Abstract
Although gastroesophageal reflux disease (GERD) has been reported to coexist with chronic rhinosinusitis (CRS), it remains controversial whether it increases risk of CRS in adults. This study accesses risk of CRS in adults with newly diagnosed GERD.
We identified 15,807 adult patients with newly diagnosed GERD from Taiwan's National Health Insurance Research Database for January 1, 2006 to December 31, 2009. We also randomly selected 47,421 subjects without this disease and matched them with patients by age, sex, index year, and comorbidity to create a control cohort. A Cox proportional hazards model was conducted to estimate the development of CRS, including CRS without nasal polyps and CRS with nasal polyps.
Subjects were followed for a median of 2.12 years. In total, CRS developed in 964 (1.52%) of the subjects: 406 patients with GERD (2.57%) and 558 without it (1.18%). After adjustment, those with GERD were found to have a 2.36 times greater risk of CRS (95% confidence interval = 2.08–2.68; P < .001). Risk of this CRS without nasal polyps was higher than the disease with polyps (adjusted hazard ratio: 2.48 vs 1.85).
The individuals with GERD in this study were at significantly greater risk of CRS, most often without nasal polyps.
INTRODUCTION
Gastroesophgeal reflux disease (GERD), which is more prevalent in western populations, is also a prevalent disorder affecting 2.5% to 7.8% of the people in East Asia.1,2 It is a disorder characterized by reflux of stomach contents causing disturbing symptoms and/or complications.3 Clinically, this disease encompasses not only classical esophageal syndrome but also an assortment of extraesophageal syndromes (EESs) including asthma, reflux cough, and chronic laryngitis.3–5 In United States, the costs of caring for patients with EESs are greater than caring for patients that have typical GERD alone.4 Because EESs do not necessarily occur with typical reflux symptoms,5,6 it is difficult to determine whether a symptom is a part of EES or an independent disease.
Chronic rhinosinusitis (CRS) is a group of disorders characterized by inflammation of sinonasal mucosa lasting for more than twelve weeks.7–9 Clinically, it is often subgrouped into CRS without and with nasal polyps,7,9 the latter characterized by intense eosinophilic infiltration and a skewing toward Th2 cytokine expression.10 CRS affects people of all ages, and rivals asthma and diabetes mellitus in prevalence.11,12 Due to its clustering of symptoms and chronicity, this disease has been associated with impaired health-related quality of life, emotional impairment, and functional limitation.12,13 The socioeconomic burden it poses from direct and indirect costs is large.12,13
Though the underlying etiology and pathogenesis of CRS remain largely unknown, it has been associated with a host of comorbidities, including GERD, particularly in investigations of children in studies with great statistical power.14–18 It is unclear whether gastroesophgeal reflux disease increases the risk of chronic rhinosinusitis (CRS) in adults. Although some studies have found that it predicts the onset of CRS,19–23 others have not.24–27 Most of these studies, unfortunately, are limited by either small sample sizes, differences in study populations, or unsound diagnostic methods.19–25 Therefore, we conducted a large-scale population-based cohort study in Taiwan to investigate the subsequent risk of CRS without nasal polyps and CRS with nasal polyps after a diagnosis of GERD.
METHODS AND MATERIAL
Ethical Consideration
This study was approved by the Institutional Review Board of Kaohsiung Veterans General Hospital. Review board requirements for written informed consent were waived because all personal identifying information had been removed from the dataset before analysis.
Database
The study samples were retrieved from the Longitudinal Health Insurance Database 2010 (LHID 2010). The National Health Insurance (NHI) program of Taiwan is a mandatory universal health insurance program offering comprehensive and easily accessible medical care to all Taiwanese residents. Its coverage rate has exceeded 99% since March 1, 1995. The National Health Insurance Research Database (NHIRD) consists of original insurance claims data maintained by National Health Research Institute (NHRI) of Taiwan. The NHIRD's accuracy has been validated28 and found to be a reliable source of data for research purposes. The LHID 2010, a subset of NHIRD, is a representative database of 1,000,000 subjects who were alive during 2010 and randomly sampled from the registry of all NHI enrollees29 from 2006 onwards. Detailed information about the database is available online at: http://nhird.nhri.org.tw/.
Study Design and Participants
In this retrospective cohort study, we enrolled a cohort of 15,807 adult subjects (older than 18 years) who were newly diagnosed as having GERD (International Classification of Diseases, 9th revision, Clinical Modification [ICD-9-CM] codes: 530.11 or 530.81) and who subsequently received proton pump inhibitor (PPI) for treatment of that disease between January 1, 2007 and December 31, 2009 from the LHID 2010. The date on which the diagnosis of GERD was recorded in the Registry was defined as the index date. This diagnosis was validated by the prescription of PPI because Taiwan's Bureau of NHI only reimburses PPI for patients with GERD confirmed by panendoscopy or 24-hour pH-meter monitoring. We created a control group of 47,421 patients without GERD from the remaining beneficiaries of LHID 2010. We used propensity score matching to match patients and controls by sex, age strata (18–44, 45–64, 65–74, 75–84, ≥85 years), index year, and comorbidity types. The selected comorbidities for which the subjects were matched are recognized to be common premorbid illness of CRS,14,15 including asthma (ICD-9-CM code: 493.X), allergic rhinitis (AR) (ICD-9-CM code: 477.X), otitis media (OM) (ICD-9-CM code: 382.X), adenotonsillitis (ICD-9-CM codes: 474.0X or 474.1X), atopic dermatitis (AD) (ICD-9-CM code: 691.8), and pneumonia (ICD-9-CM code: 480–6). In both groups, subjects with a prior diagnosis of GERD, CRS (ICD-9-CM codes: 473.X or 471.X), human immunodeficiency virus infection (ICD-9-CM code: 042), or primary immune deficiency (ICD-9-CM code: 279.X) before the index date were excluded. Ultimately, 63,228 subjects were enrolled. The flowchart for the selection process is shown in Figure 1.
FIGURE 1.
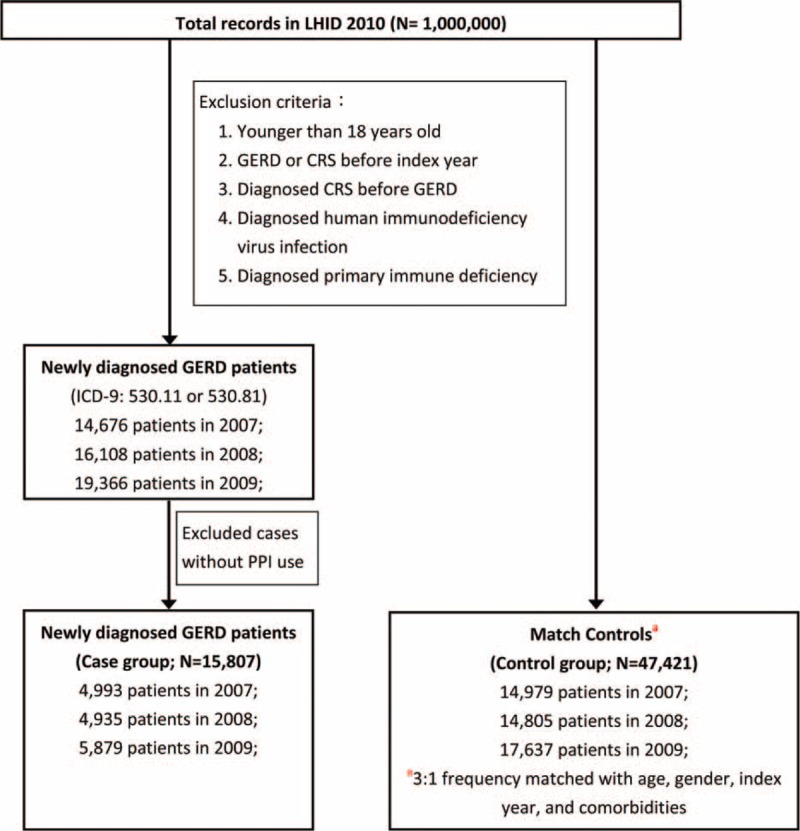
Flow chart of selection of GERD patients and matched controls from the NHIRD in Taiwan. CRS = chronic rhinosinusitis, GERD = gastroesophageal reflux disease, ICD = International Classification of Diseases.
Main Outcome Measures
The primary endpoint of the present study was a subsequent diagnosis of CRS, including CRS without nasal polyps and CRS with nasal polyps, during the study period following the GERD index date. A diagnosis of CRS was defined as diagnosis of CRS without nasal polyps followed by at least one NHI ambulatory care visit (including hospital and clinic outpatient visits). The diagnosis was certified by an otolaryngologist experienced in airway endoscopic examinations, including sinoscopoy (Specific Diagnosis and Treatment [SDT]:28003C), nasopharyngoscopy (SDT:28002C), or flexible laryngoscopy (SDT:28004C), or 1 inpatient record indicating CRS without polyps. To be included, the patients with CRS without nasal polyps could not have a prior or subsequent diagnosis of nasal polyps listed in the medical claims records throughout the study period. We only included patients with CRS with nasal polyps if they fulfilled the requirements listed above for CRS without nasal polyps and they were further diagnosed as having CRS with nasal polyps subsequent to their inclusion indicated by the date that its ICD-9 code was recorded. In other words, the subjects could be recategorized if nasal polyps developed.
We tracked each subject (N = 63228) individually until the occurrence of CRS or the date of death, dropout from the insurance program, or to the end of 2012 if no CRS was diagnosed.
Statistical Analysis
All statistical analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC) and STATA software package version 12.0 (Stata Corp., College Station, TX). Pearson χ2 tests were used to explore the differences between categorical variables and t test for continuous variables between cases and controls. We accessed the cumulative incidence of CRS using Kaplan–Meier analysis and calculated CRS incidence rates (per 10,000 person-years). Difference between cumulative curves was compared using the log-rank test. Risk of CRS following a diagnosis of GERD between the 2 cohorts was performed Cox proportional hazard regressions (stratified by gender, age strata and index year), using the controls as the reference group. Furthermore, we explored individual adjusted hazard ratios (HRs) for CRS with and without nasal polyps in both cohorts. Multiple regression analysis was performed to identify independent risk factors in GERD patients with CRS without nasal polyps and CRS with nasal polyps, respectively. A 2-tailed P-value <0.05 was considered significant.
RESULTS
Characteristics of Study Population
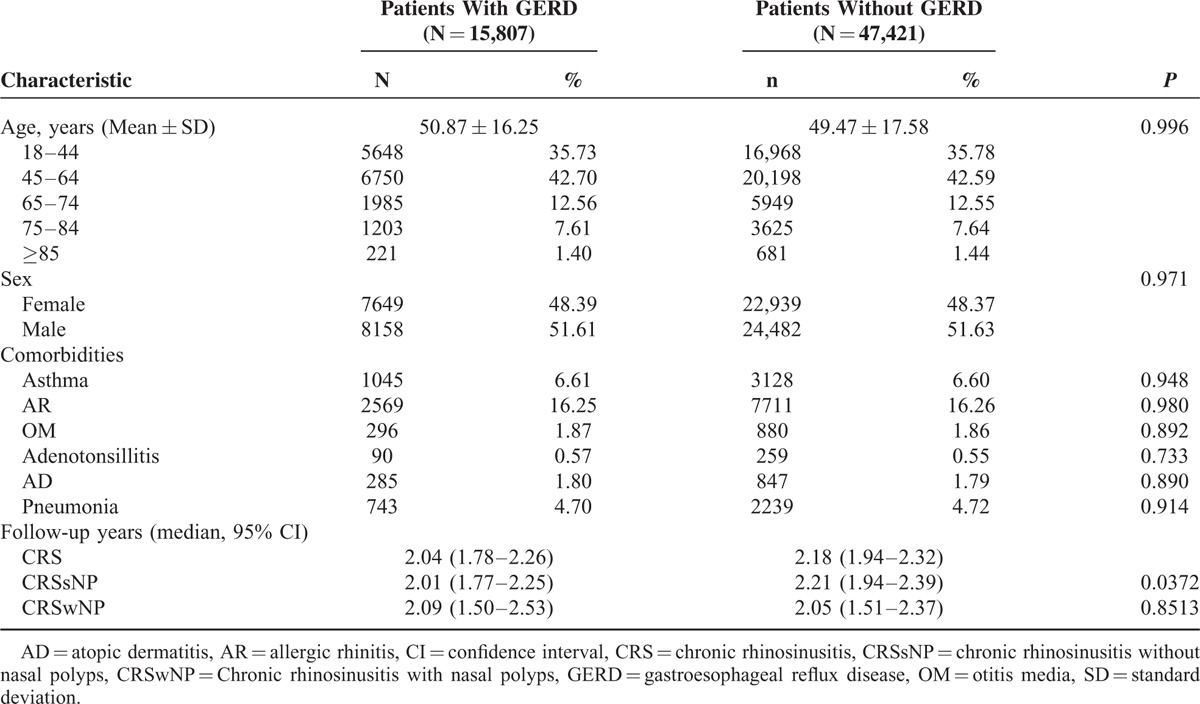
This study identified 15,807 patients newly diagnosed with GERD (mean age = 50.87 years, standard deviation [SD] = 16.25) and 47,421 match controls (mean age = 49.47 years, SD = 17.58 years) for the 2007–2009 study period. Most patients (42.62%) were aged 45 to 64 years, and asthma and AR were the most common comorbidities (6.60% and 16.26%, respectively). There were no significant statistical differences between the 2 cohorts with regard to age, sex, and comorbidity types (Table 1). However, there was a difference in duration of follow-up, which was longer in the non-GERD population without nasal polyps (P = 0.0239) (Table 1).
TABLE 1.
Demographic Characteristics and Comorbidities of GERD and Non-GERD Patients (N = 63,228)
Incidence of CRS
Out of the 63,228 patients and controls, 964 (1.52%) were diagnosed as having CRS over a mean follow-up period of 2.12 years (95% confidence interval [CI] = 1.94–2.24). A total of 406 (0.64%) patients with GERD and 558 (0.88%) of the controls developed CRS during the follow-up period. Seven hundred seventy-one of the 964 patients that developed CRS did not have nasal polyps. A greater percentage of the patients with GERD had CRS without nasal polyps than did the control group (2.12% vs 0.92%) (Table 2). The incidence rates of CRS without nasal polyps in the GERD patients and control groups were 48.80 and 19.59 persons per 10,000 person-years. GERD patients were at significantly higher risk of CRS without nasal polyps than the controls (log rank test, P < 0.001) (Fig. 2).
TABLE 2.
Crude and Adjusted Hazard Ratios for CRS Subtypes Between Patients With GERD and Without GERD
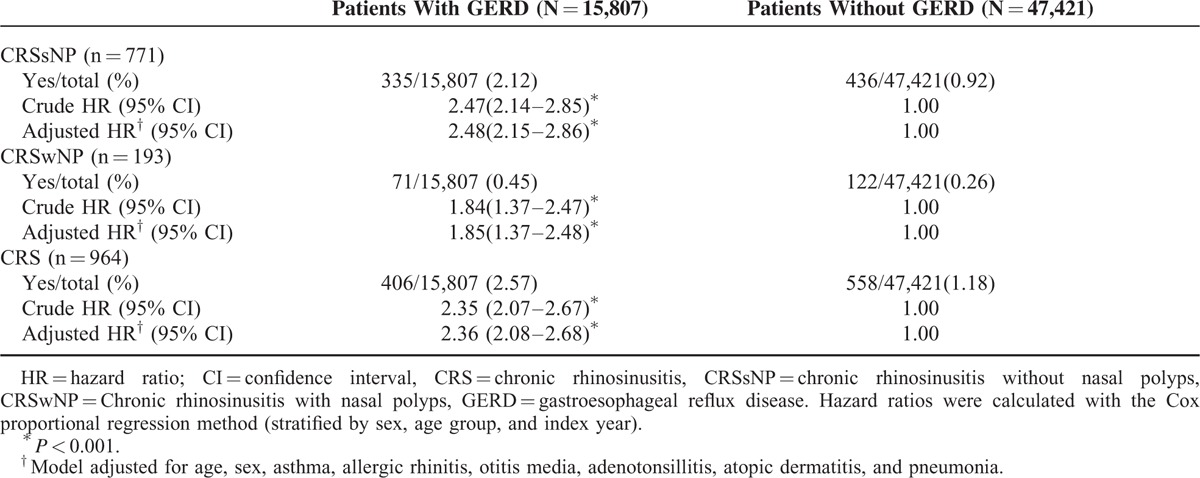
FIGURE 2.
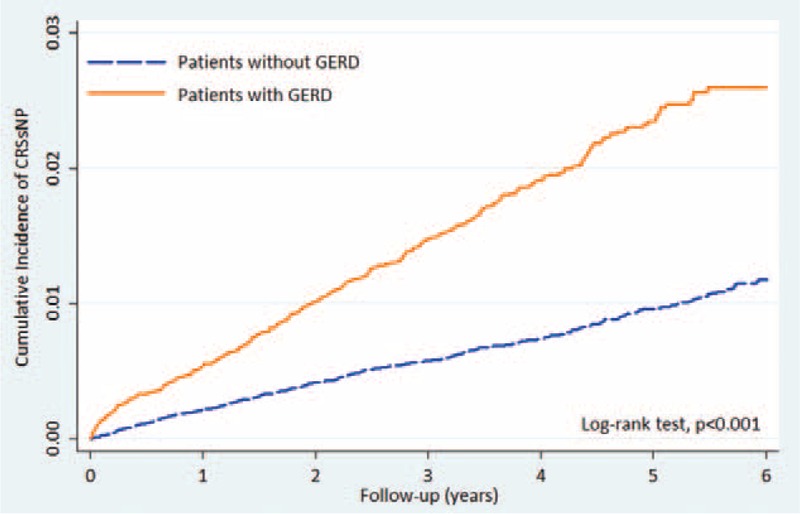
Cumulative incidence curves of CRSsNP in patients with GERD and controls. CRSsNP = chronic rhinosinusitis without nasal polyps, GERD = gastroesophageal reflux disease.
Patients with GERD had a higher prevalence of CRS with nasal polyps than those without (0.45% vs 0.26%) (Table 2). The incidence rates were 10.24 and 5.45 per 10,000 person-years, respectively (Fig. 3). The result of our Kaplan-Meier analysis of the cumulative incidence of CRS with nasal polyps shows it to be significantly higher for patients with GERD than those without (log rank test, P < 0.001).
FIGURE 3.
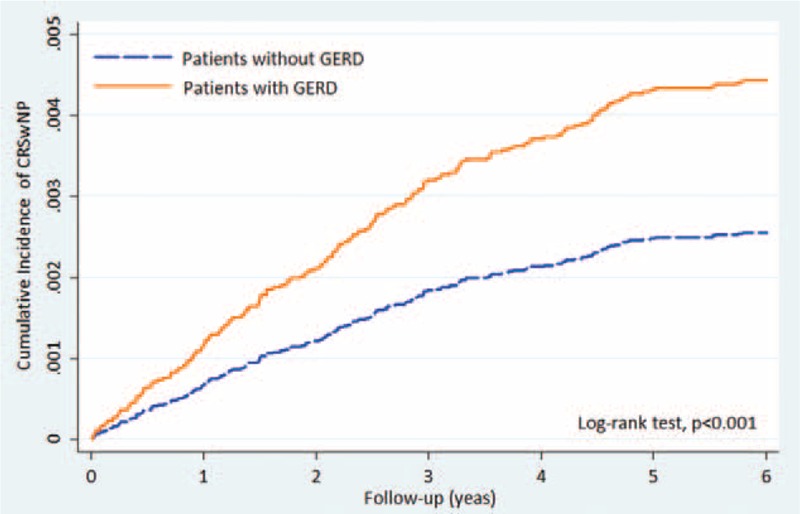
Kaplan-Meier curves showing a significant difference in cumulative incidence of CRSwNP among patients with GERD and controls. CRSwNP = Chronic rhinosinusitis with nasal polyps, GERD = gastroesophageal reflux disease.
Association Between GERD and Risk of CRS
The adjusted HRs for CRS in general, CRS without nasal polyps, and CRS with nasal polyps in patients with GERD were 2.36 (95% CI = 2.08–2.68; P < 0.001), 2.48 (95% CI = 2.15–2.86; P < 0.001), and 1.85 (95% CI = 1.37–2.48; P < 0.001), respectively, after controlling for age, sex, and comorbidities (Table 2). Patients with GERD were at risk for subsequent CRS, and they were at greater risk of CRS without nasal polyps than CRS with nasal polyps.
The results of our multivariate regression analysis showed that our GERD population had higher adjusted HRs for both CRS without and with nasal polyps in the age category of 45 to 64 years (adjusted HR = 1.59, 95% CI = 1.24–2.04 vs adjusted HR = 1.94, 95% CI = 1.09–3.44, respectively) and comorbid AR (adjusted HR = 3.15, 95% CI = 2.50–3.96 vs. adjusted HR = 2.34, 95% CI = 1.39–3.94, respectively) compared with reference groups. In addition, male patients with GERD had a 2.16-fold (95% CI = 1.31–3.58; P < .01) higher risk of CRS with nasal polyps than their female counterparts (Tables 3 and 4).
TABLE 3.
Analysis of Risk Factors for CRS Without Nasal Polyps in Patients With GERD (N = 335)
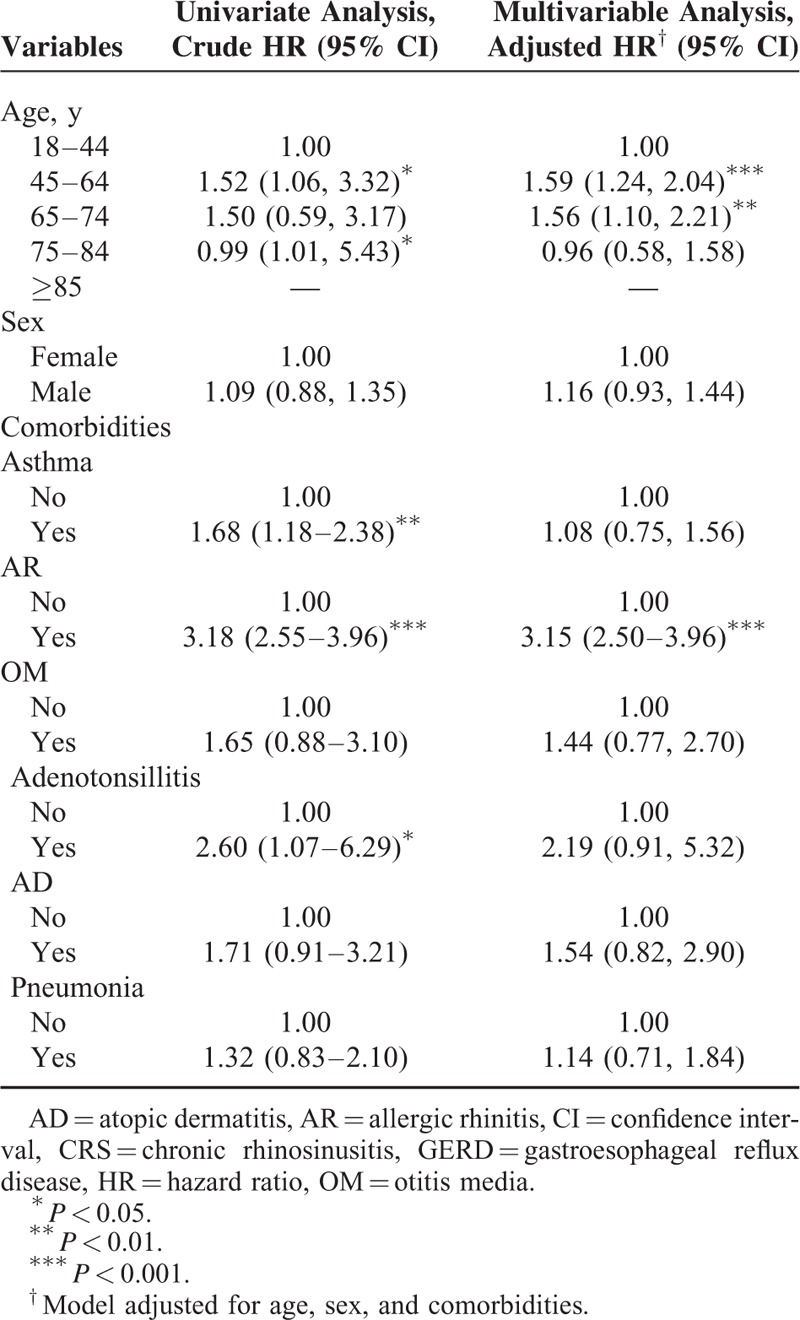
TABLE 4.
Analysis of Risk Factors for CRS With Nasal Polyps in Patients With GERD (N = 71)

DISCUSSION
This study found an independent association between newly diagnosed GERD and risk of subsequent development of CRS and estimated the risk for subsequent development of CRS in adult GERD patients. To the best of our knowledge, this is the first study to investigate the association of GERD and CRS in adults in an East Asian population. It contributes to large-scale epidemiological data regarding this association from a Chinese population to the prevailing western population studies.
Pediatric studies have found a strong association between GERD on CRS in children,17,18 whereas studies on relationship between GERD and CRS in adults have produced conflicting results. On the one hand, Jecker et al19 reported that GERD, but not laryngopharyngeal reflux, was more commonly associated with CRS. Using a 24-h pH probe, Wong et al,20 in a prospective study of 40 adult patients with newly diagnosed CRS, found that 32.4% of their CRS patients had GERD. A similar result was reported by Loehrl et al21 studying postsurgical patients with refractory CRS. Vaezi et al22 and DiBaise et al23 reported that CRS patients benefited from aggressive PPI use as evidenced by subjective symptomatic improvement. On the other hand, some studies have not found such a relationship. Durmus et al24 found that reflux had no effect on nasal mucociliary clearance. Similarly, DeConde et al25 reported that comorbid GERD had no impact on both baseline and postoperative quality of life for CRS patients undergoing endoscopic sinus surgery. Therefore, as concluded by 2 review studies, the association between the 2 diseases in adults has not been consistently demonstrated.26,27
In addition to hospital studies, there have been 2 recent population studies that have included these diseases in their research. One study, known as the GHS study, examining health records from 41 community primary practice clinics and 4 hospitals, reported that CRS patients were more prone to having antecedent GERD as well as a host of respiratory and epithelial inflammatory illness than their comparison cohort.14,15 That study noted that, in addition to the well-known risk factors for development of CRS, including asthma and AR, there are a several other diseases that can occur before a diagnosis of CRS without nasal polyps, including GERD, adenotonsillitis, OM, pneumonia, and AD.14 More recently, a prospective epidemiologic follow-up investigation, known as the RHINE study, administered a questionnaire to a randomly selected cohort of North Europeans and found people with nocturnal GERD to be 1.6 times more likely than those who did not have this disease to have further developed adult-onset noninfectious rhinitis after a follow-up period of 10 years.30 That study was limited, however, by the fact that they did not use professional society guidelines for diagnosis, did not take into account recall bias, and did not take into account differences in impact on the various phenotypes of noninfectious rhinitis.
There are several possible reasons for the increased risk of CRS in GERD patients. First, pathogenic reflux causes frequent and prolonged exposure of gastric contents to the esophagus and may reach the nasopharynx. Changes in the homeostasis of sinonasal epithelium, which could occur as result of prolonged exposure to refluxate, can contribute importantly to the pathogenesis of CRS.31 We hypothesize that acid, pepsin, trypsin, and bile contained in refluxate might directly injure sinonasal epithelium as it does esophageal mucosa, which would result in epithelial barrier dysfunction and allow micro-organisms to flourish on the surface of mucosa.32 Further studies are needed to test this hypothesis. If so, the compromised epithelium could induce a series of immune responses, including production of epithelial-derived cytokines, which contribute to both innate and adaptive type 2 inflammations through the activation of toll-like receptors.33 The resulting tissue remodeling could then be responsible for the development of rhinosinusitis as well as nasal polyps.34 Another reason that might put GERD patients at risk for GRS is that a vagal nerve-mediated reflex arc might exist between respiratory mucosa of nose and sinus cavity and the esophagus. Wong et al35 reported that esophageal parasympathetic receptors in GERD patients were hyper-responsive to different stimuli, such as acid provocation or mechanical distention. This phenomenon can be partially explained by increased expression of nerve growth factor36 and transient receptor potential vanilloid type 137. Such a esophageal-nasal reflex could be responsible for nasal congestion, excessive nasal secretions, and continuous postnasal drainage.22,35 Synergistically with other predisposing factors, neuropathic rhinitis could potentially contribute to the development of CRS in people with GERD. Still another possible reason for the increased risk of CRS in this population might be related to the association between GERD and eosinophilic esophagitis.38 This immune-mediated esophageal disease is characterized by esophageal eosinophilia resulting from overexpression of several pro-inflammatory mediators, including thymic stromal lymphopoietin,39 a key regulator in the development of CRS.40 In some cases, GERD occurs with this histologic feature of esophageal eosinophilia, and thus it may be that GERD can lead to CRS through a complex immunologic mechanism linking upper respiratory and esophageal mucosa.
Interestingly, after adjusting for demographic characteristics and selected comorbidities, this study found that patients with GERD were 2.48- and 1.85- times more likely than controls to have been subsequently diagnosed with CRS without nasal polyps and CRS with nasal polyps, respectively. As was found by the GHS study, the association between newly diagnosed GERD and risk of newly diagnosed CRS without nasal polyps was stronger than the association between GERD and CRS with nasal polyps. Given the multiplicity and complexity of the pathogeneses of GERD and CRS, consistent evidence addressing the phenomenon is limited. One possible reason for the difference in risk estimates is that the GERD's inflammatory profile resembles that of CRS without nasal polyps more closely than the profile of CRS with nasal polyps.10,41 It is also worth noting that the risk estimate values in the present study were even higher than those reported in the GHS study, especially for those with CRS without nasal polyps. These differences could be explained by differences in race and geographic region, but they could be related by how GERD was defined by the 2 studies. GHS's GERD cohort was selected based exclusively on practitioner-coded ICD-9 codes,14 whereas our study selected patients based on prescriptions of PPI, which we used as a surrogate for a diagnosis of GERD. In Taiwan, the National Health Insurance program covers the use of PPIs only for patients with reflux erosive esophagitis proven by panendoscopic examination. The duration of use depends on the severity of esophagitis. Prescription of PPIs to the nonerosive reflux disease is only indicated after esophageal pH-meter monitoring. However, this diagnostic methodology is not commonly utilized in the current medical practice in Taiwan. These differences in cohort selection may partially explain the differences in risks estimated by the two studies. This difference in selection would also result in difference the distributions of GERD subgroups. A greater proportion of the GERD population in our study may have had erosive esophagitis, and so macroscopic esophageal mucosal breach as well as the high-grade inflammation involved in this disease may contribute importantly to the development of CRS.32 A well-designed prospective study is needed to compare the effects on erosive and nonerosive reflux disease on the development of CRS with and without polyps.
This study has several limitations. One limitation was that we could not obtain information on the number and severity of symptoms as well as endoscopic findings from the administrative database we used, and thus it was not possible to determine the relationship between severity of GERD and CRS. Another limitation is that all GERD patients in this study were receiving PPI treatment, so it was not possible to determine whether the risk of developing CRS was modifiable by PPI treatment. In addition, we sampled the patients of the 2 diseases based on order of priority. Because both conditions require a period of time to develop, we could not exclude the coexistence or inverse timing sequence possibilities of the 2 conditions. Still another limitation is that we did not have access to information for some variables that contribute to propensity to CRS, such as exposure to cigarette smoke, air pollution, chemical particles, and vapors.15
CONCLUSIONS
In conclusion, patients with GERD in this population-based study were at greater risk of developing CRS, especially of CRS without nasal polys, compared with patients without GERD. Physician may want to keep the possibility of the development of CRS in mind when treating patients with GERD. Further studies may want to focus on the relationship between the types or severities of GERD and CRS.
Footnotes
Abbreviations: AD = atopic dermatitis, AR = allergic rhinitis, CI = confidence interval, CRS = chronic rhinosinusitis, CRSsNP = chronic rhinosinusitis without nasal polyps, CRSwNP = chronic rhinosinusitis with nasal polyps, EES(s) = extraesophageal syndrome(s), GERD = gastroesophageal reflux disease, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, 9th revision, Clinical Modification, LHID = Longitudinal Health Insurance Database, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, NHRI = National Health Research Institute, OM = otitis media, PPI = proton pump inhibitor, SD = standard deviation, SDT = specific diagnosis and treatment.
Authors’ contributions: Conception and design: L-YH, C-TS, L-YC; Administrative support: L-YC; Collection and assembly of data: All authors; Data analysis and interpretation: All authors; Manuscript writing: L-YH, L-YC; Final approval of manuscript: All authors.
This manuscript is original and it, or any part of it, has not been previously published; nor is it under consideration for publication elsewhere.
The authors report no conflicts of interest.
REFERENCES
- 1.El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2014; 63:871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fock KM, Talley NJ, Fass R, et al. Asia-Pacific consensus on the management of gastroesophageal reflux disease: update. J Gastroenterol Hepatol 2008; 23:8–22. [DOI] [PubMed] [Google Scholar]
- 3.Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; 101:1900–1920. [DOI] [PubMed] [Google Scholar]
- 4.Hom C, Vaezi MF. Extraesophageal manifestations of gastroesophageal reflux disease. Gastroenterol Clin North Am 2013; 42:71–91. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Sonnenberg A. Comorbid occurrence of laryngeal or pulmonary disease with esophagitis in United States military veterans. Gastroenterology 1997; 113:755–760. [DOI] [PubMed] [Google Scholar]
- 6.Poe RH, Kallay MC. Chronic cough and gastroesophageal reflux disease: experience with specific therapy for diagnosis and treatment. Chest 2003; 123:679–684. [DOI] [PubMed] [Google Scholar]
- 7.Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl 2012; 3: preceding table of contents, 1–298. [PubMed] [Google Scholar]
- 8.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg 2007; 137:S1–S31. [DOI] [PubMed] [Google Scholar]
- 9.Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol 2004; 114:155–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy 2006; 61:1280–1289. [DOI] [PubMed] [Google Scholar]
- 11.Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat 2014; 1–161. [PubMed] [Google Scholar]
- 12.Halawi AM, Smith SS, Chandra RK. Chronic rhinosinusitis: epidemiology and cost. Allergy Asthma Proc 2013; 34:328–334. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharyya N. Contemporary assessment of the disease burden of sinusitis. Am J Rhinol Allergy 2009; 23:392–395. [DOI] [PubMed] [Google Scholar]
- 14.Tan BK, Chandra RK, Pollak J, et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol 2013; 131:1350–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min JY, Tan BK. Risk factors for chronic rhinosinusitis. Curr Opin Allergy Clin Immunol 2015; 15:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandra RK, Lin D, Tan B, et al. Chronic rhinosinusitis in the setting of other chronic inflammatory diseases. Am J Otolaryngol 2011; 32:388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahdavinia M, Grammer LC., 3rd Chronic rhinosinusitis and age: is the pathogenesis different? Expert Rev Anti Infect Ther 2013; 11:1029–1040. [DOI] [PubMed] [Google Scholar]
- 18.Contencin P, Maurage C, Ployet MJ, et al. Gastroesophageal reflux and ENT disorders in childhood. Int J Pediatr Otorhinolaryngol 1995; 32 Suppl:S135–S144. [DOI] [PubMed] [Google Scholar]
- 19.Jecker P, Orloff LA, Wohlfeil M, et al. Gastroesophageal reflux disease (GERD), extraesophageal reflux (EER) and recurrent chronic rhinosinusitis. Eur Arch Otorhinolaryngo 2006; 263:664–667. [DOI] [PubMed] [Google Scholar]
- 20.Wong IW, Omari TI, Myers JC, et al. Nasopharyngeal pH monitoring in chronic sinusitis patients using a novel four channel probe. Laryngoscope 2004; 114:1582–1585. [DOI] [PubMed] [Google Scholar]
- 21.Loehrl TA, Samuels TL, Poetker DM, et al. The role of extraesophageal reflux in medically and surgically refractory rhinosinusitis. Laryngoscope 2012; 122:1425–1430. [DOI] [PubMed] [Google Scholar]
- 22.Vaezi MF, Hagaman DD, Slaughter JC, et al. Proton pump inhibitor therapy improves symptoms in post-nasal drainage. Gastroenurology 2010; 139:1887–1893. [DOI] [PubMed] [Google Scholar]
- 23.DiBaise JK, Olusola BF, Huerter JV, et al. Role of GERD in chronic resistant sinusitis: a prospective, open label, pilot trial. Am J Gastroenterol 2002; 97:843–850. [DOI] [PubMed] [Google Scholar]
- 24.Durmus R, Naiboglu B, Tek A, et al. Does reflux have an effect on nasal mucociliary transport? Acta Otolaryngol 2010; 130:1053–1057. [DOI] [PubMed] [Google Scholar]
- 25.DeConde AS, Mace JC, Smith TL. The impact of comorbid gastroesophageal reflux disease on endoscopic sinus surgery quality-of-life outcomes. Int Forum Allergy Rhinol 2014; 4:663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katle EJ, Hatlebakk JG, Steinsvåg S. Gastroesophageal reflux and rhinosinusitis. Curr Allergy Asthma Rep 2013; 13:218–223. [DOI] [PubMed] [Google Scholar]
- 27.Flook EP, Kumar BN. Is there evidence to link acid reflux with chronic sinusitis or any nasal symptoms? A review of the evidence. Rhinology 2011; 49:11–16. [DOI] [PubMed] [Google Scholar]
- 28.Cheng CL, Kao YH, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 2011; 20:236–242. [DOI] [PubMed] [Google Scholar]
- 29.National Health Research Institutes. National health insurance research database. 2007. Available at: http://nhird.nhri.org.tw/en/Data_Subsets.html Accessed May 12, 2015. [Google Scholar]
- 30.Schiöler L, Ruth M, Jõgi R, et al. Nocturnal GERD - a risk factor for rhinitis/rhinosinusitis: the RHINE study. Allergy 2015; 70:697–702. [DOI] [PubMed] [Google Scholar]
- 31.Tieu DD, Peters AT, Carter RG, et al. Evidence for diminished levels of epithelial psoriasin and calprotectin in chronic rhinosinusitis. J Allergy Clin Immunol 2010; 125:667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farré R. Pathophysiology of gastro-esophageal reflux disease: a role for mucosa integrity? Neurogastroenterol Motil 2013; 25:783–799. [DOI] [PubMed] [Google Scholar]
- 33.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol 2007; 19:711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Zhao C, Ji W, et al. Relationship of TLR2, TLR4 and tissue remodeling in chronic rhinosinusitis. Int J Clin Exp Pathol 2015; 8:1199–1212. [PMC free article] [PubMed] [Google Scholar]
- 35.Wong IW, Rees G, Greiff L, et al. Gastroesophageal reflex disease and chronic sinusitis: in search of an esophageal-nasal reflex. Am J Rhinol Allergy 2010; 24:255–259. [DOI] [PubMed] [Google Scholar]
- 36.Coffey CS, Mulligan RM, Schlosser RJ. Mucosal expression of nerve growth factor and brain-derived neurotrophic factor in chronic rhinosinusitis. Am J Rhinol Allergy 2009; 23:571–574. [DOI] [PubMed] [Google Scholar]
- 37.Yu X, Hu Y, Yu S. Effects of acid on vagal nociceptive afferent subtypes in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol 2014; 307:G471–G478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng E, Souza RF, Spechler SJ. Eosinophilic esophagitis: interactions with gastroesophageal reflux disease. Gastroenterol Clin North Am 2014; 43:243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noti M, Wojno ED, Kim BS, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med 2013; 19:1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boita M, Garzaro M, Raimondo L, et al. The expression of TSLP receptor in chronic rhinosinusitis with and without nasal polyps. Int J Immunopathol Pharmacol 2011; 24:761–768. [DOI] [PubMed] [Google Scholar]
- 41.Fitzgerald RC, Onwuegbusi BA, Bajaj-Elliott M, et al. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut 2002; 50:451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]


