Abstract
Little was known about the beneficial effects of uvulopalatopharyngoplasty (UPPP) on the outcomes after obstructive sleep apnea (OSA). The aim of this study is to investigate the effects of UPPP on reducing risk of cerebrovascular diseases in patients with OSA.
Using Taiwan's National Health Insurance Research Database, we conducted a retrospective cohort study of 10,339 patients with new OSA between January 1, 2004, and December 31, 2009. The incident cerebrovascular disease was identified during the 1-year follow-up period in patients with and without receiving UPPP. The rate ratios (RRs) and 95% confidence intervals (CIs) of cerebrovascular disease associated with receiving UPPP in patients with OSA were calculated in multivariate Poisson regression.
The 1-year incidences of cerebrovascular disease for OSA patients with and without UPPP were 1.06% and 5.14%, respectively. Patients with OSA receiving UPPP had lower risk of cerebrovascular disease compared with those without UPPP (RR, 0.45; 95% CI, 0.33–0.61). The decreased risk of cerebrovascular disease following UPPP was observed in both sexes and all age groups. In the stratified analysis of medical conditions, the RR of cerebrovascular disease associated with UPPP for patients with 0, 1, ≥ 2 medical conditions were 0.28 (95% CI 0.12–0.68), 0.39 (95% CI 0.21–0.73), and 0.63 (95% CI 0.43–0.93), respectively.
Patients with OSA who received UPPP had lower risk of cerebrovascular disease within 1 year after surgery compared with patients not receiving UPPP. Clinical physicians could have more evidence to persuade patients to receive surgical intervention, especially those who have severe OSA symptoms or do not acquire adequate symptom relief under conservative treatments.
INTRODUCTION
Obstructive sleep apnea (OSA) is the most common form of sleep-disordered breathing. It is estimated to affect about 4% of men and 2% of women in Western communities.1,2 Obstructive sleep apnea is associated with sleep disturbance from snoring, choking, and wakefulness and with excessive daytime sleepiness and fatigue, all affecting physical and psycho-social well-being. Hypertension, coronary artery disease, arrhythmia, heart attack, heart failure, cerebrovascular disease, cancer, diabetes, obesity, pneumonia, anxiety, and depression have been identified as complications for OSA patients.3–8
Cerebrovascular disease is the leading cause of acquired disability in adults and the second leading cause of death worldwide.9,10 Risk factors such as cardiac diseases, hypertension, diabetes mellitus, smoking, alcohol intake, unhealthy diet, abdominal obesity, lack of exercise, psychosocial stress, and depression contribute 90% of stroke risk.10 Molecular markers of coagulation and fibrinolysis, arterial stiffness, immune-inflammatory factors, and biochemical profiles were also found to be associated with cerebrovascular disease.11–16
Several studies suggest that people with OSA have increased risk of cerebrovascular disease.17–20 When treating people with OSA, continuous positive airway pressure is considered the first-line treatment for moderate to severe OSA and has many treatment benefits.21 However, many patients have poor compliance with using continuous positive airway pressure due to discomfort from the apparatus.5 Surgical interventions such as uvulopalatopharyngoplasty (UPPP), maxillo-mandibular advancement, radio frequency ablation, and palatal implants are alternative OSA treatments. Uvulopalatopharyngoplasty is the most common surgical procedure used to treat patients with OSA, with success rates ranges from 36% to 62% and improved apnea–hypopnea index according to various procedure modifications.22 However, limited information was available on the real effectiveness of UPPP for improving OSA-related disease, particularly cerebrovascular disease. Therefore, we conducted this nationwide population-based study to investigate the effectiveness of UPPP in reducing risk of cerebrovascular disease among patients with OSA.
METHODS
Source of Data
Taiwan's National Health Insurance Program has integrated medical claims since 1996, and this database is available to researchers with identification numbers of those insured scrambled to protect patient privacy. Sets of information available for this study include sex, birth dates, diagnoses, health care received, medications prescribed, admissions, discharges, medical institutions, and physicians providing services. For research and administrative purposes, Taiwan National Health Research Institute has released a data subset of claims data for 1 million randomly selected insurance enrollees aged 0 to 113 years in 2005. This random subgroup represents about 5 % of Taiwan's insured population. Information on health care was collected from 1996 to 2008.23–25
Ethical Approval
Insurance reimbursement claims used in this study were from Taiwan's National Health Insurance Research Database. To protect personal privacy, the electronic database was decoded with patient identifications scrambled for further public access for research. This study was evaluated and approved by Taiwan's National Health Research Institutes (NHIRD-103-121) and the Institutional Review Board of Taipei Medical University (TMU-JIRB-201404070); informed consent was exempted because patient identification has been decoded and scrambled. This study was conducted in accordance with the Declaration of Helsinki.23–25
Study Design
In this longitudinal cohort of 1 million insured individuals, we identified an intervention cohort of patients aged 18 years and older with primary new diagnosis of OSA receiving UPPP between 2005 and 2007 (without any previous record of diagnosis or treatment for OSA from the database since 1996) and without a history of cerebrovascular disease before the index date. Patients with OSA who did not receive UPPP and had no history of cerebrovascular disease were identified as the nonintervention group.
Patients with any diagnosis of cerebrovascular disease before the index date were excluded to ensure that all study participants were free of cerebrovascular disease at the start of both cohorts. Follow-up for 12 months started at the new diagnosis of OSA for 1 year in patients who did not receive UPPP. To reduce the immortal time bias in the intervention group,26 patients with OSA receiving UPPP were followed for 12 months starting at surgery. We sought to determine whether individuals with OSA who received UPPP faced a reduced risk of cerebrovascular disease compared with those who did not.
Measures and Definitions
We identified income status by defining low-income patients as those qualifying for waived medical copayment as verified by the Bureau of National Health Insurance. Population density was calculated by dividing the population (persons) by the area (square kilometers) for each administrative unit of Taiwan and then sorting these areas into quartiles of low, moderate, high, and very high urbanization. These categories were used as surrogates for residential urbanization. Medications, such as aspirin, anticoagulant, coumadin, enoxaparin, heparin, statin (included atorvastatin, simvastatin, rosuvastatin, fluvastatin, lovastatin, pravastatin), and anti-hypertension (included beta-blocker, angiotensin converting enzyme inhibitor, calcium channel blockers, diuretics) were also considered in this study.
Following previous reports,23–25 we used codes from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) to define medical conditions. Cerebrovascular disease (ICD-9-CM 430–438) and OSA (ICD-9-CM 780.51, 780.53, 780.57) were defined as outcome and exposure, respectively. Coexisting medical conditions were determined from medical claims for the follow-up period and pre-OSA period within 24 months, such as hypertension (ICD-9-CM 401–405), mental disorders (ICD-9-CM 290–319), chronic obstructive pulmonary disease (ICD-9-CM490–496), hyperlipidemia (ICD-9-CM 272.0, 272.1, and 272.2), ischemic heart disease (ICD-9-CM 410–414), asthma (ICD-9-CM 493), diabetes (ICD-9-CM 250), atherosclerosis (440), liver cirrhosis (ICD-9-CM 571), traumatic brain injury (ICD-9-CM 800–804, 850–854), pneumonia (480–486), congestive heart failure (ICD-9-CM 428), epilepsy (ICD-9-CM 345), and atrial fibrillation (ICD-9-CM 427.31). Alcohol-related illness included alcoholic psychoses (ICD-9-CM 291), alcohol dependence syndrome (ICD-9-CM 303), alcohol abuse (ICD-9-CM 305), alcoholic fatty liver (ICD-9-CM 571.0), acute alcoholic hepatitis (ICD-9-CM 571.1), alcoholic cirrhosis of the liver (ICD-9-CM 571.2), and alcoholic liver damage (ICD-9-CM 571.3). Renal dialysis was identified by the administration code (D8, D9).
Statistical Analysis
Our study used chi-square tests to compare sociodemographic characteristics and coexisting medical conditions between people who did and did not receive UPPP. The multivariate Poisson regression analysis was used to calculate the adjusted rate ratio (RR) and 95% confidence interval (CI) of cerebrovascular disease in these patients with adjustment for sociodemographics and coexisting medical conditions. To investigate the effects of potential confounding factors on the association between UPPP and risk of cerebrovascular disease, we performed model 1 (unadjusted), model 2 (adjusted for age and sex), model 3 (adjusted for age, sex, low income, and urbanization), model 4 (all sociodemographics and medical conditions), and model 5 (all sociodemographics, medical conditions, and medications) to calculate RRs and 95% CIs of risk of cerebrovascular disease associated with UPPP.
In the age- and sex-stratified analysis of age, sex, and coexisting medical conditions, the multivariate Poisson regression analysis was also used to calculate RRs and 95% CIs of cerebrovascular disease associated with receiving UPPP after adjustment for age, sex, low income, urbanization, mental disorders, hypertension, chronic obstructive pulmonary disease, ischemic heart disease, hyperlipidemia, diabetes, asthma, smoking cessation, atherosclerosis, traumatic brain injury, pneumonia, alcohol-related illness, liver cirrhosis, congestive heart failure, epilepsy, renal dialysis, atrial fibrillation, and medications.
RESULTS
Compared with OSA patients without UPPP (Table 1), patients with OSA receiving UPPP had lower proportions of people aged ≥65 years (1.5% vs 14.0%, P < 0.0001), females (18.5% vs 38.3%, P < 0.0001), and people with low-income status (1.5% vs 3.3%, P < 0.0001), mental disorders (22.1% vs 39.5%, P < 0.0001), hypertension (20.1% vs 32.1%, P < 0.0001), chronic obstructive pulmonary disease (10.3% vs 22.4%, P < 0.0001), ischemic heart disease (8.3% vs 17.5%, P < 0.0001), hyperlipidemia (7.2% vs 11.9%, P < 0.0001), diabetes (5.7% vs 12.4%, P < 0.0001), asthma (5.6% vs 11.0%, P < 0.0001), atherosclerosis (3.6% vs 8.0%, P < 0.0001), traumatic brain injury, (2.8% vs 5.8%, P < 0.0001), pneumonia (2.7% vs 5.0%, P < 0.0001), alcohol-related illness (2.5% vs 4.4%, P < 0.0001), liver cirrhosis (2.3% vs 5.1%, P < 0.0001), congestive heart failure (0.5% vs 2.4%, P < 0.0001), epilepsy (0.5% vs 1.2%, P < 0.0001), atrial fibrillation (0.2% vs 0.6%, P < 0.0001), and renal dialysis (0.1% vs 0.8%, P < 0.0001).
TABLE 1.
Sociodemographic Factors and Coexisting Medical Conditions in Obstructive Sleep Apnea Patients With and Without UPPP
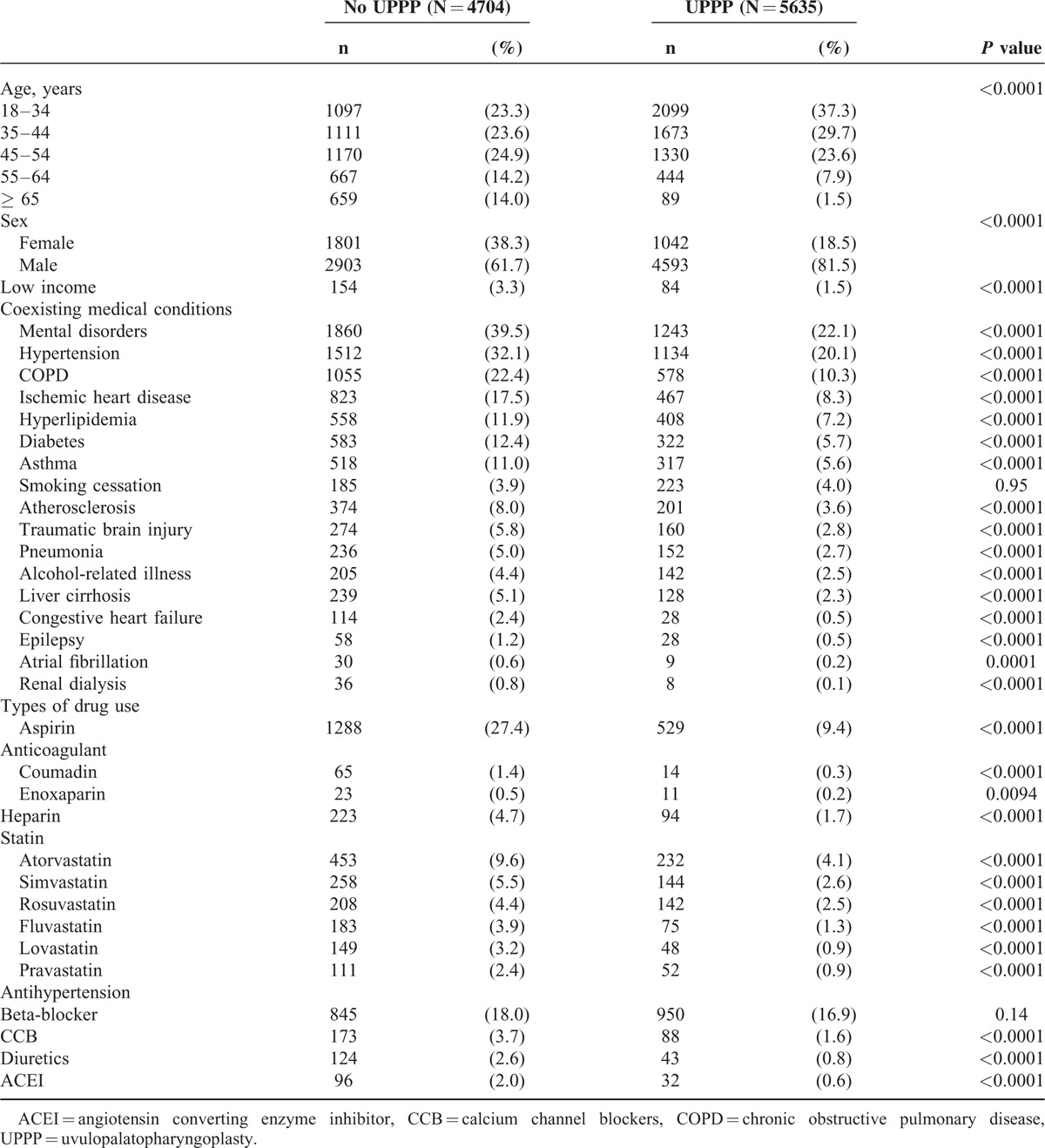
Among patients with OSA, patients receiving UPPP had lower 1-year incidence of cerebrovascular disease than those without UPPP treatment (1.06% vs 5.14%, P < 0.0001) (Table 2). The 1-year incidences of hemorrhagic stroke (0.07% vs 0.47%, P < 0.0001), ischemic stroke (0.30% vs 1.76%, P < 0.0001), other stroke (0.35% vs 1.45%, P < 0.0001), transient ischemic attack (0.25% vs 0.68%, P < 0.0001), and late effects of cerebrovascular disease (0.09% vs 0.79%, P < 0.0001) were also lower in patients receiving UPPP than in those without.
TABLE 2.
One-year Incidence of Cerebrovascular Disease Among Patients With Obstructive Sleep Apnea in 2004 to 2009
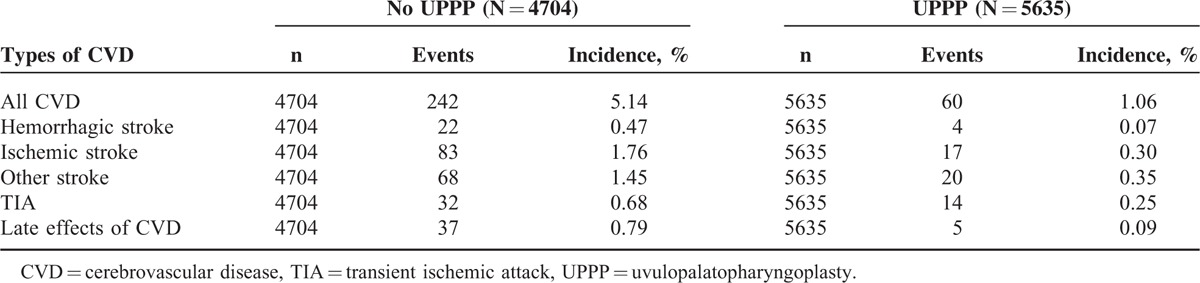
In model 1 with univariate Poisson regression analysis (Table 3), patients with OSA receiving UPPP had lower RRs of cerebrovascular disease compared with those who did not receive UPPP (RR 0.21, 95% CI 0.16–0.27). After adjustment for all sociodemographic factors, coexisting medical conditions, and medications in the multivariate Poisson regression (model 5), UPPP in patients with OSA was associated with reduced risk of cerebrovascular disease with an adjusted RR of 0.45 (95% CI 0.33–0.61).
TABLE 3.
Risk of Cerebrovascular Disease Among Patients With Obstructive Sleep Apnea Receiving UPPP in 2004 to 2009∗
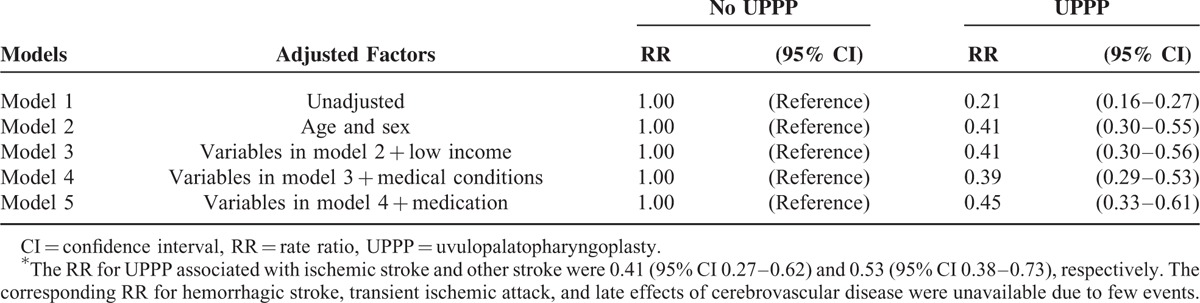
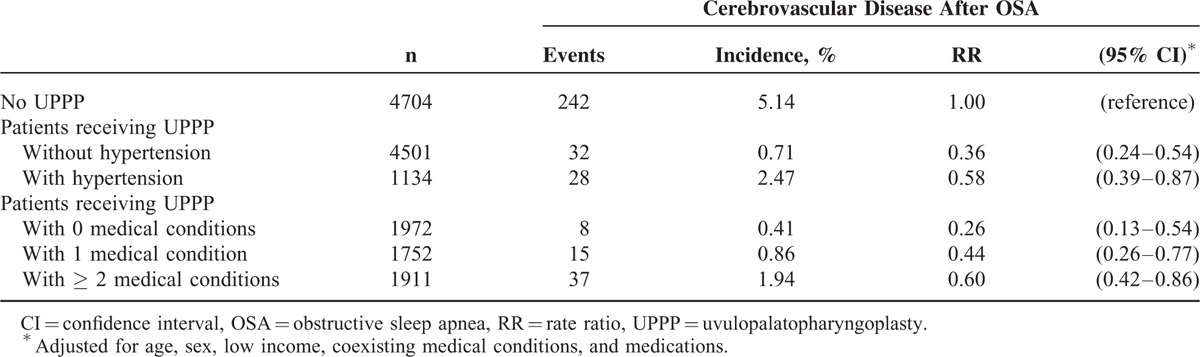
In patients with OSA (Table 4), receiving UPPP was associated with reduced risk of cerebrovascular disease in both women (RR 0.41, 95% CI 0.20–0.85) and men (RR 0.47, 95% CI 0.33–0.66), and in those aged 45–54 (RR 0.43, 95% CI 0.25–0.72), and ≥ 55 years (RR 0.33, 95% CI 0.21–0.53). Regarding coexisting medical conditions, whether patients with OSA had 0 (RR 0.28, 95% CI 0.12–0.68), 1 (RR 0.39, 95% CI 0.21–0.73), or ≥ 2 (RR 0.63, 95% CI 0.43–0.93) medical conditions, those receiving UPPP showed protective effect regarding risk of cerebrovascular disease. Compared with those who did not receive UPPP (Table 5), the RRs of patients receiving UPPP who had hypertension and ≥ 2 medical conditions were 0.58 (95% CI 0.39–0.87) and 0.60 (95% CI 0.42–0.86), respectively.
TABLE 4.
Stratification Analysis for the Association Between UPPP and Risk of Cerebrovascular Disease in Patients With Obstructive Sleep Apnea
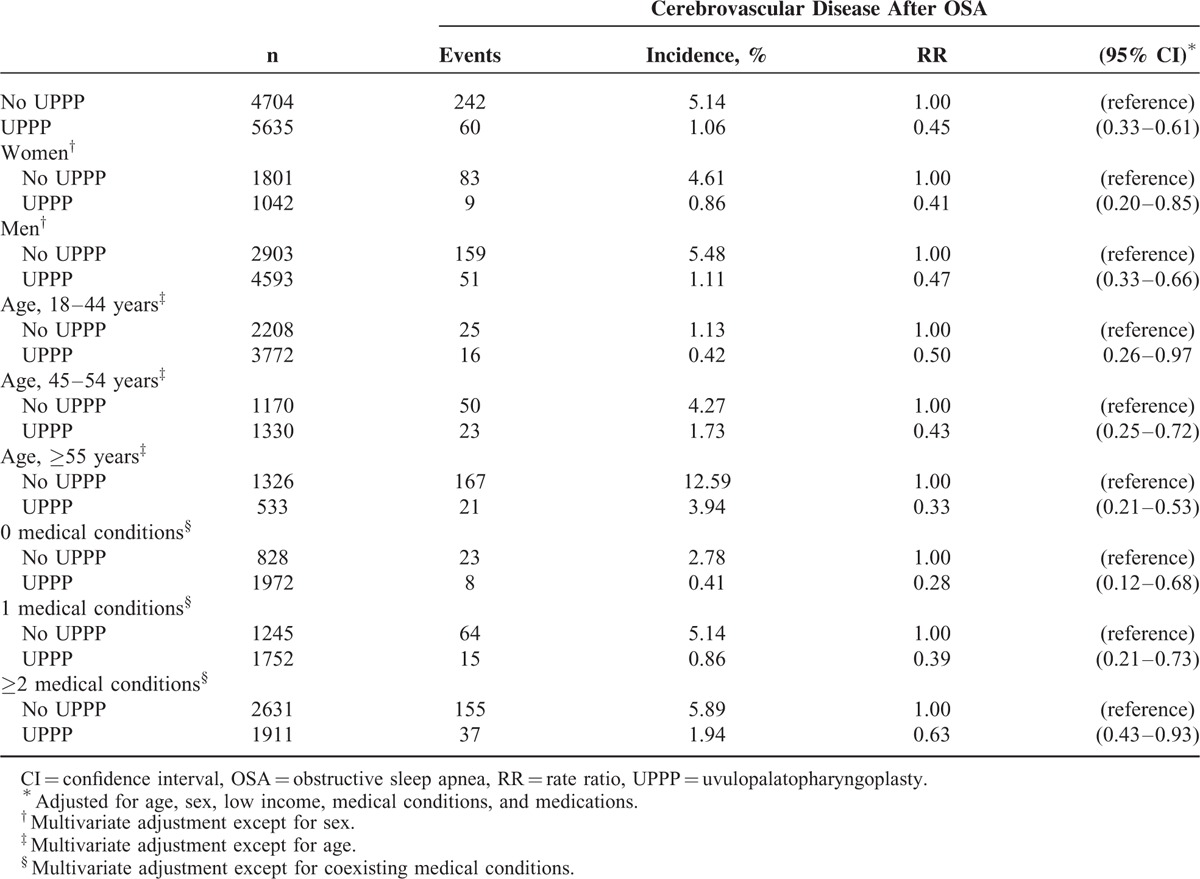
TABLE 5.
Impact of UPPP on Risk of Cerebrovascular Disease in OSA Patients With Medical Conditions
DISCUSSION
Using claims data from Taiwan's National Health Insurance Research Database, our results showed that patients with OSA who received UPPP had a significantly reduced risk of cerebrovascular disease within the first year after UPPP. This benefit was observed in both genders and in those with various coexisting medical conditions. To the best of our knowledge, this is the first study reporting the benefit of UPPP regarding reduced risk of cerebrovascular disease for patients with OSA.
When investigating the relationship between OSA and risk of cerebrovascular disease, potential confounding factors need to be considered. Age, gender, and socioeconomic status have been related to OSA, and these factors are also associated with cerebrovascular disease.1,2,27–29 Coexisting medical conditions, such as hypertension,3,10 diabetes,4,10 cardiovascular diseases,10,20 asthma,30 pneumonia,8 and mental disorders,30 have been related to sleep apnea and cerebrovascular disease. Moreover, patients with OSA and cerebrovascular disease may take antiplatelet or anticoagulant medications to prevent thrombo-embolism. To eliminate confounding effects from age, gender, low income, urbanization, coexisting medical conditions, and medications, we used the multivariate Poisson regression model to adjust these potential confounding factors when assessing risk of cerebrovascular disease in OSA patients who did or did not receive UPPP. Older patients and those with major medical conditions might not receive surgical interventions due to higher risks of mortality and morbidity from anesthetics and surgery. This may explain why patients who did not receive UPPP were older and had more coexisting medical conditions than those who received UPPP in the present study. In addition, patients who had more severe OSA may have more medical conditions, such as hypertension.3,10 In this study, we found that the benefit of UPPP in reducing risk of cerebrovascular disease decreased in those who had more medical conditions. However, limited data from the National Health Insurance Research Database prevented us from considering the respiratory disturbance index and the apnea-hypopnea index, as this information is not available in the database.
The stratification analysis found RRs of 1-year incidence of cerebrovascular disease in patients with UPPP are significantly lower than those without UPPP in both genders as well as in patients with zero, 1, and 2 or more coexisting medical conditions. As in general, older people had higher risk of cerebrovascular disease compared with younger populations,10 we found UPPP showed more significant protective effects regarding cerebrovascular disease in older patients with OSA.
To clarify the beneficial effects of UPPP in reducing the cerebrovascular disease risk in patients with OSA, we propose some possible explanations. First, chronic hypoxemia may cause increased erythropoiesis, blood viscosity, and coagulability that may lead to thrombosis and cerebrovascular disease.31 Uvulopalatopharyngoplasty improves oxygenation in patients with OSA, prevents patients from intermittent hypoxemia during apnea episodes, and thus reduces the risk of cerebrovascular disease. Second, intermittent hypoxemia, chemoreceptor stimulation, sympathetic activation, and the renin–angiotensin system activation are possible mechanisms by which OSA might lead to hypertension and arrhythmias, increasing the risk of cerebrovascular disease.32–35 The UPPP treatment may prevent hypopnea or apnea and subsequent hypoventilation in patients with OSA, and therefore improve hypertension and arrhythmias. Third, frequent waking and sleep deprivation may cause a stressed mood and further exacerbate hypertension, thus increasing cerebrovascular disease risk.32,36 Patients who receive UPPP may have improved sleep quality and maintain stable blood pressure. Fourth, as OSA has been associated with increased platelet activation, increased fibrinogen, and other potential markers of thrombotic risk,37,38 UPPP may benefit patients with OSA as another surgical treatment reducing prothrombotic effects.39 In addition, OSA has been reported to increase the incidence of pneumonia, since apnea results in negative intra-thoracic pressure that increases the risk of pulmonary aspiration and subsequent pneumonia,8 and pneumonia appeared to confer a higher risk of cerebrovascular disease in another population-based study.40 Uvulopalatopharyngoplasty improves apnea symptoms and, as a consequence, may prevent pneumonia and decrease cerebrovascular disease risk.41 Finally, patients with OSA showed greater signs of early atherosclerosis, increased arterial stiffness, increased carotid intima-media thickness, and increased prevalence of silent brain infarcts.20,42 A decrease in cardiac output during obstructive apnea may cause cerebral perfusion impairment.20 These conditions may predispose to ischemic events in patients with cerebral blood flow limitations.20 Considering these possible benefits, it was reasonable that UPPP reduced the post-operative risk of cerebrovascular disease among patients with OSA.
Among people with OSA, the observation that 1-year incidence of hemorrhagic stroke is lower in patients receiving UPPP than in those without UPPP treatment indicates that patients with OSA might be more likely to have less-severe cerebrovascular disease. However, the present study may be limited because we could not use animal or human trials to provide the mechanism of this phenomenon. The results of this study were similar to a previous investigation reporting higher proportion of ischemic stroke than hemorrhage among all stroke patients in Taiwan.43
There are some limitations in this study. First it is a relatively small number of older patients in our analysis, so we did not investigate UPPP's effects regarding risk of cerebrovascular disease in the elderly. Second, the insurance reimbursement claims data lack detailed information that might help to demarcate OSA severity, as they have no data on biomedical measures, risk scores, lifestyle factors such as smoking or drinking alcohol, or clinical examination results such as prior abnormal cerebrovascular symptoms, blood pressure, respiratory disturbance index, apnea–hypopnea index, and biochemical or image study reports. Third, although polysomnography is the gold standard for OSA diagnosis and covered under National Health Insurance, patients may choose to use selfpay clinics for this service, and this could result in underestimation of this intervention for the study population. Fourth, some extra-paid (not covered by Taiwan's National Health Insurance) OSA treatments are not documented in the National Health Insurance Research Database. These include continuous positive airway pressure ventilator support, maxillo-mandibular advancement, and palatal implants. Patients who did not receive UPPP may have benefited from continuous positive airway pressure treatment or other selfpay interventions or remained untreated. We believe that the comparison cohort may have had a higher proportion of extra-paid OSA treatments than patients with UPPP, and this phenomenon may result in underestimation of the beneficial effects of UPPP in this study. Finally, the classification of subtypes of cerebrovascular disease in the emergency departments of Taiwan's medical system in Taiwan was limited because the proportion of other subtypes of cerebrovascular disease was higher than in other countries.27
Our nationwide retrospective cohort study found that OSA patients who received UPPP had a significant reduction of 1-year risk of cerebrovascular disease. This implies that surgical intervention for OSA treatment may reduce the likelihood of future cerebrovascular events and subsequent disabilities or mortality. Clinical physicians could have more evidence to persuade patients to receive surgical intervention, especially those who have severe OSA symptoms or do not acquire adequate symptom relief under conservative treatments. However, further investigations are warranted to evaluate the treatment efficacy of other surgical procedures on OSA complications such as reduction of cerebrovascular disease, as well as on cardiovascular, metabolic, respiratory, or psychosocial complications.
ACKNOWLEDGMENTS
This study is based on data obtained from the National Health Insurance Research Database provided by the Bureau of National Health Insurance of Taiwan's Ministry of Health and Welfare and managed by the National Health Research Institutes. The authors’ interpretation and conclusions do not represent viewpoints of these agencies.
Footnotes
Abbreviations: CI = confidence interval, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, OSA = obstructive sleep apnea, RR = rate ratio, UPPP = uvulopalatopharyngoplasty.
Dr. Shih-Yu Huang has equal contribution with the first author.
Dr. Chien-Chang Liao has equal contribution with corresponding author.
This research was supported in part by Shuang Ho Hospital, Taipei Medical University (104TMU-SHH-23), and by Taiwan's Ministry of Science and Technology (MOST104-2314-B-038-027-MY2; NSC102-2314-B-038-021-MY3).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-age adults. N Engl J Med 1993; 328:1230–1235. [DOI] [PubMed] [Google Scholar]
- 2.Duran J, Esnaola S, Rubio R, et al. Obstructive sleep apneahypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med 2001; 163:685–689. [DOI] [PubMed] [Google Scholar]
- 3.Kapur VK, Weaver EM. Filling in the pieces of the sleep apnea-hypertension puzzle. JAMA 2012; 307:2197–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin Proc 2011; 86:549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durgan DJ, Bryan RM., Jr Cerebrovascular consequences of obstructive sleep apnea. J Am Heart Assoc 2012; 1:e000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice TB, Strollo PJ, Jr, Morrell MJ. Update in sleep medicine 2011. Am J Respir Crit Care Med 2012; 185:1271–1274. [DOI] [PubMed] [Google Scholar]
- 7.Campos-Rodriguez F, Martinez-Garcia MA, Martinez M, et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter Spanish cohort. Am J Respir Crit Care Med 2013; 187:99–105. [DOI] [PubMed] [Google Scholar]
- 8.Su VY, Liu CJ, Wang HK, et al. Sleep apnea and risk of pneumonia: a nationwide population-based study. CMAJ 2014; 186:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol 2007; 6:182–187. [DOI] [PubMed] [Google Scholar]
- 10.O’Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 2010; 376:112–123. [DOI] [PubMed] [Google Scholar]
- 11.Tuttolomondo A, Di Raimondo D, Di Sciacca R, et al. Arterial stiffness and ischemic stroke in subjects with and without metabolic syndrome. Atherosclerosis 2012; 225:216–219. [DOI] [PubMed] [Google Scholar]
- 12.Tuttolomondo A, Di Raimondo D, Pecoraro R, et al. Immune-inflammatory markers and arterial stiffness indexes in subjects with acute ischemic stroke. Atherosclerosis 2010; 213:311–318. [DOI] [PubMed] [Google Scholar]
- 13.Pinto A, Tuttolomondo A, Di Raimondo D, et al. Cardiovascular risk profile and morbidity in subjects affected by type 2 diabetes mellitus with and without diabetic foot. Metabolism 2008; 57:676–682. [DOI] [PubMed] [Google Scholar]
- 14.Strano A, Hoppensteadt D, Walenga JM, et al. Plasma levels of the molecular markers of coagulation and fibrinolysis in patients with peripheral arterial disease. Semin Thromb Hemost 1996; 22 Suppl 1:35–40. [PubMed] [Google Scholar]
- 15.Crepaldi G, Fellin R, Calabrò A, et al. Double-blind multicenter trial on a new medium molecular weight glycosaminoglycan. Current therapeutic effects and perspectives for clinical use. Atherosclerosis 1990; 81:233–243. [DOI] [PubMed] [Google Scholar]
- 16.Pinto A, Tuttolomondo A, Di Raimondo D, et al. Risk factors profile and clinical outcome of ischemic stroke patients admitted in a Department of Internal Medicine and classified by TOAST classification. Int Angiol 2006; 25:261–267. [PubMed] [Google Scholar]
- 17.Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005; 353:2034–2041. [DOI] [PubMed] [Google Scholar]
- 18.Capampangan DJ, Wellik KE, Parish JM, et al. Is obstructive sleep apnea an independent risk factor for stroke? A critically appraised topic. Neurologist 2010; 16:269–273. [DOI] [PubMed] [Google Scholar]
- 19.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the Sleep Heart Health Study. Am J Respir Crit Care Med 2010; 182:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Ouyang Y, Wang Z, et al. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Int J Cardiol 2013; 169:207–214. [DOI] [PubMed] [Google Scholar]
- 21.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365:1046–1053. [DOI] [PubMed] [Google Scholar]
- 22.Handler E, Hamans E, Goldberg AN, et al. Tongue suspension: an evidence-based review and comparison to hypopharyngeal surgery for OSA. Laryngoscope 2014; 124:329–336. [DOI] [PubMed] [Google Scholar]
- 23.Liao CC, Chou YC, Yeh CC, et al. Stroke risk and outcomes in patients with traumatic brain injury: 2 nationwide studies. Mayo Clin Proc 2014; 89:163–172. [DOI] [PubMed] [Google Scholar]
- 24.Liao CC, Chang PY, Yeh CC, et al. Outcomes after surgery in patients with previous stroke. Br J Surg 2014; 101:1616–1622. [DOI] [PubMed] [Google Scholar]
- 25.Liao CC, Su TC, Sung FC, et al. Does hepatitis C virus infection increase risk for stroke? A population-based cohort study. PLoS One 2012; 7:e31527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lévesque LE, Hanley JA, Kezouh A, et al. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. Br Med J 2010; 340:b5087. [DOI] [PubMed] [Google Scholar]
- 27.Bhatnagar P, Scarborough P, Smeeton NC, et al. The incidence of all stroke and stroke subtype in the United Kingdom, 1985 to 2008: a systematic review. BMC Public Health 2010; 10:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engels T, Baglione Q, Audibert M, et al. Socioeconomic status and stroke prevalence in Morocco: results from the Rabat-Casablanca study. PLoS One 2014; 9:e89271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Sundquist K, Sundquist J. Socioeconomic status and occupation as risk factors for obstructive sleep apnea in Sweden: a population-based study. Sleep Med 2008; 9:129–136. [DOI] [PubMed] [Google Scholar]
- 30.Bhattacharyya N, Kepnes LJ. Ambulatory office visits and medical comorbidities associated with obstructive sleep apnea. Otolaryngol Head Neck Surg 2012; 147:1154–1157. [DOI] [PubMed] [Google Scholar]
- 31.Liak C, Fitzpatrick M. Coagulability in obstructive sleep apnea. Can Respir J 2011; 18:338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease. Circulation 2008; 118:1080–1111. [DOI] [PubMed] [Google Scholar]
- 33.Lesske J, Fletcher EC, Bao G, et al. Hypertension caused by chronic intermittent hypoxia: influence of chemoreceptors and sympathetic nervous system. J Hypertens 1997; 15:1593–1603. [DOI] [PubMed] [Google Scholar]
- 34.Fletcher EC, Lesske J, Culman J, et al. Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension 1992; 20:612–619. [DOI] [PubMed] [Google Scholar]
- 35.Engström G, Hedblad B, Juul-Möller S, et al. Cardiac arrhythmias and stroke: increased risk in men with high frequency of atrial ectopic beats. Stroke 2000; 31:2925–2929. [DOI] [PubMed] [Google Scholar]
- 36.Everson-Rose SA, Roetker NS, Lutsey PL, et al. Chronic stress, depressive symptoms, anger, hostility, and risk of stroke and transient ischemic attack in the multi-ethnic study of atherosclerosis. Stroke 2014; 45:2318–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Von Känel R, Loredo JS, Ancoli-Israel S, et al. Association between polysomnographic measures of disrupted sleep and prothrombotic factors. Chest 2007; 131:733–739. [DOI] [PubMed] [Google Scholar]
- 38.Von Känel R, Natarajan L, Ancoli-Israel S, et al. Day/night rhythm of hemostatic factors in obstructive sleep apnea. Sleep 2010; 33:371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niżankowska-Jędrzejczyk A, Almeida FR, Lowe AA, et al. Modulation of inflammatory and hemostatic markers in obstructive sleep apnea patients treated with mandibular advancement splints: a parallel, controlled trial. J Clin Sleep Med 2014; 10:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen LF, Chen HP, Huang YS, et al. Pneumococcal pneumonia and the risk of stroke: a population-based follow-up study. PLoS One 2012; 7:e51452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep 2010; 33:1396–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drager LF, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: an emerging risk factor for atherosclerosis. Chest 2011; 140:534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee HC, Chang KC, Huang YC, et al. Readmission, mortality, and first-year medical costs after stroke. J Chin Med Assoc 2013; 76:703–714. [DOI] [PubMed] [Google Scholar]


