Abstract
Combined arterial resection during pancreatectomy can be a challenging treatment, and outcome would be more favorable if the tumor becomes technically removable from the artery. Neoadjuvant chemoradiotherapy (NACRT) is expected to achieve locoregional control and enable margin-negative resection. To investigate the effects of NACRT in patients with pancreatic adenocarcinoma (PDAC) which were deemed borderline resectable through preoperative imaging due to abutment of the major artery, including the superior mesenteric artery (SMA) or common hepatic artery (CHA), but were still considered to be technically removable. In the current study, comparisons were make between 71 patients who underwent upfront surgery and 21 patients who underwent NACRT followed by surgery in the strategy to preserve the artery, using unmatched and inverse probability of treatment weighting analysis (UMIN000017115). Fifty patients in the upfront surgery group and 18 in the NACRT group underwent curative resection (70% vs 86%, respectively; P = 0.16). The results of the propensity score weighted logistic regressions indicated that the incidences of pathological lymph node metastasis and a pathological positive resection margin were significantly lower in the NACRT group (odds ratio, 0.006; P < 0.001 and odds ratio, 0.007; P < 0.001, respectively). Among the propensity-score matched patients, the estimated 1- and 2-year survival rates in the upfront surgery group were 66.7% and 16.0%, respectively, and those in the NACRT group were 80.0% and 65.2%, respectively. In conclusion, it was suggested that chemoradiotherapy followed by surgery provided clinical benefits in patients with PDACs in contact with the SMA or CHA.
INTRODUCTION
Pancreatic adenocarcinoma (PDAC) is the fourth leading cause of cancer-related death worldwide and has the worst prognosis, with only 3% of patients surviving for 5 years after diagnosis.1,2 Although the treatment strategies for PDAC have changed over recent years, especially with the development of antineoplastic agents such as gemcitabine,3 surgery with curative intent remains the only therapeutic option with potential for cure.4–8 However, PDAC often invades major arteries and portal vein (PV) system and this renders the surgical resection complex and technically demanding.
The National Comprehensive Cancer Network, an alliance of 25 cancer centers in the United States, has updated its guidelines regarding the diagnosis and treatment of PDAC.9 In these guidelines, PDACs are classified as resectable, borderline resectable (BR), or unresectable in which BR is defined in short as cancer that infiltrated the adjacent major vessels. BR tumors are at high risk of having positive resection margins following de novo surgery as a result of tumor involvement with these adjacent vasculatures. Moreover, radical pancreatectomy with coresection of major vasculature seems to have a negative effect on survival partially due to postoperative morbidity.10 In the guidelines, therefore, neoadjuvant therapy followed by restaging and resection only in patients without disease progression had been mentioned as one of the treatment options for this disease entity (category 2B). Evans et al reported in their experience with neoadjuvant chemoradiotherapy (NACRT) for BR-PDAC that cancer developed into metastatic or unresectable locally advanced disease during the neoadjuvant treatment in 17% and 2% of patients, respectively, meaning that the remaining 80% still had BR disease which could be indicated for resection.11,12 Subsequently, 80 of 115 (70%) BR patients received pancreatic resection, of which as many as 95% turned out to be R0 resection.18 The usefulness of neoadjuvant therapy for BR-PDAC has thereafter been highlighted in several other articles.13–19 Unfortunately, these reports often analyzed mixed cohorts of patients that include BR and locally advanced PDAC, or of BR-PDAC due to the infiltration of celiac and/or superior mesentery arteries and those due only to the infiltration of portal system. The surgical strategy and outcome definitely differ between PDAC abutted to the major artery and PDAC involved exclusively with the PV system, and it seems inappropriate to discuss efficacy of the treatment strategy using such admixture.
In the present study, only patients with BR-PDAC abutting the major artery but were still considered technically resectable by the surgical team were eligible. There have been no comprehensive analyses focusing on surgical strategy for this cohort. With this cohort, perioperative outcomes and survival were compared between patients who underwent NACRT followed by surgery (NACRT group) and patients who underwent upfront surgery (upfront surgery group). Inverse probability of treatment weighting (IPTW) analysis was used to reduce the impact of treatment-selection bias and the potential confounding factors inherent to an observational study.
METHODS
Patient Characteristics
A prospectively maintained pancreatic resection database was queried to identify all cases of PDAC in contact with the major arteries. Between February 2002 and September 2014, 493 patients underwent curative intent surgery for PDAC at the Department of Gastroenterological Surgery (Surgery II), Nagoya University Graduate School of Medicine, Nagoya, Japan. Of these, 92 patients diagnosed with PDAC in contact with the superior mesenteric artery (SMA) and/or common hepatic artery (CHA) as the BR disease according to the National Comprehensive Cancer Network Guidelines,9 were enrolled in this study (Figure 1). Patients eligibility was rigorously defined using the following thin-slice multidetector-row computed tomography criteria: no distant metastases; direct abutment with the CHA without extension to the celiac axis (Figure 2A); and tumor abutment with the SMA not to exceed 180 degrees of the circumference of the vessel wall (Figure 2B). Tumors with encasement or stenosis of the arteries, those abutted with the celiac axis, and those only defined as BR diseases because of the superior mesenteric vein/PV factor were excluded from the study. All images were reviewed by 2 or more experienced radiologists to reaffirm the findings of abutment with the artery for this study. Written informed consent for inclusion in the study, as required by the Institutional Review Board of Nagoya University, was obtained from all patients.
FIGURE 1.

Study profile and clinical course of the upfront surgery and neoadjuvant chemoradiotherapy (NACRT) groups.
FIGURE 2.

Representative multidetector high-resolution CT scans. (A) Direct abutment of the CHA without extension to the celiac axis and (B) tumor abutment of the SMA not to exceeding 180° of the circumference of the vessel wall. CHA = common hepatic artery, CT = computed tomography, SMA = superior mesenteric artery.
Neoadjuvant CRT
In patients where PDAC exhibited contact with the major arteries, surgery was undertaken without preoperative therapy until 2009 whereas NACRT was exclusively administered in all cases after 2010. The NACRT regimen consisted of radiation therapy (50.4 gray in 28 fractions) combined with systemic chemotherapy involving oral S-1, the oral 5-fluorouracil prodrug tegafur with oteracil, and gimeracil.20 S-1 was orally administered, twice daily (80 mg/m2/day) on days 1 to 14, and days 22 to 35. At the completion of CRT, restaging imaging including multidetector-row computed tomography was performed to explore distant metastases or the operative indication.
Surgical Technique
Pancreatectomy and systematic lymphadenectomy were performed with curative intent in all patients after confirming the absence of peritoneal dissemination or distant metastases. In patients who underwent pancreaticoduodenectomy, the procedure began via the mesenteric approach to achieve en bloc pancreaticoduodenectomy using a nontouch isolation technique as previously described.21–24 In patients who underwent distal pancreatectomy, closure of the main pancreatic duct was performed with placement of a continuous suture.25,26 Superior mesenteric vein and/or PV resection were carried out in cases with possible or definitive tumor invasion.27 For the abutted site of the artery, tumor dissection was attempted to preserve the artery. In principle, combined arterial resection was not undertaken. All operations were performed by the same surgical team, and invariably involved either or both of the 2 experienced surgeons (TF and AN). All operative procedures were carried out in the same manner throughout the study period.
Postoperative adjuvant chemotherapy was basically applied unless contraindicated by the patient's condition. In short, the patients received treatments according to the protocols which were available at the time of treatment. Thus, the patients were given gemcitabine or S-1.3,28 Gemcitabine at a dose of 1000 mg/m2 was administered weekly for 3 weeks followed by 1 week of rest; oral S-1 (80 mg/m2/day) was administered from days 1 to 14 followed by a 1-week rest period. Chemotherapy was initiated at <2 months after the operation in all patients who were considered eligible for the treatment.
Factors Evaluated
Pretreatment factors included age, sex, comorbidity, American Society of Anesthesiologists physical status score, body mass index,29 biliary drainage,30 tumor size and an abutted major artery on preoperative computed tomography, and blood test results including carbohydrate antigen (CA) 19–9 level. Perioperative factors included type of surgery, operative time, blood loss volume, blood transfusion, number of harvested lymph nodes, and the incidence of postoperative complications according to the Clavien–Dindo classification.31
The tumor-node-metastasis staging system for pancreatic tumors of the 7th edition of the Union for International Cancer Control was applied.32 The pathological data collected included tumor grade, number of positive lymph nodes, resection margins, perineural invasion, and PV invasion. The surgical margin in this study denoted either the stump of the pancreas or the bile duct, or the dissected plane around the pancreas as described by Staley et al.33 If viable cancer cells were detected microscopically at the tip of any of these sites, the surgical margin was determined as being positive. If the tumor was located at a distance of >1 mm from the surgical margin, it was determined as negative. Pathological diagnoses were made by experienced pathologists using standard techniques.
Statistical Analysis
A biostatistician (KM) was responsible for statistical analysis. Before matching, baseline characteristics and surgical outcomes were compared between the upfront surgery and NACRT groups. Differences in the numerical data between the 2 groups were examined using the Chi-squared test or Fisher exact test when the number of patients was <5. Differences in quantitative variables between the 2 groups were evaluated using Student's t-test, or the Mann–Whitney U test if the distribution was abnormal.
To evaluate the effect of NACRT regarding patient survival, and to reduce the impact of treatment-selection bias and potential confounding effects in an observational study, significant differences in patient characteristics were rigorously adjusted using IPTW propensity score method.34 A logistic regression model involving 7 covariates was used to estimate the propensity score. The 7 selected variables were clinicopathologic factors that could affect treatment selection.35,36 These included 3 continuous variables (age [years], pretreatment serum CA19–9 level [U/mL], and intraoperative blood loss volume [mL]) and 4 categorical variables (sex [male or female], major artery abutted by cancer [SMA or CHA], propriety of resection, and Union for International Cancer Control stage). Analysis involving propensity score weighted linear regressions for operative time, number of harvested lymph nodes and the length of the postoperative hospital stay, and weighted logistic regressions for the presence of postoperative complications (Clavien–Dindo classification), completion of adjuvant chemotherapy, and pathological factors (including lymph node metastasis, resection margin, perineural invasion, and PV invasion), were performed with adjustment for propensity scores. IPTW for NACRT was applied to estimate the hazard ratios regarding treatment effect using Cox proportional hazard model. Survival rates were estimated using Kaplan–Meier survival curves and the log-rank test. Statistical analysis was performed using SAS software, version 9.3 (SAS Institute, Inc., Cary, NC). A P value of <0.05 was considered statistically significant.
RESULTS
Characteristics of All Unmatched Patients
Of the 92 patients who had PDAC in contact with the SMA and/or CHA, 71 underwent upfront surgery and 21 received NACRT (Table 1). The 2 groups did not differ significantly in terms of preoperative comorbidities, body mass index, distribution of the major artery abutted, and pretreatment level of CA19–9. Consequently, 50 patients in the upfront surgery group and 18 in the NACRT group underwent curative resection (70% vs 86%, respectively; P = 0.16; Table 1; Figure 2).
TABLE 1.
Clinical Characteristics of the Enrolled Patients

NACRT Patients
Only 2 (9.5%) patients developed severe treatment-related toxicity resulting in the disruption of therapy. The relative dose intensity of S-1 ranged from 21% to 100% with a mean value of 93% (median, 100%). Patients receiving NACRT exhibited a significant decrease in pre- and posttreatment median CA19–9 levels from 284 to 63 U/mL (P = 0.042). This resulted in a CA19–9 level of <40 U/mL in 43% of patients as compared with only 5% of pre-NACRT patients (P = 0.004). A significant decrease in tumor diameter on computed tomography from a median value of 33 to 28 mm was also observed (P = 0.002). Of the 21 NACRT patients, imaging after treatment identified a partial response in 1 patient, stable disease in 18, and progressive disease (multiple liver metastases) in 2 using the response evaluation criteria in solid tumor analysis. Of the 19 patients who underwent surgical exploration regarding attempted resection, 1 was found to have locally advanced cancer that was unresectable after open laparotomy, and 18 underwent pancreatectomy.
Operative and Pathological Results
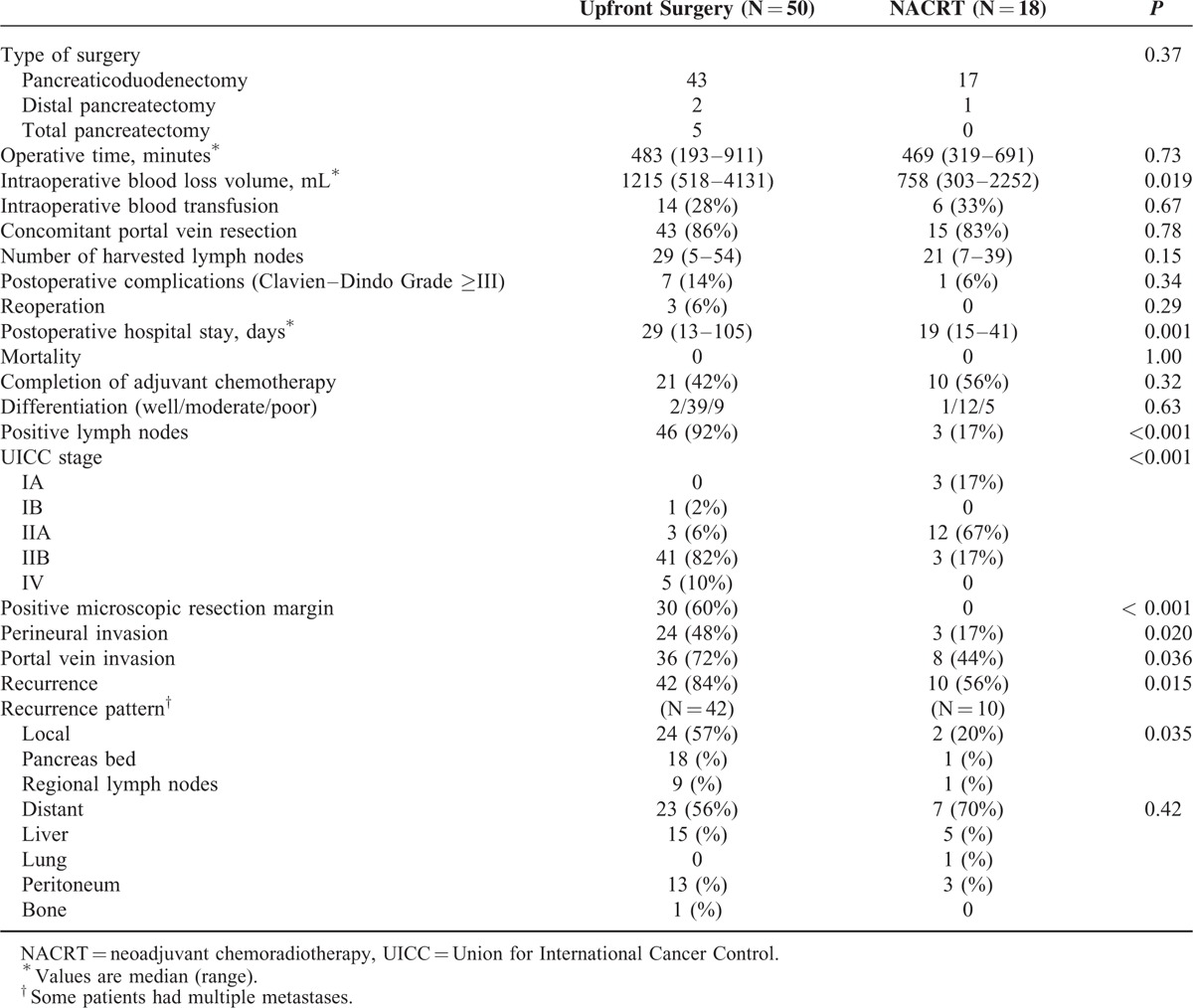
Fifty patients in the upfront surgery group and 18 in the NACRT group underwent curative pancreatectomy (Table 2). Median operative time, the incidence of concomitant PV resection, and the number of harvested lymph nodes were comparable between the 2 groups. The median blood loss volume and length of postoperative hospital stay in the NACRT group was significantly lower than in the upfront surgery group (758 vs 1215 mL; P = 0.019 and 19 vs 29 days; P = 0.001, respectively). The incidences of postoperative complications and reoperation tended to be higher in the upfront surgery group, although the differences did not reach statistical significance. There were no operative or hospital deaths. In terms of pathological results, the incidence of lymph node metastasis in the upfront surgery group was 92% (46/50), whereas that the incidence in the NACRT group was only 17% (3/18; P < 0.001), possibly reflecting significant downstaging by the neoadjuvant treatment. A positive microscopic resection margin was found in 30 patients in the upfront surgery group, but there were no patients with a positive microscopic resection margin in the NACRT group (60% vs 0%, respectively; P < 0.001). Patients in the NACRT group had significantly lower rates of perineural and PV invasion (P = 0.020 and 0.036, respectively).
TABLE 2.
Operative and Pathological Results
Regressions Weighted by Propensity Scores
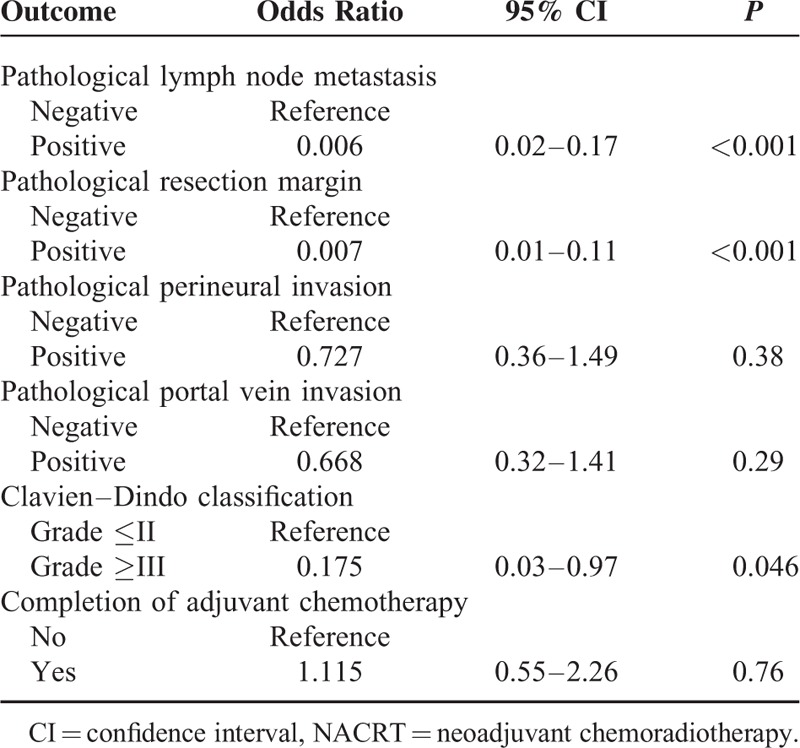
To reduce the impact of selection bias, propensity score analysis was performed using 7 selected baseline characteristics. The results of the propensity score weighted linear regressions are detailed in Table 3. In the NACRT group, length of postoperative hospital stay was 13.8 days less than in the upfront surgery group (95% confidence interval, –21.42 to –6.25; P < 0.001). The results of the propensity score weighted logistic regressions are presented in Table 4. The incidences of pathological lymph node metastasis and a pathological positive resection margin were significantly lower in the NACRT group (odds ratio, 0.006; 95% confidence interval, 0.02–0.17; P < 0.001 and odds ratio, 0.007; 95% confidence interval, 0.01–0.11; P < 0.001, respectively). On the other hand, the delivery of NACRT did not affect the completion rate of postoperative adjuvant chemotherapy.
TABLE 3.
Propensity Score Weighted Linear Regressions of the NACRT Group With Reference to the Upfront Surgery Group
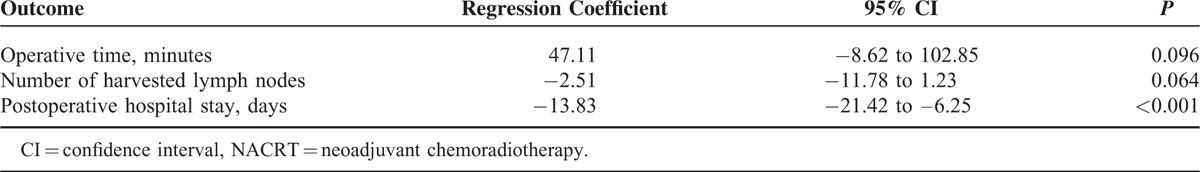
TABLE 4.
Propensity Score Weighted Logistic Regressions of the NACRT Group With Reference to the Upfront Surgery Group
Survival Analysis
The median follow-up period for the entire cohort was 15.5 months. The patterns of first recurrence for the patients who underwent pancreatectomy are listed in Table 2. The incidence of local recurrence including in the pancreas bed and the regional lymph nodes was lower in the NACRT group (20% and 57%, respectively; P = 0.035); conversely, the incidence of distant metastasis was similar between the 2 groups. The median disease specific survival times for the unmatched patients in the NACRT and the upfront surgery groups were 29.1 and 13.1 months, respectively (Figure 3A). After 17 patients from each group were selected by propensity score matching, the median disease specific survival time for the upfront surgery group was 13.9 months; in the NACRT group it could not be calculated, because the survival curve did not reach the 50% line before the end of the study (Figure 3B). Among the matched patients, the estimated 1- and 2-year survival rates in the upfront surgery group were 66.7% and 16.0%, respectively, and those in the NACRT group were 80.0% and 65.2%, respectively. Both the unmatched and matched patients in the NACRT group had significantly longer survival times than in the upfront surgery group (P = 0.001 and 0.007, respectively).
FIGURE 3.

(A) Kaplan–Meier curves for disease specific survival after initiation of treatment for unmatched patients who underwent upfront surgery (N = 71) or neoadjuvant chemoradiotherapy (NACRT) (N = 21; P = 0.001). The median survival times in the upfront surgery and NACRT groups were 13.1 and 29.1 months, respectively. (B) Kaplan–Meier survival curves for the propensity score-matched upfront surgery (N = 17) and NACRT (N = 17) groups (P = 0.007). In the upfront surgery group, the median survival time was 13.9 months, and the estimated 1- and 2-year survival rates were 66.7% and 16.0%, respectively. In the NACRT group, the median survival time could not be calculated because the survival curve did not reach the 50% line before the end of the study, and the estimated 1- and 2-year survival rates were 80.0% and 65.2%, respectively.
In Table 5, the cumulative hazards in evaluating the effect of NACRT on disease specific survival using unadjusted and adjusted multivariate analyses are detailed. Unadjusted and adjusted Cox regression models revealed significantly longer survival time in the NACRT group (hazard ratio, 0.323; 95% confidence interval, 0.157–0.663; P = 0.002 and hazard ratio, 0.281; 95% confidence interval, 0.116–0.680; P = 0.005, respectively); in the IPTW and the propensity score-matched analyses, the adjusted hazard ratios were 0.398 (95% confidence interval, 0.255–0.620; P < 0.001) and 0.278 (95% confidence interval, 0.103–0.751; P = 0.012), respectively.
TABLE 5.
Hazard Ratios of Overall Survival of Patients in the NACRT Group Compared with the Upfront Surgery Group

DISCUSSION
Surgery for locally advanced PDAC with involvement of the major artery including the hepatic artery and the SMA is highly controversial. The survival of patients who underwent combined resection and reconstruction of major arteries was reportedly comparable with that of patients who underwent standard pancreatectomy in small cohort studies carried out at high-volume centers; however, combined arterial resection was reported to be associated with significantly higher mortality rates.37–39 A systematic review by Mollberg et al10 revealed that the median rates of perioperative morbidity, reoperation, and mortality were 53.6% (range, 16.7%–100%), 12.5% (range, 0%–68.8%), and 11.8% (range, 0%–45.5%), respectively. Combined arterial resection during pancreatectomy may have potential clinical benefits in highly selected patients; however, it could be a challenging treatment that can be performed only in selected institutions. A greater proportion of patients could have access to surgical treatment if removal of the tumor without coresection of the arteries could be deemed oncologically feasible. However, little information is available on treatment strategy featuring artery-preserving surgery for BR-PDAC in contact with the major artery.
In the present study, comparisons were made between upfront surgery and NACRT followed by surgery in patients with PDAC in contact with the SMA or the CHA. In both groups, the treatment strategy entailed preservation of the artery. Unmatched and propensity score-matched analyses were used to adjust for patient characteristics. Twenty-one (30%) patients in the upfront surgery group were determined to be unresectable at laparotomy, whereas a total of 3 (14%) patients in the NACRT group (2 prior to surgery and 1 at laparotomy) were unresectable. No significant difference in terms of the resection rate was found between the groups. However, the incidence of lymph node metastasis was found to be significantly lower and the R0 resection rate significantly higher in the NACRT group when evaluated using the propensity score-weighted logistic regression model. A systematic review by Mollberg et al10 of PDAC associated with arterial infiltration showed that the incidence of lymph node metastasis and the R0 resection rate were 66.7% (range, 41.1%–100%) and 60.0% (range, 13.3%–100%), respectively, in the absence of neoadjuvant treatment. Comparing these findings with the NACRT results obtained in the current study (17% and 100% for the incidence of lymph node metastasis and the R0 resection rate, respectively) clearly implies that the tumor cells in the lymph node and at the boundary between the normal tissue had disappeared after NACRT. Moreover in the present study, the intraoperative blood loss volume was significantly lower in the NACRT, presumably as a result of the status the resection margin. The NACRT group had a cancer-negative margin in all cases, and it was suggested that the dissection of the tumor from the artery was technically easier than was the case in the upfront surgery group.
Recently, a number of studies have reported the results of treatment using neoadjuvant FOLFIRINOX, an exceedingly promising chemotherapy regimen.40,41 Ferrone et al reported outstanding short-term outcomes in patients with BR and locally advanced PDAC. The efficacy of neoadjuvant FOLFIRINOX was found to be potentially superior to that in the current study with a remarkable decrease in pre- and posttreatment median CA19–9 level from 169 to 0.17 U/mL and a resection rate of 100%.40 The incidence of lymph node metastasis and R0 resection rate were equivalent between the 2 studies. Neoadjuvant FOLFIRINOX provided a radiographic response in 90% (36/40) of patients,40 whereas the response was confined to stable disease in 86% (18/21) of patients in the current study in which, compatible with previous reports,12,42 NACRT was found to illicit little radiographic response as determined using response evaluation criteria in solid tumor. Nevertheless, the lower incidence of local recurrence may denote the locoregional control effect of NACRT, possibly resulting in better survival than the upfront surgery strategy.
A recent review by Franke et al43 revealed that the median overall survival time was 10 to 35 months in patients undergoing NACRT for BR-PDAC. NACRT potentially has clinical and survival benefits although precise methodical comparison is difficult because heterogeneity, including treatment protocols, clinical endpoints, inclusion criteria, and the metrics used to define resectability, exists between each study. Nevertheless, neoadjuvant therapy has the advantage that it may identify patients with rapidly progressive disease or with early metastasis who would likely not benefit from surgical treatment. The cost-effectiveness of NACRT was also found to be superior to a surgery-first approach in the treatment of PDAC.44
A limitation of the present study was its nonrandomized design. Despite efforts to control for baseline factors using IPTW analysis, this was not a prospective randomized trial and the 2 groups of patients were not the same. In addition, the length of postoperative stay in the current study was slightly longer than reported in previous studies. This probably reflects the differences in medical insurance systems including lower hospitalization fees which, as previously described,45,46 preclude comparison with other developed countries.
CONCLUSIONS
In conclusions, findings suggested that CRT followed by surgery, rather than upfront surgery, provided short-term clinical benefits in patients with PDACs in contact with the SMA or CHA, which were expected to be technically removable in pretreatment imaging. Further comparative studies will be required involving combined arterial resection and promising neoadjuvant systemic chemotherapy including FOLFIRINOX.
Footnotes
Abbreviations: BR = borderline resectable, CA19–9 = carbohydrate antigen 19–9, CHA = common hepatic artery, IPTW = inverse probability of treatment weighting, MDCT = multidetector-row computed tomography, NACRT = neoadjuvant chemoradiotherapy, PDAC = pancreatic adenocarcinoma, PV = portal vein, SMA = superior mesenteric artery, UICC = Union for International Cancer Control.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29. [DOI] [PubMed] [Google Scholar]
- 2.Wray CJ, Ahmad SA, Matthews JB, et al. Surgery for pancreatic cancer: recent controversies and current practice. Gastroenterology 2005; 128:1626–1641. [DOI] [PubMed] [Google Scholar]
- 3.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013; 310:1473–1481. [DOI] [PubMed] [Google Scholar]
- 4.Nakao A, Takeda S, Inoue S, et al. Indications and techniques of extended resection for pancreatic cancer. World J Surg 2006; 30:976–982. [DOI] [PubMed] [Google Scholar]
- 5.Cameron JL, Riall TS, Coleman J, et al. One thousand consecutive pancreaticoduodenectomies. Ann Surg 2006; 244:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg 1995; 221:721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg 2004; 91:586–594. [DOI] [PubMed] [Google Scholar]
- 8.Kanda M, Fujii T, Kodera Y, et al. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg 2011; 98:268–274. [DOI] [PubMed] [Google Scholar]
- 9.Tempero MA, Arnoletti JP, Behrman SW, et al. Pancreatic Adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2012; 10:703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mollberg N, Rahbari NN, Koch M, et al. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg 2011; 254:882–893. [DOI] [PubMed] [Google Scholar]
- 11.Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg 1992; 127:1335–1339. [DOI] [PubMed] [Google Scholar]
- 12.Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012; 118:5749–5756. [DOI] [PubMed] [Google Scholar]
- 13.White RR, Hurwitz HI, Morse MA, et al. Neoadjuvant chemoradiation for localized adenocarcinoma of the pancreas. Ann Surg Oncol 2001; 8:758–765. [DOI] [PubMed] [Google Scholar]
- 14.Breslin TM, Hess KR, Harbison DB, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol 2001; 8:123–132. [DOI] [PubMed] [Google Scholar]
- 15.Pisters PW, Wolff RA, Janjan NA, et al. Preoperative paclitaxel and concurrent rapid-fractionation radiation for resectable pancreatic adenocarcinoma: toxicities, histologic response rates, and event-free outcome. J Clin Oncol 2002; 20:2537–2544. [DOI] [PubMed] [Google Scholar]
- 16.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008; 26:3496–3502. [DOI] [PubMed] [Google Scholar]
- 17.Satoi S, Toyokawa H, Yanagimoto H, et al. Neo-adjuvant chemoradiation therapy using S-1 followed by surgical resection in patients with pancreatic cancer. J Gastrointest Surg 2012; 16:784–792. [DOI] [PubMed] [Google Scholar]
- 18.Homma Y, Taniguchi K, Murakami T, et al. Immunological impact of neoadjuvant chemoradiotherapy in patients with borderline resectable pancreatic ductal adenocarcinoma. Ann Surg Oncol 2014; 21:670–676. [DOI] [PubMed] [Google Scholar]
- 19.Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010; 7:e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikeda M, Okusaka T, Ito Y, et al. A phase I trial of S-1 with concurrent radiotherapy for locally advanced pancreatic cancer. Br J Cancer 2007; 96:1650–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakao A, Kanzaki A, Fujii T, et al. Correlation between radiographic classification and pathological grade of portal vein wall invasion in pancreatic head cancer. Ann Surg 2012; 255:103–108. [DOI] [PubMed] [Google Scholar]
- 22.Fujii T, Kanda M, Kodera Y, et al. Preservation of the pyloric ring has little value in surgery for pancreatic head cancer: a comparative study comparing three surgical procedures. Ann Surg Oncol 2012; 19:176–183. [DOI] [PubMed] [Google Scholar]
- 23.Fujii T, Kato K, Kodera Y, et al. Prognostic impact of pancreatic margin status in the intraductal papillary mucinous neoplasms of the pancreas. Surgery 2010; 148:285–290. [DOI] [PubMed] [Google Scholar]
- 24.Fujii T, Sugimoto H, Yamada S, et al. Modified Blumgart anastomosis for pancreaticojejunostomy: technical improvement in matched historical control study. J Gastrointest Surg 2014; 18:1108–1115. [DOI] [PubMed] [Google Scholar]
- 25.Sahin TT, Fujii T, Kanda M, et al. Prognostic implications of lymph node metastases in carcinoma of the body and tail of the pancreas. Pancreas 2011; 40:1029–1033. [DOI] [PubMed] [Google Scholar]
- 26.Kanda M, Fujii T, Sahin TT, et al. Invasion of the splenic artery is a crucial prognostic factor in carcinoma of the body and tail of the pancreas. Ann Surg 2010; 251:483–487. [DOI] [PubMed] [Google Scholar]
- 27.Fujii T, Nakao A, Yamada S, et al. Vein resections >3 cm during pancreatectomy are associated with poor 1-year patency rates. Surgery 2015; 157:708–715. [DOI] [PubMed] [Google Scholar]
- 28.Maeda A, Boku N, Fukutomi A, et al. Randomized phase III trial of adjuvant chemotherapy with gemcitabine versus S-1 in patients with resected pancreatic cancer: Japan Adjuvant Study Group of Pancreatic Cancer (JASPAC-01). Jpn J Clin Oncol 2008; 38:227–229. [DOI] [PubMed] [Google Scholar]
- 29.Fujii T, Kanda M, Nagai S, et al. Excess weight adversely influences treatment length of postoperative pancreatic fistula: a retrospective study of 900 patients. Pancreas 2015; 44:971–976. [DOI] [PubMed] [Google Scholar]
- 30.Fujii T, Yamada S, Suenaga M, et al. Preoperative internal biliary drainage increases the risk of bile juice infection and pancreatic fistula after pancreatoduodenectomy: a prospective observational study. Pancreas 2015; 44:465–470. [DOI] [PubMed] [Google Scholar]
- 31.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.International Union Against Cancer. TNM Classification of Malignant Tumors. 7th ed. New York: Wiley-Blackwell; 2009. [Google Scholar]
- 33.Staley CA, Cleary KR, Abbruzzese JL, et al. The need for standardized pathologic staging of pancreaticoduodenectomy specimens. Pancreas 1996; 12:373–380. [DOI] [PubMed] [Google Scholar]
- 34.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000; 11:550–560. [DOI] [PubMed] [Google Scholar]
- 35.Bonjer HJ, Hop WC, Nelson H, et al. Laparoscopically assisted vs open colectomy for colon cancer: a meta-analysis. Arch Surg 2007; 142:298–303. [DOI] [PubMed] [Google Scholar]
- 36.Thorpe H, Jayne DG, Guillou PJ, et al. Medical Research Council Conventional versus Laparoscopic-Assisted Surgery in Colorectal Cancer Trial Group. Patient factors influencing conversion from laparoscopically assisted to open surgery for colorectal cancer. Br J Surg 2008; 95:199–205. [DOI] [PubMed] [Google Scholar]
- 37.Bachellier P, Rosso E, Lucescu I, et al. Is the need for an arterial resection a contraindication to pancreatic resection for locally advanced pancreatic adenocarcinoma? A case-matched controlled study. J Surg Oncol 2011; 103:75–84. [DOI] [PubMed] [Google Scholar]
- 38.Bockhorn M, Burdelski C, Bogoevski D, et al. Arterial en bloc resection for pancreatic carcinoma. Br J Surg 2011; 98:86–92. [DOI] [PubMed] [Google Scholar]
- 39.Christians KK, Pilgrim CH, Tsai S, et al. Arterial resection at the time of pancreatectomy for cancer. Surgery 2014; 155:919–926. [DOI] [PubMed] [Google Scholar]
- 40.Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015; 261:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paniccia A, Edil BH, Schulick RD, et al. Neoadjuvant FOLFIRINOX application in borderline resectable pancreatic adenocarcinoma: a retrospective cohort study. Medicine (Baltimore) 2014; 93:e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sa Cunha A, Rault A, Laurent C, et al. Surgical resection after radiochemotherapy in patients with unresectable adenocarcinoma of the pancreas. J Am Coll Surg 2005; 201:359–365. [DOI] [PubMed] [Google Scholar]
- 43.Franke AJ, Rosati LM, Pawlik TM, et al. The role of radiation therapy in pancreatic ductal adenocarcinoma in the neoadjuvant and adjuvant settings. Semin Oncol 2015; 42:144–162. [DOI] [PubMed] [Google Scholar]
- 44.Abbott DE, Tzeng CW, Merkow RP, et al. The cost-effectiveness of neoadjuvant chemoradiation is superior to a surgery-first approach in the treatment of pancreatic head adenocarcinoma. Ann Surg Oncol 2013; 20 Suppl 3:S500–S508. [DOI] [PubMed] [Google Scholar]
- 45.Tokunaga J, Imanaka Y. Influence of length of stay on patient satisfaction with hospital care in Japan. Int J Qual Health Care 2002; 14:493–502. [DOI] [PubMed] [Google Scholar]
- 46.Kondo A, Zierler BK, Isokawa Y, et al. Comparison of outcomes and costs after hip fracture surgery in three hospitals that have different care systems in Japan. Health Policy 2009; 91:204–210. [DOI] [PubMed] [Google Scholar]


