses to a sudden, unexpected postural perturbation in the DCD population. Williams and Woollacott8 were the first to use a translating platform to elicit lower limb postural muscle responses in children with and without DCD. They found that the average onset latency of postural muscle activation was similar between the 2 groups. Some years later, Geuze9 used a more functional setup (subjects were perturbed by a ball lightly hitting the back) to measure the EMG activation timing of lower limb muscles in children with DCD and controls. They also reported that the lower limb muscle EMG onset latencies did not differ between the 2 groups. However, in a recent study by our research team using a motor control test, we found that the latency time between the platform translation and the onset of postural response was longer in children with DCD than in controls (effect size = 0.42–0.71).10 We postulated that prolonged EMG onset latencies in the lower limb muscles might be a contributing factor to the mechanical delay in postural response, and that children with DCD might have longer EMG onset latencies in their lower limb muscles. The insignificant findings in the previous studies8,9 were probably due to the relatively small sample size (13 with DCD vs 13 controls)9 and large intragroup variability.8 It is thus necessary to use a larger sample to verify the results.
Based on the results of our previous study,7 it is plausible that another motor timing deficit—slowed muscle force production—in the lower limb muscles might also contribute to the inferior balance and motor skills performance in children with DCD. We found that increasing the time to reach peak force in the hamstring muscles was associated with atypical muscle synergy (excessive use of hip strategy) in this group of children.7 Regarding muscle strength per se, Raynor11 reported that children with DCD produced lower levels of maximal muscle strength (peak force/torque) and power during isokinetic knee flexion and extension testing when compared with typically developing controls. Because lower limb muscle strength correlates significantly with balance performance in adults,12 weaker lower limb muscles might also compromise the balance and gross motor skills performance of children affected with DCD. However, no study has examined the relationships between neuromuscular (motor) deficits, balance, and gross motor skills performances in this particular group of children thus far.
Therefore, the objectives of this study were to compare the neuromuscular (motor) performance indices, balance and motor skills performance scores of DCD, and control participants and determine the associations of these neuromuscular factors with balance and motor skills performance among children with DCD. It was hypothesized that the neuromuscular and motor functional outcomes would be significantly different between the DCD and control groups and that the neuromuscular factors would be significantly associated with balance and motor skills performance in the DCD population.
METHODS
Participants
This was a large-scale, cross-sectional, and exploratory study. Children with DCD were recruited from local child assessment centers, hospitals, nongovernmental organizations with pediatric rehabilitation services, mainstream primary schools, parents’ groups (via poster and website advertising), and our research team's database of DCD participants (via phone calls). From January through June 2014, all children volunteers were screened by 2 physiotherapists (over the phone first and then face-to-face) to determine whether the following eligibility criteria were met. The inclusion criteria were a diagnosis of DCD according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR)1; a total impairment score of <5th percentile on the Movement Assessment Battery for Children (MABC)13 or a gross motor composite score of ≤42 on the Bruininks–Oseretsky Test of Motor Proficiency14; a total score of <46 (5–7 years 11 months old), <55 (8–9 years 11 months old), or <57 (10–15 years old) on the 2007 version of the DCD questionnaire15; ages between 6 and 10 years old; and studying in a regular education framework. Exclusion criteria were diagnosis of emotional, neurological, or other movement disorders (comorbid attention deficit/hyperactivity disorder, dyslexia, and suspected autism spectrum disorder were acceptable); significant musculoskeletal, cardiopulmonary, neurological, visual, vestibular, or other sensorimotor disorders that might affect motor performances; receiving active physical training; demonstrating excessive disruptive behaviors; or unable to follow instructions thoroughly during assessments or cannot complete the assessments.
Typically developing healthy control participants were recruited from primary schools and an existing database of participants who had participated in our previous studies. The eligibility criteria were the same as those for the DCD group except that the control participants could not have had a diagnosis of DCD; a total impairment score of >15th percentile on the MABC13; and a total score of >46 (5–7 years 11 months old), >55 (8–9 years 11 months old), or >57 (10–15 years old) on the DCD questionnaire.15 Ethical approval for this study (EA160913) was provided by the Human Research Ethics Committee of the University of Hong Kong, Hong Kong, on 25 September 2013. Each participant and parent gave informed written consent before participating in the study. Data collection was performed by an experienced physiotherapist and a trained research assistant in the Physical Activity Laboratory of the University of Hong Kong, and all experimental procedures were conducted in accordance with the Declaration of Helsinki.
OUTCOME MEASUREMENTS
Demographics
Demographic and relevant information such as medical history was obtained by interviewing the parent and child. In addition, physical activity level (in metabolic equivalent [MET] hours per week) was estimated based on the self-reported activity intensity level (light, moderate, or hard), duration (in hours), frequency (times per week), and the assigned MET value of the activity according to the Compendium of Energy Expenditures for Youth.16 After the interview, the body height and weight of each child were measured and body mass index (BMI, in kg/m2) was calculated.
Lower Limb Muscle Activation Onset Latency (Muscle Reflex Contraction Latency)
Lower limb postural muscle responses following unexpected posterior-to-anterior (PA) trunk perturbation were measured using surface EMG (Biometrics, Newport, UK). A triaxle accelerometer (ACL300, Biometrics) was attached to the sternum of the participant to register the onset of trunk perturbation.17 Physiologically, a sudden PA perturbation to the trunk would trigger reflexive contractions of primarily the hamstrings and gastrocnemius, allowing the participant to maintain postural stability.18 To record the muscle activities of these 2 major postural muscles (medial hamstrings and gastrocnemius) in the dominant lower limb (defined as the leg used to kick a ball) in response to a PA trunk perturbation,17 circular Ag/AgCl bipolar surface EMG electrodes (EMG sensor SX230-1000, Biometrics) were used. Active electrode location sites on the skin were identified following the recommendations of Barbero et al19 and prepared by shaving of hair, lightly abrading with fine sandpaper and cleansing using alcohol swabs to reduce skin impedance. The EMG active electrodes were fixed over the center of each muscle belly and placed in a line parallel with the longitudinal axis of the lower extremity. The diameter of each active electrode was 1 cm, and the center-to-center inter-electrode distance was 2 cm. The EMG signals were filtered with a bandwidth of 20 to 460 Hz, sampled at 1000 Hz and amplified by a gain factor of 1000 using a single differential amplifier with an input impedance of >1015 and a common mode rejection ratio of >96 dB.20 A reference electrode (R506, Biometrics) was placed on the ipsilateral lateral malleolus. All of the electrodes were connected to the DataLOG (Biometrics), which was securely attached to the participant's waist during the perturbation test to minimize artifacts. The DataLOG uses both a high-pass filter (20 Hz) to remove DC offsets due to membrane potential and a low-pass filter for frequencies >450 Hz. It also stores EMG data for offline analysis.20
The PA trunk perturbation test was modified from our previous study17 and that of Geuze.9 Each participant was blindfolded and stood with bare feet apart at shoulder width and arms resting by the side of the trunk. The participant was instructed to stand still and not to take any corrective steps during the test. Then, the same assessor gave a sudden and light horizontal push to the back (at T12 level) of the participant to disturb his/her balance. The acceleration of the trunk in the PA direction was recorded by the accelerometer attached to the sternum. To prevent falls, a parent stood in front of the participant and provided support if absolutely necessary. Postural muscle activity was measured for 5 seconds before the perturbation (ie, baseline EMG signals) and 5 seconds after the onset of the unexpected perturbation (ie, reflex muscle contraction EMG signals). Only 1 perturbation trial was performed to avoid anticipation and learning.9
The EMG signals of each muscle and the accelerometer signal were postprocessed using the Biometrics EMG analysis software for DataLOG version 8.51. The nonrectified and non-normalized EMG raw data were extracted, and the mean and standard deviation of the resting EMG signals of the 2 muscles were calculated. Then, the onset of muscle activation, defined as the starting point of the EMG activity of each muscle that lasts for more than 25 ms and is 2 standard deviations away from the mean resting EMG value,17 was marked. In addition, the onset of the accelerometer signal, defined as the time point at which the signal amplitude is 0.20 ms−2 away from the resting value,21 was identified. This point represents the onset of trunk perturbation.17 Finally, the muscle activation onset latency, defined as the time interval (in milliseconds) between the onset of the accelerometer signal and the first discernible EMG activities of each muscle, was calculated and used for analysis.17
Lower Limb Muscle Peak Force and Time to Peak Force
The maximum isometric muscle strength (peak force, in kg) of the participants’ dominant knee flexors (hamstrings) and ankle plantar flexors (gastrocnemius) was measured using the Lafayette Manual Muscle Test System (Model 01165, Lafayette Instrument Company, Lafayette, IN) with standardized manual muscle testing procedures22 and dynamometer placements.23 Good to perfect reliability (ICC range: 0.81–0.98) has been reported for lower limb muscle strength measurements using this method in young people.24 The participants completed 2 trials of manual muscle testing in which the peak force was generated for 2 to 3 seconds for each muscle group. They were instructed to voluntarily contract their muscles as hard and as fast as possible. The average peak force of the 2 trials of each muscle group was used for analysis. Time to peak force (in seconds), defined as the time elapsed from the start of the test until the maximum force has been reached,23 was also documented for data analysis.
Balance and Motor Skills Performances
The MABC was used to evaluate the balance and motor proficiencies of the participants as it is a standardized, validated, and reliable instrument for measuring motor performances in children.13,25 This assessment tool consists of 8 fine and gross motor tasks for each of 4 age bands (ie, 4–6 years, 7–8 years, 9–10 years, and 11–12 years). The 8 tasks are divided into 3 domains: manual dexterity, ball skills, and balance. The manual dexterity tasks access various fine motor skills such as threading, drawing, and cutting; the ball skills tasks test the participant's bouncing, catching, and throwing abilities; and the balance tests assess single-leg balance, hopping, walking with heels raised or tandem walking abilities. The assessment procedures are described in detail in Henderson and Sugden.13 Each participant was assessed with the appropriate age-band motor skill tests. The raw score for each test item was summed to obtain a total impairment score. In addition, the raw scores of the 2 ball skills items and the 3 balance items were summed to obtain a ball skill subscore and a balance subscore, respectively. A lower score represents better motor performance in general, and a higher score represents more severe motor impairment.13 The total impairment score, ball skills subscore, and balance subscore were used for analysis.
Statistical Analyses
All sample size calculations were based on a statistical power of 80% and a 2-tailed alpha level of 5%. For objective 1 (t test), according to our previous studies,7,10 a medium effect size of 0.40 was assumed. So, the minimum sample size required to detect a significant between-group difference in the neuromuscular outcomes was 100 participants per group. For objective 2 (regression analysis), our previous study7 showed that the neuromuscular factor (time to peak force of knee flexors) accounted for 19.8% of the variance in balance performance among children with DCD. This translated into a medium effect size (F2 = 0.25). Thus, a minimum of 58 children with DCD was needed to detect a significant association of the neuromuscular outcome with the MABC motor impairment subscore, after accounting for age, sex, BMI, and physical activity level (ie, total number of predictors = 5).
Statistical analyses were conducted using SPSS 20.0 software (IBM, Armonk, NY), and a level of significance at 5% (2-tailed) was set. Descriptive statistics (eg, mean and standard deviations) were used to describe all of the variables of interest. Kolmogorov–Smirnov tests and histograms were used to check the normality of the data. Independent t tests and chi-squared test were used to compare the continuous and categorical demographic variables, respectively, between the 2 groups. Since no significant covariates were present (Table 1), independent t tests were also used to compare the muscle onset latency, time to peak force, peak force, and MABC outcome variables between the 2 groups. The alpha level was Bonferroni adjusted in each outcome category to avoid an inflation of type I error. The effect size (Cohen's d) was also presented for each of the outcomes.
TABLE 1.
Characteristics of the DCD and Control Groups

Pearson product-moment correlation (r) was performed to assess the degree of association of MABC-derived scores with all neuromuscular outcome variables within the DCD group. We focused on the MABC gross motor skills impairment scores (ie, balance and ball skills subscores) as they are functional, clinically meaningful and more relevant to lower limb neuromuscular performances.17,18 Additionally, multiple linear regression analyses were performed to identify the determinants of the MABC balance subscore and ball skills subscore among the children with DCD. Age, sex, BMI, and physical activity level were first forced into the regression model (Enter method) as these factors may influence MABC motor skills performance.26 Then, the neuromuscular outcomes that were significantly associated with the MABC balance subscore or ball skills subscore in the bivariate correlational analysis were entered into the regression model. The tolerance approach and the variance inflation factor were used to check for multicollinearity. Any predictor variables that had a tolerance value of <0.1 and a variance inflation factor of >10 were not included in the same regression model.
RESULTS
A total of 270 children were screened and 130 children with DCD and 117 typically developing children were eligible and participated in the study voluntarily. No significant differences were found in the various demographic variables between the 2 groups, except that the DCD group scored significantly lower (mean total score = 44.6 points) on the DCD questionnaire than the control group (mean total score = 58.5 points) (Table 1). This finding was expected and actually was 1 of our criteria used to classify the children with DCD versus the controls.
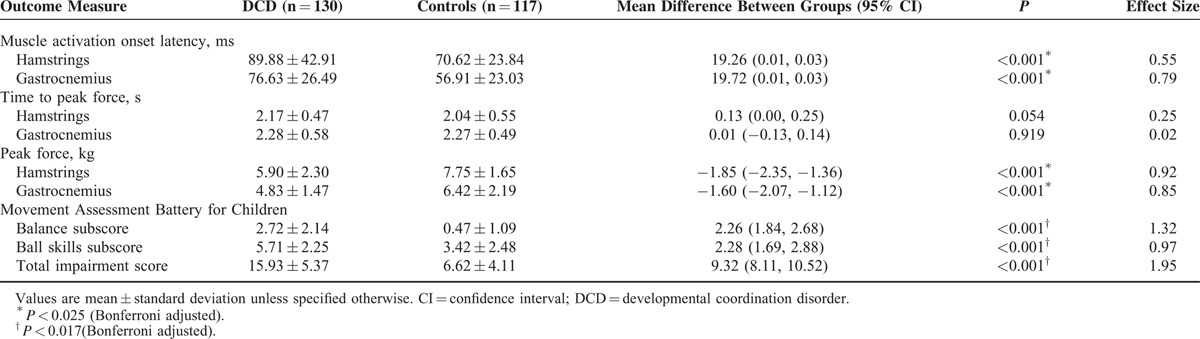
Our results revealed that the hamstring and gastrocnemius muscle activation onset latencies were prolonged in the children with DCD compared with their typically developing peers (all P < 0.001). However, the time periods required to reach peak force for both the hamstring and gastrocnemius muscles were comparable between the 2 groups (P > 0.025, Bonferroni adjusted) (exact P values in Table 2), reflecting that the speed of force production did not differ between the children with and without DCD. For the isometric peak force of hamstrings and gastrocnemius, the DCD group achieved a lower value than the control group (all P < 0.001), indicating that the children with DCD had weaker lower limb muscle strength than that of their typically developing peers. As anticipated, the DCD group had a significantly higher MABC total impairment score (P < 0.001), balance subscore (P < 0.001), and ball skills subscore (P < 0.001) than the control group. The Cohen's d values ranged from 0.97 to 1.95, indicating large effect sizes (Table 2).
TABLE 2.
Group Differences in Neuromuscular and Motor Performances Among Children With and Without DCD
Bivariate correlation analysis showed that the MABC balance subscore correlated inversely with gastrocnemius peak force (r = −0.254, P = 0.004) exclusively in the children with DCD. In addition, the MABC ball skills subscore correlated positively with gastrocnemius muscle activation onset latency (r = 0.336, P < 0.001) and negatively with gastrocnemius peak force (r = −0.314, P < 0.001). No significant correlations were found between the MABC subscores and hamstrings-related scores (P > 0.05) (Table 3).
TABLE 3.
Pearson's Correlation Matrix for the Key Variables Among Children With DCD (n = 130)

Multiple regression analyses were performed to identify the determinants of the MABC balance subscore and ball skills subscore in the children with DCD. We did not enter all of the neuromuscular outcomes into a single regression model due to concerns of collinearity (Table 4). In the first set of the regression (Table 4, model 1), gastrocnemius peak force was used to predict the MABC balance subscore. We first accounted for demographics including age, sex, BMI, and physical activity level. We then found that the gastrocnemius peak force was a significant predictor of the MABC balance subscore, accounting for 5.7% of its variance.
TABLE 4.
Multiple Regression Analyses for Predicting Motor Skills Impairments in Children With DCD (n = 130)

In the next sets of the regression model (Table 4, models 2 and 3), we used gastrocnemius muscle activation onset latency and peak force to predict the MABC ball skills subscore. After accounting for age, sex, BMI, and physical activity level, gastrocnemius muscle onset latency (model 2) and gastrocnemius peak force (model 3) remained independently associated with the MABC ball skills subscore, explaining 11.4% and 8.5% of its variance, respectively. When comparing the 2 regression models, gastrocnemius muscle onset latency was a stronger determinant of the MABC ball skills subscore than was gastrocnemius peak force, as reflected by the greater magnitude of change in the R2 value (11.4%) and beta weight (0.340).
DISCUSSION
DCD is widely acknowledged to impair the motor ability of children.1 As expected, our DCD group demonstrated poorer body balance, ball skills, and general motor performance than their typically developing peers. In addition, their hamstrings and gastrocnemius muscles reacted more slowly when standing balance was being challenged unexpectedly in the PA direction. Our present findings obtained from a large sample of children confirmed our hypothesis and supported our previous finding that children with DCD took more time to recover from postural disturbance.10 The causes of prolonged postural muscle onset latencies and longer time to recover from a postural disturbance in children with DCD might be related to their suboptimal cerebellar function and atypical development of autonomous balance control.9 Muscle contractile speed may not be a contributing factor as demonstrated in the present study. Although the exact causes of the neuromuscular timing deficit are still not known, this deficit may adversely affect the postural synergies used among children with DCD. For example, children with DCD over rely on hip strategy to maintain body balance perhaps because they fail to activate the hamstrings muscles in a timely manner to control postural (hip) sway,7 resulting in suboptimal balance performance. Further studies are needed to identify the underlying causes and functional consequences of this neuromuscular timing deficit in children with DCD.
In some contrast to our previous study suggesting that children with DCD had slower knee muscle force production,7 this study demonstrated that the time required to reach peak force (produce maximum muscle force) in the hamstring and gastrocnemius muscles was similar between the children with DCD and controls. This discrepancy could be attributed to the fact that we measured time to peak torque (force) isokinetically (at 180° per seconds) in our previous study10 but isometrically in the present study. Because the rate of force development depends very much on the type of muscle contraction performed,27 it is logical to find that the time required to achieve peak force in the lower limb muscles differed between the 2 studies.
We also found that the maximum isometric force of the hamstrings and gastrocnemius was lower in the children with DCD compared with the controls. Again, this finding is different from our previous study reporting that the isokinetic muscle strength of knee flexors (at 180° per seconds) was comparable between children with and without DCD.10 Isometric torque is always higher than isokinetic torque, whereas torque declines with increasing isokinetic velocity.28 Thus, it is plausible that the true maximum (isometric) peak force of the lower limb muscles is lower in the DCD population, which may not be reflected in isokinetic testing. In addition, the effect of age may also influence the lower limb muscle (isometric and isokinetic) peak force among children with DCD.11 All of these factors may explain the different findings across our 2 studies.
Among the neuromuscular deficits identified in the children with DCD, only gastrocnemius peak force was independently associated with balance performance, explaining 5.7% of its variance. Because our balance tests primarily include jumping, hopping, and tip-toe walking activities,13 gastrocnemius muscle strength is particularly important for maintaining posture, balance, and gait pattern.29
We also found that gastrocnemius peak force alone accounted for 8.5% of the variance in ball skills performance, whereas gastrocnemius muscle activation onset latency explained 11.4% of the variance in the children with DCD. Therefore, both the amplitude and timing of gastrocnemius muscle contraction were important for ball throwing, catching, and bouncing maneuvers. This finding was expected because before these forward-oriented movements (eg, ball catching and throwing), the gastrocnemius muscle must contract first to maintain posture and balance.30 Gastrocnemius muscle activation timing, which is particularly important to provide a stable base of support for catching and throwing activities,30 was compromised in the children with DCD. Therefore, improving the timing of gastrocnemius muscle activation and strengthening of this important postural muscle should be included in the rehabilitation treatments for children with DCD to improve their postural control.
The major limitation of this study is that our various regression models explained only 5.7% to 11.4% of the variance in balance and ball skills difficulties in the children with DCD, indicating that some potentially important factors were not captured. Indeed, other factors such as inconsistent or absent anticipatory trunk muscle activation31 and visual perceptual deficits32 may also be associated with these motor difficulties in the DCD population. A second limitation is that we measured the lower limb muscle activation onset latency (ie, reactive postural control) and correlated it with balance and ball skills performance that require primarily anticipatory postural control ability. It would be better in a future study to measure the spatio-temporal muscle activation sequence associated with the various balance and gross motor activity and the associated balance strategy instead.30 Moreover, further studies may include Teager–Kaiser energy operator signal conditioning to improve the accuracy of EMG onset detection.33 Another technical limitation of this study is the use of manual muscle testing and hand-held dynamometer to assess time to peak force of the strong gastrocnemius muscle. This method might be unable to detect subtle differences in muscle contractile speed between the 2 groups because of the inherent difficulties with stabilization and possibly insufficient strength of the assessor.34 Further studies may use isokinetic dynamometry instead.34 Finally, our results can only be generalized to children with DCD, but not children with other types of movement deficits.
CONCLUSIONS
In summary, the children with DCD demonstrated both the delayed onset of hamstring and gastrocnemius muscle activation in response to an unexpected PA trunk perturbation and lower isometric peak forces in these muscles. Gastrocnemius peak force was independently associated with balance and ball skills performance, whereas the timing of gastrocnemius muscle activation was a more important determinant of ball skills performance in this group of children.
ACKNOWLEDGMENTS
The authors would like to acknowledge TWGHs Hok Shan School, Chinese YMCA of Hong Kong, Hong Kong Christian Service, Heep Hong Society, Department of Health of Hong Kong (Child Assessment Service), Watchdog Early Education Centre, Caritas Nursery School (Tsui Lam), Aplichau Kaifong Primary School, Hennessy Road Government Primary School (AM and PM), SKH St Matthew's Primary School and Tsung Tsin Primary School and Kindergarten for enabling the recruitment of the participants.
Footnotes
Abbreviations: BMI = body mass index, DCD = developmental coordination disorder, EMG = electromyographic or electromyography, MABC = Movement Assessment Battery for Children, MET = metabolic equivalent, PA = posterior-to-anterior.
This study was partially supported by an ECS grant (number 27100614) from the Research Grants Council of Hong Kong.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental, Disorders. 4th edWashington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.Macnab J, Miller LT, Polatajko HJ. The search for subtypes of DCD: is cluster analysis the answer? Hum Mov Sci 2001; 20:49–72. [DOI] [PubMed] [Google Scholar]
- 3.Geuze RH. Postural control in children with DCD. Neural Plast 2005; 12:183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fong SSM, Lee VYL, Pang MYC. Sensory organization of balance control in children with developmental coordination disorder. Res Dev Disabil 2011; 32:2376–2382. [DOI] [PubMed] [Google Scholar]
- 5.Fong SSM, Tsang WWN, Ng GYF. Taekwondo training improves sensory organization and balance control in children with developmental coordination disorder: a randomized controlled trial. Res Dev Disabil 2012; 33:85–95. [DOI] [PubMed] [Google Scholar]
- 6.Fong SSM, Tsang WWN, Ng GYF. Altered postural control strategies and sensory organization in children with DCD. Hum Mov Sci 2012; 31:1317–1327. [DOI] [PubMed] [Google Scholar]
- 7.Fong SSM, Ng SSM, Yiu BPHL. Slowed muscle force production and sensory organization deficits contribute to altered postural control strategies in children with developmental coordination disorder. Res Dev Disabil 2013; 34:3040–3048. [DOI] [PubMed] [Google Scholar]
- 8.Williams H, Woollacott M. Characteristics of neuromuscular responses underlying posture control in clumsy children. Mot Dev Res Rev 1997; 1:8–23. [Google Scholar]
- 9.Geuze RH. Static balance and developmental coordination disorder. Hum Mov Sci 2003; 22:527–548. [DOI] [PubMed] [Google Scholar]
- 10.Fong SSM, Chung JWY, Chow LPY, et al. Differential effect of taekwondo training on knee muscle strength and reactive and static balance control in children with developmental coordination disorder: a randomized controlled trial. Res Dev Disabil 2013; 34:1446–1455. [DOI] [PubMed] [Google Scholar]
- 11.Raynor AJ. Strength, power, and coactivation in children with developmental coordination disorder. Dev Med Child Neurol 2001; 43:676–684. [DOI] [PubMed] [Google Scholar]
- 12.Fukagawa NK, Wolfson CL, Judge J, et al. Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol A Biol Sci Med Sci 1995; 50:64–67. [DOI] [PubMed] [Google Scholar]
- 13.Henderson SE, Sugden DA. Movement Assessment Battery for Children Manual. London: The Psychological Corporation Ltd; 1992. [Google Scholar]
- 14.Bruininks RH. Bruininks–Oseretsky Test of Motor Proficiency: Examiner's Manual. Circle Pines, MN: American Guidance Service; 1978. [Google Scholar]
- 15.Wilson BN, Crawford SG, Green D, et al. Psychometric properties of the revised developmental coordination disorder questionnaire. Phys Occup Ther Pediatr 2009; 29:182–202. [DOI] [PubMed] [Google Scholar]
- 16.Ridley K, Ainsworth BE, Olds TS. Development of a compendium of energy expenditures for youth. Int J Behav Nutr Phys Act 2008; 5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fong SM, Ng GYF. The effects on sensorimotor performance and balance with Tai Chi training. Arch Phys Med Rehabil 2006; 87:82–87. [DOI] [PubMed] [Google Scholar]
- 18.Shumway-Cook A, Woollacott MH. Motor Control: Translating Research into Clinical Practice. 3rd edPhiladelphia, PA: Lippincott Williams and Wilkins; 2007. [Google Scholar]
- 19.Barbero M, Merletti R, Rainoldi A. Atlas of Muscle Innervation Zones—Understanding Surface Electromyography and Its Applications. Milan, Italy: Springer-Verlag Italia; 2012. [Google Scholar]
- 20.Biometrics Ltd. EMG Sensor and DataLOG Operating Manual. Newport, UK: Biometrics Ltd.; 2012. [Google Scholar]
- 21.Biometrics Ltd. Accelerometer Sensor Operating Manual (Type No. ACL300). Newport, UK: Biometrics Ltd.; 2007. [Google Scholar]
- 22.Kendall FP, McCreary EK, Provance PG. Muscle Testing and Function With Posture and Pain. 4th edBaltimore, MD: Lippincott Williams and Wilkins; 1993. [Google Scholar]
- 23.Lafayette Instrument. Lafayette Manual Muscle Test System User, Instructions. Lafayette, IN: Lafayette Instrument Company; 2012. [Google Scholar]
- 24.Taylor NF, Dodd KJ, Graham HK. Test-retest reliability of hand-held dynamometric strength testing in young people with cerebral palsy. Arch Phys Med Rehabil 2004; 85:77–80. [DOI] [PubMed] [Google Scholar]
- 25.Crock RV, Horvat M, McCarthy E. Reliability and concurrent validity of the Movement Assessment Battery for Children. Percept Mot Skills 2001; 93:275–280. [DOI] [PubMed] [Google Scholar]
- 26.Fong SSM, Lee VYL, Chan NNC, et al. Motor ability and weight status are determinants of out-of-school activity participation for children with developmental coordination disorder. Res Dev Disabil 2011; 32:2614–2623. [DOI] [PubMed] [Google Scholar]
- 27.Kawamori N, Rossi SJ, Justice BD, et al. Peak force and rate of force development during isometric and dynamic mid-thigh clean pulls performed at various intensities. J Strength Cond Res 2006; 20:483–491. [DOI] [PubMed] [Google Scholar]
- 28.Knapik JJ, Wright JE, Mawdsley RH, et al. Isometric, isotonic, and isokinetic torque variations in four muscle groups through a range of joint motion. Phys Ther 1983; 63:938–947. [DOI] [PubMed] [Google Scholar]
- 29.Moore KL, Dalley AR. Clinically Oriented Anatomy. 5th edPhiladelphia, PA: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 30.Crenna P, Frigo C. A motor programme for the initiation of forward-oriented movements in humans. J Physiol 1991; 437:635–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kane K, Barden J. Frequency of anticipatory trunk muscle onsets in children with and without developmental coordination disorder. Phys Occup Ther Pediatr 2014; 34:75–89. [DOI] [PubMed] [Google Scholar]
- 32.Van Waelvelde H, De Weerdt W, De Cock P, et al. Association between visual perceptual deficits and motor deficits in children with developmental coordination disorder. Dev Med Child Neurol 2004; 46:661–666. [DOI] [PubMed] [Google Scholar]
- 33.Solnik S, Rider P, Steinweg K, et al. Teager-Kaiser energy operator signal conditioning improves EMG onset detection. Eur J Appl Physiol 2010; 110:489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarkson HM. Musculoskeletal Assessment Joint Range of Motion and Manual Muscle Strength. 2nd edPhiladelphia, PA: Lippincott Williams and Wilkins; 2000. [Google Scholar]


