Abstract
Studies have suggested that statin use is related to cancer risk and prostate cancer mortality. We conducted a population-based cohort study to determine whether using statins in prostate cancer patients is associated with reduced all-cause mortality rates.
Data were obtained from the Taiwan National Health Insurance Research Database. The study cohort comprised 5179 patients diagnosed with prostate cancer who used statins for at least 6 months between January 1, 1998 and December 31, 2010. To form a comparison group, each patient was randomly frequency-matched (according to age and index date) with a prostate cancer patient who did not use any type of statin-based drugs during the study period. The study endpoint was mortality. The hazard ratio (HR) and 95% confidence interval (CI) were estimated using Cox regression models.
Among prostate cancer patients, statin use was associated with significantly decreased all-cause mortality (adjusted HR = 0.65; 95% CI = 0.60–0.71). This phenomenon was observed among various types of statin, age groups, and treatment methods. Analyzing the defined daily dose of statins indicated that both low- and high-dose groups exhibited significantly decreased death rates compared with nonusers, suggesting a dose–response relationship.
The results of this population-based cohort study suggest that using statins reduces all-cause mortality among prostate cancer patients, and a dose–response relationship may exist.
INTRODUCTION
Statins are competitive inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase; this class of drug is commonly used to reduce serum cholesterol levels, and the effectiveness of statins in preventing heart attack and stroke was documented.1 Preclinical studies have reported that statins potentiate antitumor effects2,3; however, the chemopreventive role of statins has remained ambiguous.4–9 Regarding prostate cancer, studies have indicated that statin use is associated with risk reduction10–12; however, contrasting reports have suggested that statins do not reduce13,14 or even increase the risk15 of prostate cancer development.
Several observational studies have investigated the role of statins in the recurrence of prostate cancer, yielding inconsistent results.16–22 Some reports have indicated decreased risks of biochemical recurrence after treatment,16–18 whereas other reports have not.19–22 A population-based observational study in Denmark revealed that statin use is associated with reduced cancer-related mortality among cancer patients.23 An updated study from 1995 to 2009 among adults older than 40 years in the Danish population indicated that patients using statins before cancer diagnosis were 15% less likely to die from any cause, and particularly cancer, than were those who did not take statins.24 However, a meta-analysis indicated that statin use did not influence cancer-related mortality.25 Two epidemiologic investigations focusing on prostate cancer have indicated that using statins after diagnosis is associated with a decreased risk of prostate cancer mortality.26,27 A recent review highlighted that some studies that adjust for confounding factors have demonstrated statin therapy to be associated with favorable clinical outcomes of prostate cancer.28
Prostate cancer is the most common malignancy among men in Western countries and is the second leading cause of death.29 Although the incidence and mortality rates of prostate cancer among Asian men are much lower compared with men in Western countries, these rates have increased rapidly in the past 2 decades in most native Asian populations. In spite of enhanced detection, much of the increased incidence may be associated with westernization of the lifestyle, with increasing obesity and increased consumption of fat.30 Prostate cancer is the fifth most prevalent cancer and the seventh leading cause of cancer death among Taiwanese men. The age-adjusted incidence and mortality of prostate cancer among Taiwanese men in 2011 were 29.66 people per 100,000 and 6.36 people per 100,000, respectively.31 To determine whether statin use is associated with a reduced risk of death among Taiwanese men diagnosed with prostate cancer, we conducted a study using the database of the National Health Insurance (NHI) system of Taiwan.
METHODS
Data Sources
In this cohort study, data were obtained from the Taiwan NHI electronic records system, which contains all medical claims from 1996 to 2011. The NHI program covers 99% of the Taiwanese population, and 97% of medical institutions in Taiwan are NHI-contracted providers (http://www.nhi.gov.tw/english/index.aspx). The National Health Research Institutes (NHRI) manages the insurance claims data reported to the Bureau of Health Insurance.
The NHRI has established the National Health Insurance Research Database (NHIRD) and releases annual data for use in research. The NHIRD includes complete information regarding ambulatory and inpatient care. Researchers are provided with scrambled identification numbers associated with the relevant claim information, including information on the sex, date of birth, received medical services, and medication prescriptions of patients. The diagnostic codes are formatted according to the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM).
As part of the NHI program, insurants who suffer from certain severe diseases or conditions, such as malignancies, organ transplants, or autoimmune diseases, can apply for a catastrophic illness certificate. Applying for a catastrophic illness certificate for malignancies requires cytological or pathological evidence supporting the diagnosis. Multiple NHI databases are integrated to form the catastrophic illness database, providing comprehensive information on all patients with severe diseases who obtain copayment exemptions from the NHI program.
Patient consent is not required to access the NHIRD. This study was approved by the Institutional Review Board of China Medical University in Central Taiwan (CMU-REC-101-012).
Study Sample
By using the Registry of Catastrophic Illness and NHI databases, we identified prostate cancer patients (ICD-9-CM code 185) between January 1, 1998 and December 31, 2010. Patients younger than 20 years were excluded. The date of application for a catastrophic illness exemption was defined as the index date. The patients were divided into 2 cohorts based on their statin use before the diagnosis of prostate cancer: the statin (at least 6 months of statin therapy before the index date) and nonstatin (no statin therapy before the index date) cohorts. To establish the nonstatin cohort, we randomly selected 1 nonstatin patient from the same period and matched this patient with a statin patient according to year of receiving statin treatment, age (per 5 years), and index year.
Definitions of the Endpoint, Comorbidities, and Covariables
The key variable of interest was the overall survival rate. The study endpoints were death, withdrawal from the database, or the end of 2011.
The baseline comorbidities were diabetes (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401–405), stroke (ICD-9-CM codes 430–438), coronary artery disease (CAD; 410-414) (CAD; ICD-9-CM codes 410–414), and chronic obstructive pulmonary disease (COPD; ICD-9-CM codes 490–496). The treatments for prostate cancer involved chemotherapy, radiotherapy, prostatectomy, and hormone therapy.
Statistical Analysis
The distributions of categorical sociodemographic characteristics and comorbidities were compared between the statin and nonstatin cohorts, and the differences were examined using the χ2 test for categorical variables and the t test for continuous variables. For each variable, the follow-up person-years were used to estimate the incidence rate. Cox proportional hazard regressions were used to assess the mortality rates associated with statin use. Furthermore, we divided each statin type into 2 groups according to the quartile of cumulative defined daily dose (DDD). Cox proportional hazard regression was used to assess how the statin dose affected the relative risk of mortality. The overall survival curves were plotted using the Kaplan-Meier method, and statistical significance was examined using a log-rank test. A P value of <0.05 indicated statistical significance. All statistical analyses were performed using SAS statistical software (version 9.3 for Windows; SAS Institute, Inc., Cary, NC).
RESULTS
In all, 10,358 prostate cancer patients were followed up for an average of 7.75 years (±3.31 years). The mean ages of the nonstatin and statin cohorts were 68.6 (±8.76 years) and 68.5 years (±8.14 years), respectively (Table 1). Approximately, 84.8% of the patients were older than 60 years.
TABLE 1.
Demographic Characteristics of Study Subjects With and Without Statin Use
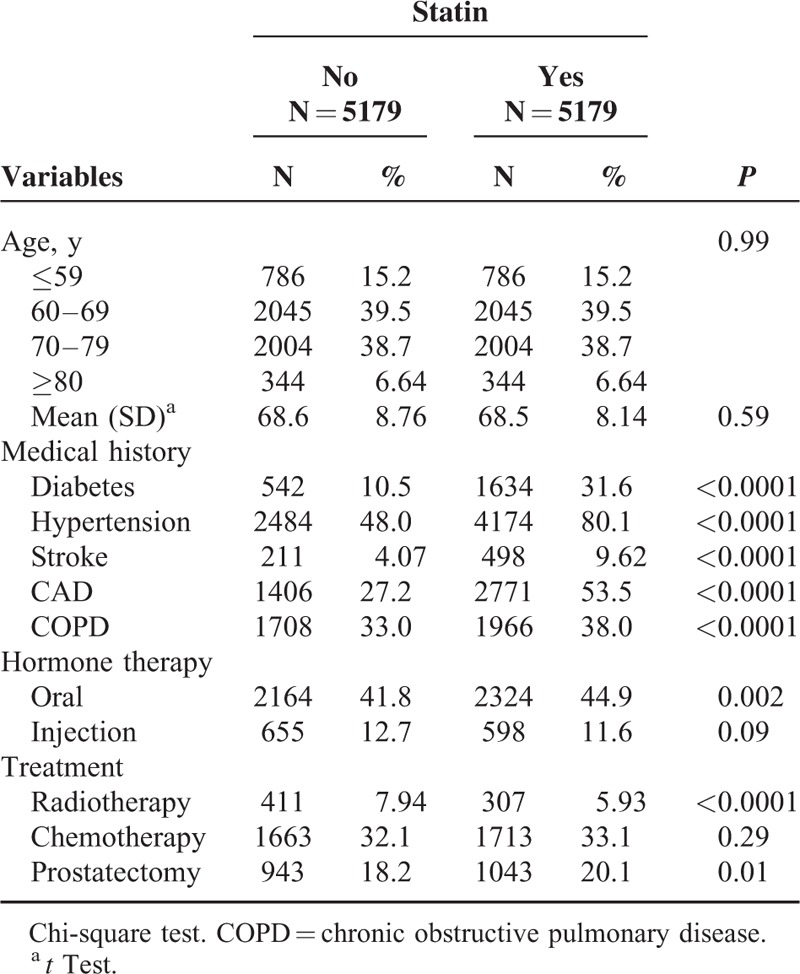
Table 1 shows the baseline comorbidities of the cohorts. Compared with the nonstatin users, the statin users were more likely to have diabetes (10.5% vs 31.6%, P < 0.0001), hypertension (48.0% vs 80.1%, P < 0.0001), stroke (4.07% vs 9.62%, P < 0.0001), CAD (27.2% vs 53.5%, P < 0.0001), and COPD (33.0% vs 38.0%, P < 0.0001). Regarding the treatment for prostate cancer, the statin users exhibited a higher rate of oral hormone therapy (41.8% vs 44.9%, P = 0.002) and prostatectomy (18.2% vs 20.1%) and a lower rate of radiotherapy (7.94% vs. 5.93%, P < 0.0001) than the nonstatin users did.
Table 2 shows the overall and age-, comorbidity-, and treatment-specific incidence rates of mortality among the cohorts. Overall, 1367 nonstatin users and 1025 statin users became deceased during the follow-up period, and the incidence rates were 34.8 per 1000 person-years and 25 per 1000 person-years, respectively. When the participants were stratified according to age, the death incidence rate increased as age increased in the statin user cohort (8.97 at ≤59 years, 16.9 at 60–69 years, 37.6 at 70–79 years, and 60.1 at ≥80 years). Among patients who lacked comorbidities, the statin cohort exhibited the lowest incidence rate of death (12.3 per 1000 person-years).
TABLE 2.
Comparison of Incidence and Hazard Ratio of Mortality Stratified by Age, Comorbidity, and Treatment According to Medication Status Among Prostate Cancer Patients
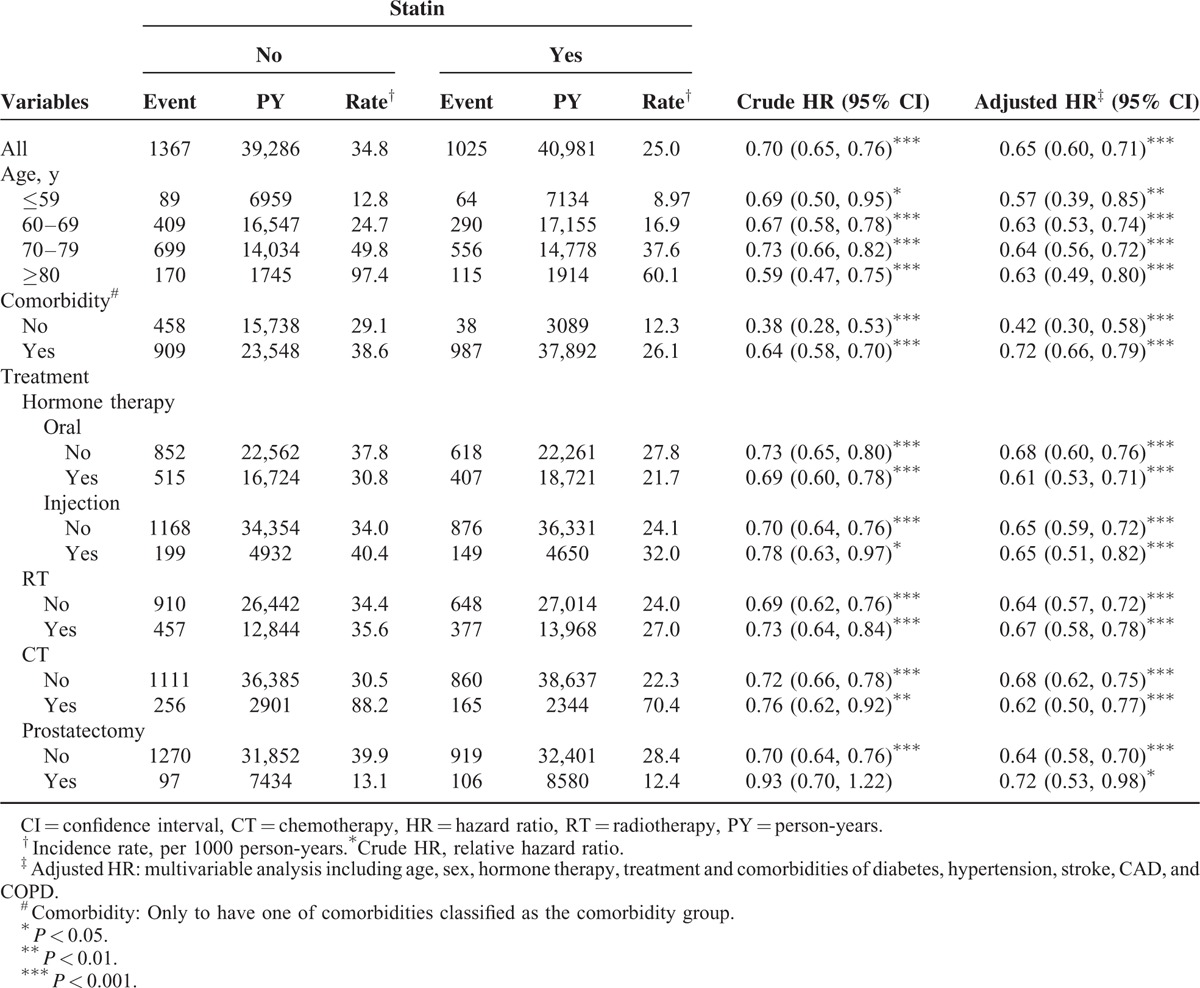
Table 2 shows the crude and adjusted hazard ratios (HRs) for the risk of mortality among the comparison cohorts. The statin users exhibited a significantly lower risk of mortality than did the nonstatin users (crude HR = 0.70, 95% confidence interval [CI] = 0.65–0.76). Figure 1 shows the results of the log-rank test and overall survival curve. The rate of overall survival was significantly higher in the statin cohort than in the nonstatin cohort (log-rank P < 0.001; Figure 1). After we controlled for variables (Table 1), the statin cohort was associated with significantly decreased risk of mortality (adjusted HR = 0.65, 95% CI = 0.60–0.71).
FIGURE 1.
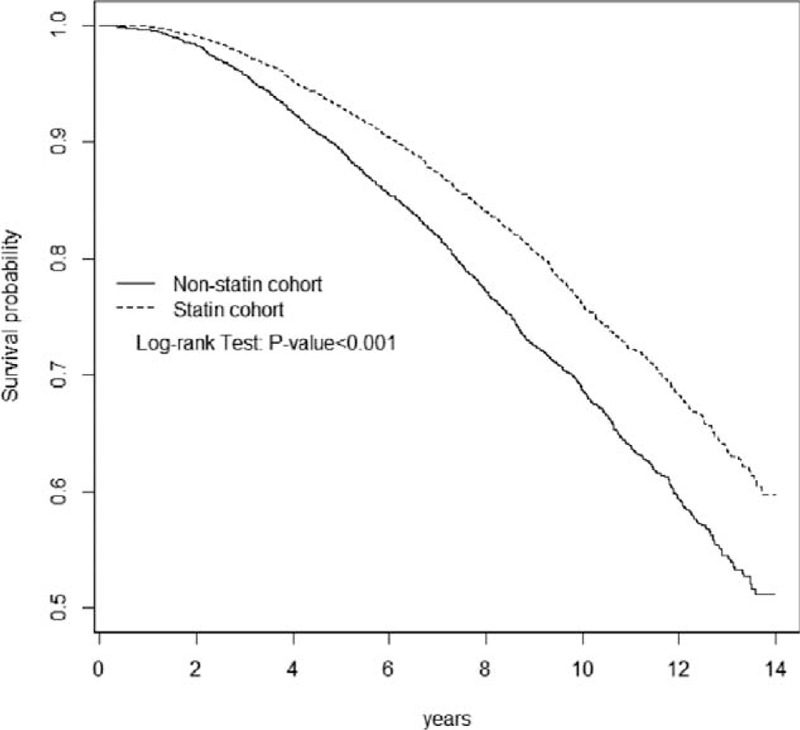
Overall survival for statin cohort (dashed line) and nonstatin cohort (solid line).
Stratified analyses indicated that compared with the nonstatin users, the statin users exhibited a lower risk of mortality among both the comorbidity (HR = 0.42, 95% CI = 0.30–0.58) and noncomorbidity (HR = 0.72, 95% CI = 0.66–0.79; Table 2) groups. Regardless of treatment with hormone therapy, radiotherapy, chemotherapy, or prostatectomy, statins exerted the same protective effects against the risk of mortality.
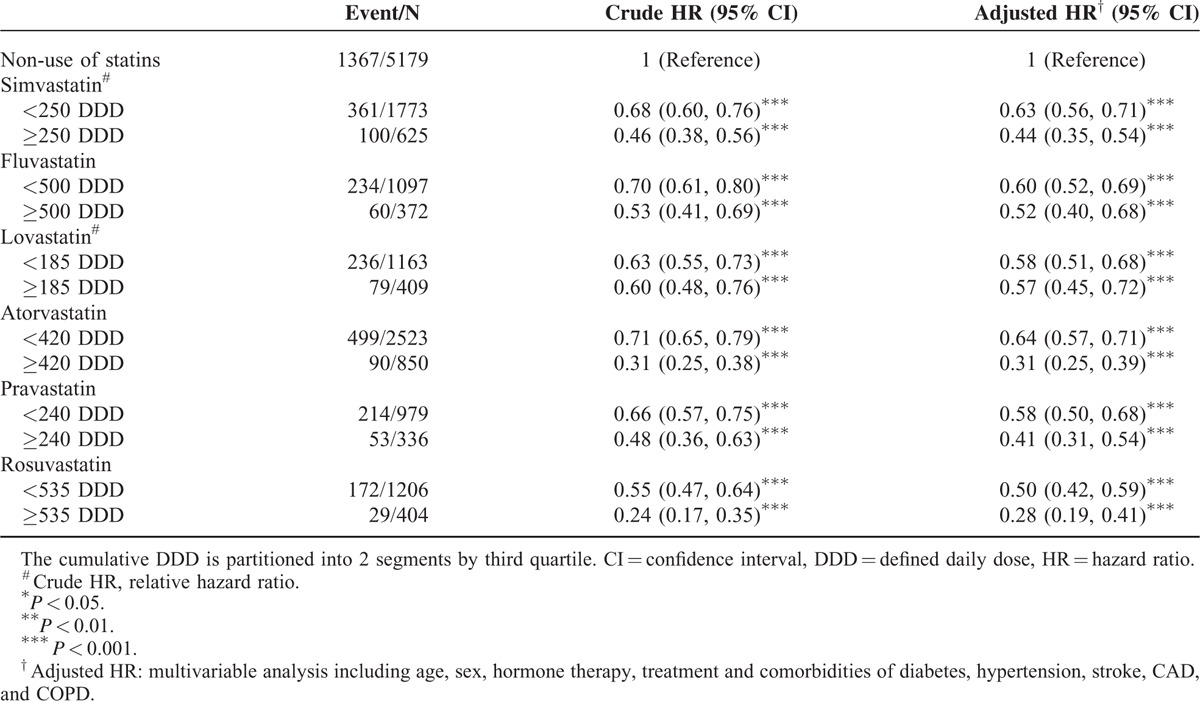
Table 3 shows the association between the cumulative DDD and the risk of mortality. Patients who exhibited high cumulative DDDs exhibited a decreased risk of mortality (43%–72%). Compared with nonstatin users, patients who underwent treatment with rosuvastatin at a DDD ≥535 (HR = 0.28, 95% CI = 0.19–0.41) exhibited the lowest risk of mortality, followed by treatment with atorvastatin at ≥420 DDD (HR = 0.31, 95% CI = 0.25–0.39), and pravastatin at ≥240 DDD (HR = 0.41, 95% CI = 0.31–0.54).
TABLE 3.
Hazard Ratio and 95% Confidence Intervals of Mortality Associated With Cumulative DDD of Individual Statins
DISCUSSION
In this nationwide population-based cohort study, prostate cancer patients in Taiwan who used statins exhibited significantly lower all-cause mortality than did nonusers, regardless of the type of statin. The results suggested a dose-response relationship regarding the cumulative DDD.
The NHI program provides insurants with comprehensive coverage; thus, the NHIRD contains outpatient and inpatient records and prescription claim data. This database enabled appropriately selecting matched patients to represent the underlying population. We have used the NHIRD to perform several cohort studies evaluating the risk factors of or protectors against cancer.32–34 Because statins are commonly prescribed throughout the world, a small hazard-benefit ratio can yield crucial clinical implications of interest to the public and medical practitioners. A large population-based study can elucidate how statin use is associated with mortality in prostate cancer patients. Therefore, a cohort design was employed to identify the relationship between statin use and prostate cancer all-cause mortality.
Statins inhibit cholesterol synthesis within cells by inhibiting HMG-CoA reductase, a crucial rate-limiting enzyme involved in the mevalonate synthesis pathway that is essential for synthesizing various compounds, including cholesterol.35 By inhibiting HMG-CoA reductase, statins block the pathway of synthesizing cholesterol in the liver and reduce systemic cholesterol. Statins indirectly reduce cellular cholesterol levels in multiple cell types through lowering circulating cholesterol, consequently impacting on membrane microdomains and steroidogenesis.28 Disruptions of these processes in malignant cells result in inhibited cancer growth and metastasis23,36,37; laboratory studies have supported this phenomenon, indicating that statins exhibit broad anticancer properties.2,3,38 Regarding statins and prostate cancer in vitro, preclinical data suggest that statins might exert a chemopreventive role against prostate cancer by inhibiting the proliferation and inducing apoptosis of prostate cancer cells and also inhibiting angiogenesis, inflammation, and metastasis.39 Yokomizo et al reported that statins reduce the androgen sensitivity and cell proliferation by decreasing the androgen receptor protein in prostate cancer cells.40 In addition, a recent study found that statins competitively reduce dehydroepiandrosterone sulfate uptake, thus effectively decreasing the available intratumoral androgen pool, affords a plausible mechanism to support the clinical observation of prolonged time to progression of prostate cancer in statin users.41 Several observational epidemiologic studies have explored the chemopreventive effects of statin use in different cancers, yielding inconsistent results.4–9 The role of statins in the risk of prostate cancer has remained undetermined in several observational and clinical studies.10–15 Furthermore, a study using the NHIRD indicated that statin use increased the risk of prostate cancer.15
In addition to cancer risk, researchers have explored how statin use affects prostate cancer outcomes. Previous researchers have investigated biochemical recurrence, but no definite conclusion can be reached based on the inconclusive results of observational studies.16–22 A review indicated that after adjusting for confounding factors, some studies have demonstrated statin therapy to be associated with favorable clinical outcomes in prostate cancer patients.28 Harshman et al demonstrated that men with prostate cancer taking statins had a significantly longer median time to progression during androgen deprivation therapy compared with nonusers after adjusting for predefined prognostic factors.41 The current study focused on the influence of statin use on the all-cause mortality of prostate cancer; among prostate cancer patients, statin use was associated with decreased all-cause mortality, regardless of the type of statin used. Both low- and high-dose levels were associated with significantly reduced mortality, but the high-dose groups consistently exhibited lower HRs than did the low-dose groups regardless of the statin type, suggesting a dose-response relationship. Our results correspond with those of the cohort study of Yu et al, who indicated that the rates of prostate cancer mortality and all-cause mortality significantly decreased by 24% and 14%, respectively, among statin users, exhibiting a dose-response relationship.26 In an ongoing prospective cohort study in the United States, Platz et al42 indicated that cancer-related mortality was reduced among advanced prostate cancer patients who used statins. A matched case-control study showed an inverse association between statin use and prostate cancer death.43 Furthermore, a population-based epidemiologic study performed in the United States (Washington state) indicated that using statins before the diagnosis of prostate cancer was associated with a decreased risk of prostate-cancer specific mortality.27 Our data revealed that, compared with nonusers, statins users exhibit a significantly lower risk of prostate cancer death regardless of the prescribed treatment. A previous study reported that, among patients who underwent radiotherapy and radical prostatectomy, statins were associated with significantly decreased all-cause mortality risks of 41% and 65%, respectively.44
Klop et al indicated that observational studies have reported the beneficial effects of statins on various health outcomes, whereas randomized controlled trials have not. The discrepancies between observational studies and randomized controlled trials may be attributable to healthy-user bias in observational studies.45 No data are available from randomized clinical trials evaluating the role of statins in protecting against prostate cancer death.
The strength of this study is the population-based design, which yielded strong generalizability; however, several limitations must be considered based on the incompleteness of the employed database. First, the NHIRD lacks information regarding the lifestyles and behaviors of patients, making it impossible to adjust for health behavior-related factors such as smoking and alcohol consumption. Smoking is a strong risk factor in cancer-related death, and a survey among general practitioners in the United Kingdom indicated that statins are selectively underprescribed because of the unhealthy lifestyles of patients who smoke.46 Second, the NHIRD records do not contain data on the prostate cancer stage, Gleason score, or prostate-specific antigen levels. Thus, we could not conduct sophisticated tests adjusting for these variables. Although these factors are vital predictors of the prostate cancer outcome, it remains unclear if they can be considered confounders because they may not be associated with statin exposure.26 Moreover, the analyses were adjusted based on the provided cancer treatment, and the treatment method correlated with the tumor characteristics. Third, the cause of death is not documented in the Registry of Catastrophic Illness; therefore, we could analyze the all-cause mortality, but not the cancer-specific mortality.
In conclusion, the results of this population-based observational study indicate that statin use may reduce all-cause mortality among prostate cancer patients. Large-scale, prospective, well-controlled randomized trials are required to validate these findings.
Acknowledgments
None.
Footnotes
Abbreviations: CAD = coronary artery disease, CI = confidence interval, COPD = chronic obstructive pulmonary disease, DDD = defined daily dose, HMG-CoA = 3-hydroxy-3-methylglutaryl-coenzyme A, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, SD = standard deviation.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039 -006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and Health, and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TDU-B-212-124-002, Taiwan). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
Author Contributions: Conception/Design: L-MS, M-CL,C-HK; Provision of study material or patients: C-HK; Collection and/or assembly of data: C-LL; Data analysis and interpretation: All authors; Manuscript writing: All authors; Final approval of manuscript: All authors.
L-MS and M-CL contributed equally to this work.
I-CL and C-HK contributed equally to this work.
The authors report no conflicts of interests.
REFERENCES
- 1.Hebert PR, Gaziano JM, Chan KS, et al. Cholesterol lowering with statin drugs, risk of stroke, and total mortality. An overview of randomized trials. JAMA 1997; 278:313–321. [PubMed] [Google Scholar]
- 2.Sassano A, Platanias LC. Statins in tumor suppression. Cancer Lett 2008; 260:11–19. [DOI] [PubMed] [Google Scholar]
- 3.Jakobisiak M, Golab J. Potential antitumor effects of statins (Review). Int J Oncol 2003; 23:1055–1069. [PubMed] [Google Scholar]
- 4.Bonovas S, Filioussi K, Tsavaris N, et al. Statins and cancer risk: a literature-based meta-analysis and meta-regression analysis of 35 randomized controlled trials. J Clin Oncol 2006; 24:4808–4817. [DOI] [PubMed] [Google Scholar]
- 5.Bonovas S, Filioussi K, Tsavaris N, et al. Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol 2005; 23:8606–8612. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Tang W, Wang J, et al. Association between statin use and colorectal cancer risk: a meta-analysis of 42 studies. Cancer Causes Control 2014; 25:237–249. [DOI] [PubMed] [Google Scholar]
- 7.Cui X, Xie Y, Chen M, et al. Statin use and risk of pancreatic cancer: a meta-analysis. Cancer Causes Control 2012; 23:1099–1111. [DOI] [PubMed] [Google Scholar]
- 8.Cheng MH, Chiu HF, Ho SC, et al. Statin use and the risk of female lung cancer: a population-based case-control study. Lung Cancer 2012; 75:275–279. [DOI] [PubMed] [Google Scholar]
- 9.Chiu HF, Ho SC, Chen CC, et al. Statin use and the risk of liver cancer: a population-based case–control study. Am J Gastroenterol 2011; 106:894–898. [DOI] [PubMed] [Google Scholar]
- 10.Jespersen CG, Nørgaard M, Friis S, et al. Statin use and risk of prostate cancer: A Danish population-based case-control study, 1997–2010. Cancer Epidemiol 2014; 38:42–47. [DOI] [PubMed] [Google Scholar]
- 11.Flick ED, Habel LA, Chan KA, et al. Statin use and risk of prostate cancer in the California Men's Health Study cohort. Cancer Epidemiol Biomarkers Prev 2007; 16:2218–2225. [DOI] [PubMed] [Google Scholar]
- 12.Bansal D, Undela K, D’Cruz S, et al. Statin use and risk of prostate cancer: a meta-analysis of observational studies. PLoS One 2012; 7:e46691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonovas S, Filioussi K, Sitaras NM. Statin use and the risk of prostate cancer: A metaanalysis of 6 randomized clinical trials and 13 observational studies. Int J Cancer 2008; 123:899–904. [DOI] [PubMed] [Google Scholar]
- 14.Boudreau DM, Yu O, Buist DS, et al. Statin use and prostate cancer risk in a large population-based setting. Cancer Causes Control 2008; 19:767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang CC, Ho SC, Chiu HF, et al. Statins increase the risk of prostate cancer: a population-based case-control study. Prostate 2011; 71:1818–1824. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton RJ, Banez LL, Aronson WJ, et al. Statin medication use and the risk of biochemical recurrence after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer 2010; 116:3389–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutt R, Tonlaar N, Kunnavakkam R, et al. Statin use and risk of prostate cancer recurrence in men treated with radiation therapy. J Clin Oncol 2010; 28:2653–2659. [DOI] [PubMed] [Google Scholar]
- 18.Ritch CR, Hruby G, Badani KK, et al. Effect of statin use on biochemical outcome following radical prostatectomy. BJU Int 2011; 108:E211–E216. [DOI] [PubMed] [Google Scholar]
- 19.Scosyrev E, Tobis S, Donsky H, et al. Statin use and the risk of biochemical recurrence of prostate cancer after definitive local therapy: a meta-analysis of eight cohort studies. BJU Int 2013; 111:E71–E77. [DOI] [PubMed] [Google Scholar]
- 20.Chao C, Jacobsen SJ, Xu L, et al. Use of statins and prostate cancer recurrence among patients treated with radical prostatectomy. BJU Int 2013; 111:954–962. [DOI] [PubMed] [Google Scholar]
- 21.Mass AY, Agalliu I, Laze J, et al. Preoperative statin therapy is not associated with biochemical recurrence after radical prostatectomy: our experience and meta-analysis. J Urol 2012; 188:786–791. [DOI] [PubMed] [Google Scholar]
- 22.Ku JH, Jeong CW, Park YH, et al. Relationship of statins to clinical presentation and biochemical outcomes after radical prostatectomy in Korean patients. Prostate Cancer Prostatic Dis 2011; 14:63–68. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med 2012; 367:1792–1802. [DOI] [PubMed] [Google Scholar]
- 24.Vallianou NG, Kostantinou A, Kougias M, et al. Statins and Cancer. Anticancer Agents Med Chem 2014; 14:706–712. [DOI] [PubMed] [Google Scholar]
- 25.Emberson JR, Kearney PM, Blackwell L, et al. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One 2012; 7:e29849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu O, Eberg M, Benayoun S, et al. Use of statins and the risk of death in patients with prostate cancer. J Clin Oncol 2014; 32:5–11. [DOI] [PubMed] [Google Scholar]
- 27.Geybels MS, Wright JL, Holt SK, et al. Statin use in relation to prostate cancer outcomes in a population-based patient cohort study. Prostate 2013; 73:1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moon H, Hill MM, Roberts MJ, et al. Statins: protectors or pretenders in prostate cancer? Trends Endocrinol Metab 2014; 25:188–196. [DOI] [PubMed] [Google Scholar]
- 29.Siegel R, Ward E, Brawley O, et al. Cancer statistics 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011; 61:212–236. [DOI] [PubMed] [Google Scholar]
- 30.Pu YS, Chiang HS, Lin CC, et al. Changing trends of prostate cancer in Asia. Aging Male 2004; 7:120–132. [DOI] [PubMed] [Google Scholar]
- 31.Cancer Statistics Annual Report. (2012) Taiwan Cancer Registry. http://tcr.cph.ntu.edu.tw/main.php?Page=N2 (Accessed August 22, 2015). [Google Scholar]
- 32.Sun LM, Lin CL, Chang YJ, et al. Urinary tract stone raises subsequent risk for urinary tract cancer: a population-based cohort study. BJU Int 2013; 112:1150–1155. [DOI] [PubMed] [Google Scholar]
- 33.Kao CH, Sun LM, Chen PC, et al. A population-based cohort study in Taiwan-use of insulin sensitizers can decrease cancer risk in diabetic patients? Ann Oncol 2013; 24:523–530. [DOI] [PubMed] [Google Scholar]
- 34.Lee WY, Sun LM, Lin MC, et al. A higher dosage of oral alendronate will increase the subsequent cancer risk of osteoporosis patients in Taiwan: a population-based cohort study. PLoS One 2012; 7:e53032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong WWL, Dimitroulakos J, Minden MD, et al. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 2002; 16:508–519. [DOI] [PubMed] [Google Scholar]
- 36.Chan KKW, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res 2003; 9:10–19. [PubMed] [Google Scholar]
- 37.Demierre MF, Higgins PD, Gruber SB, et al. Statins and cancer prevention. Nat Rev Cancer 2005; 5:930–942. [DOI] [PubMed] [Google Scholar]
- 38.Graaf MR, Richel DJ, van Noorden CJ, et al. Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat Rev 2004; 30:609–641. [DOI] [PubMed] [Google Scholar]
- 39.Papadopoulos G, Delakas D, Nakopoulou L, et al. Statins and prostate cancer: molecular and clinical aspects. Eur J Cancer 2011; 47:819–830. [DOI] [PubMed] [Google Scholar]
- 40.Yokomizo A, Shiota M, Kashiwagi E, et al. Statins reduce androgen sensitivity and cell proliferation by decreasing the androgen receptor protein in prostate cancer cells. Prostate 2011; 71:298–304. [DOI] [PubMed] [Google Scholar]
- 41.Harshman LC, Wang X, Nakabayashi M, et al. Statin use at the time of initiation of androgen deprivation therapy and time to progression in patients with hormone-sensitive prostate cancer. JAMA Oncol 2015; 1:494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Platz EA, Leitzmann MF, Visvanathan K, et al. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst 2006; 98:1819–1825. [DOI] [PubMed] [Google Scholar]
- 43.Marcella SW, David A, Ohman-Strickland PA, et al. Statin use and fatal prostate cancer: a matched case-control study. Cancer 2012; 118:4046–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katz MS, Carroll PR, Cowan JE, et al. Association of statin and nonsteroidal anti-inflammatory drug use with prostate cancer outcomes: Results from CaPSURE. BJU Int 2010; 106:627–632. [DOI] [PubMed] [Google Scholar]
- 45.Klop C, Driessen JH, de Vries F. Statin use and reduced cancer-related mortality. Correspondence. N Engl J Med 2013; 368:574. [DOI] [PubMed] [Google Scholar]
- 46.Evans JS, Harries C, Dennis I, et al. General practitioners’ tacit and stated policies in the prescription of lipid lowering agents. Br J Gen Pract 1995; 45:15–18. [PMC free article] [PubMed] [Google Scholar]


