Abstract
Bone morphogenic protein 4 (BMP-4) is a known pro-inflammatory and pro-atherogenic cytokine. Here, we investigated whether the serum BMP-4 level predicts coronary artery disease (CAD) severity in humans.
We measured serum BMP-4 concentrations in 1044 consecutive patients who underwent elective coronary angiography and percutaneous coronary intervention. CAD severity was estimated by the number of diseased vessels showing ≥50% diameter stenosis.
Among males, the serum BMP-4 level was significantly lower in patients with multivessel disease (MVD) compared with those with single-vessel disease (SVD) (16.3 ± 22.6 vs. 22.0 ± 28.4 pg/mL, P < 0.01). After adjustment for other cardiovascular risk factors, a high serum BMP-4 level was an independent predictor for a decreased risk of MVD (odds ratio, 0.992; 95% confidence interval [CI], 0.985–0.998; P = 0.01) and patients in the lower tertile were 1.55-fold more likely to have MVD compared with upper tertile patients. Receiver-operating characteristic curve analysis demonstrated that the serum BMP-4 level had a 54% sensitivity and 54% specificity for predicting MVD (area under the curve [AUC], 56.5%; 95% CI, 51.9–61.0%; P < 0.01). Serum BMP-4 improved the predictive capability of risk factors for MVD (AUC with and without BMP-4: 64.9 and 63.6%, respectively). Considering the likelihood ratio and number of parameters, adding the serum BMP-4 level provided a better-fit model for predicting MVD compared with the model consisting of conventional risk factors (likelihood ratio χ2 = 6.20, P = 0.01). However, an association between serum BMP-4 and CAD was not observed in females.
Serum BMP-4 levels are independently associated with CAD severity and contribute to discriminating CAD severity in males.
INTRODUCTION
Atherosclerosis is an inflammatory disease,1,2 associated with multiple risk factors and a complex pathophysiology. It is well known that vessel bifurcations or branched regions are prone to atherosclerosis.3–5 In these regions, a disruption in flow induces bone morphogenic protein-4 (BMP-4), which causes endothelial dysfunction and inflammation in a nuclear factor-κB- and nicotinamide adenine dinucleotide phosphate oxidase-dependent manner.6–8 However, inhibition of BMP-4 has been shown to restore endothelial function.9 BMP-2 and BMP-4 expression is increased in human atherosclerotic plaques and the overlying endothelium of early atherosclerotic lesions within the coronary artery.8,10 Moreover, in a diabetic animal model, hyperglycemia reportedly induced BMP-4 expression, resulting in vascular calcification.11
Although BMP-4 has been proposed as a proinflammatory and proatherosclerotic cytokine, previous in vitro or animal studies focused on the role of BMP-4 in early atherogenesis, such as initiation of endothelial dysfunction,7–9,12 and monocyte adhesion.7 There is a paucity of data regarding the association between serum BMP-4 level and the progression of atherosclerosis, especially in humans. We previously reported contradictory data on the association between serum BMP-4 level and atherosclerotic parameters in different study subjects. In a general population of individuals without diabetes, serum BMP-4 level was positively correlated with adiposity and insulin resistance,13 while it was inversely correlated with intima-media thickness and cardio-ankle vascular index in patients with type 2 diabetes.14 In a recent small study,15 serum BMP-4 levels were increased in subjects with chronic kidney disease and coronary artery disease (CAD) compared with those without CAD, but there was no difference among patients without chronic kidney disease. These human studies raise the possibility that BMP-4 might have a different role in the context of disease type or progression of atherosclerosis.
CAD is one of the leading causes of death worldwide,16 and there are many branching regions of the coronary arteries vulnerable to BMP-4 expression–related atherosclerosis. Therefore, we measured serum BMP-4 concentrations in subjects who underwent elective coronary angiography and investigated whether the serum BMP-4 level was associated with CAD severity and contributed to CAD discrimination in humans.
METHODS
Subjects and Coronary Angiography
We included 1044 consecutive subjects who underwent elective coronary angiography and percutaneous coronary intervention at Yeouido Saint Mary's Hospital (Seoul, Korea) between March 2007 and March 2009. We excluded patients with myocardial infarction defined as having an elevated troponin T level (>0.1 μg/L) with or without ST elevation, as well as patients who underwent a previous revascularization, who were on dialysis, had cancer, or had an infectious disease. Elective coronary angiography was performed in all subjects. CAD severity was estimated by the number of vessels showing ≥50% diameter stenosis on quantitative coronary analysis. We classified the subjects with CAD according to the number of diseased vessels into single-vessel disease (SVD) and multivessel disease (MVD) groups. All laboratory and clinical parameter data were obtained from a careful review of the patients’ medical records at index admission. This study protocol complied with the principles of the Declaration of Helsinki revised in 2000, and ethical approval was obtained from the Institutional Review Board of Yeouido Saint Mary's Hospital, the Catholic University of Korea (Seoul, Korea). Written informed consent was obtained from all subjects prior to participation.
Blood Sampling and Measurement of Serum BMP-4 Concentration
After an overnight fast, arterial blood was obtained via an arterial sheath just before the routine coronary angiography procedure. The serum was separated and stored at −70°C. Serum BMP-4 concentrations were measured using a Quantikine Human BMP-4 Immunoassay Kit (R&D Systems, Minneapolis, MN) with intra- and interassay coefficients of variation (CVs) of 5.3 and 5.8%, respectively. Serum BMP-4 measurements were performed in duplicate by researchers who were blinded to the coronary angiography results.
Statistical Analysis
Since the association between serum BMP-4 and CAD severity was significantly different in males and females, all analyses were performed separately by gender. All data are presented as means ± standard deviation (SD) or frequency and percentage. We compared the clinical characteristics and serum BMP-4 concentrations between the SVD and MVD groups using an independent t-test or χ2 test. One-way analysis of variance (ANOVA) with a post-hoc and χ2 test was used to compare clinical characteristics across the groups divided by serum BMP-4 level tertile. To estimate the association between serum BMP-4 level and CAD severity, we used multiple logistic regression analysis to discriminate MVD from SVD after adjustment for traditional cardiovascular risk factors defined as age, diabetes mellitus (DM), hypertension, smoking, LDL. We evaluated improvements in the discrimination after adding serum BMP-4 level to a model consisting of traditional cardiovascular risk factors with an increased area under the curve (AUC) in receiver-operating characteristic analysis. We assessed whether the serum BMP-4 level provided a better-fit model to discriminate CAD severity compared with known conventional risk factors using the likelihood ratio χ2 test for nested models. The results were considered statistically significant at P ≤0.05. All statistical analyses were performed using SPSS 19.0 and R statistical language v. 2.15.0.
RESULTS
Baseline Clinical Characteristics
The clinical characteristics of SVD and MVD are described in Table 1. Among males, MVD patients were older with a higher prevalence of DM. However, there were no significant differences in other laboratory or demographic parameters. Among females, MVD patients showed higher fasting blood glucose, hemoglobin (Hb) A1c, total cholesterol, and low-density lipoprotein (LDL) levels with a higher prevalence of DM. The serum BMP-4 concentration was significantly decreased in MVD compared with SVD patients among males (16.3 ± 22.6 vs. 22.0 ± 28.4 pg/mL, P < 0.01), but not among females (19.8 ± 25.2 vs. 18.4 ± 23.7 pg/mL, P = 0.57, Figure 1).
TABLE 1.
Clinical Characteristics of Patients With SVD or MVD
FIGURE 1.
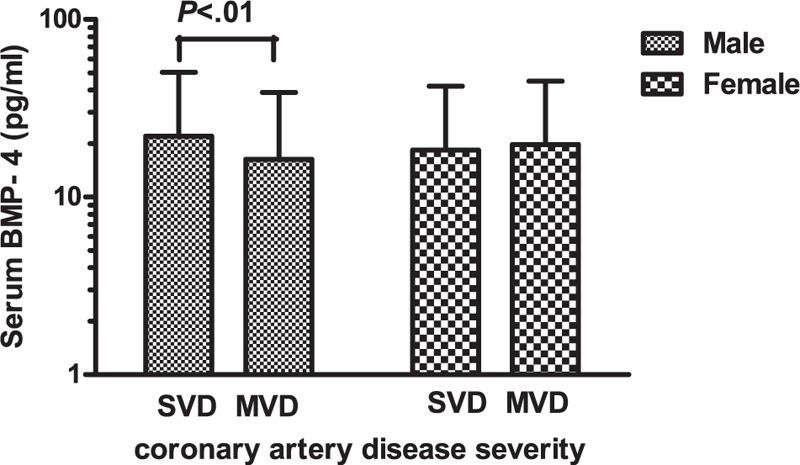
Serum BMP-4 levels grouped by coronary artery disease severity in males and females. Data are expressed as means ± standard deviation (SD). BMP-4 = bone morphogenic protein-4, MVD = multivessel disease, SVD = single-vessel disease.
Association of Serum BMP-4 With Cardiovascular Risk Factors
Since BMP-4 is a pro-inflammatory cytokine, we investigated the relationship between serum BMP-4 level and cardiovascular risk factors. As shown in Table 2, among males and females, there were no significant differences in age, prevalence of DM, hypertension, smoking status, creatinine, lipid profile, and inflammatory markers, such as white blood cell count and high-sensitivity C-reactive protein level, across the serum BMP-4 level tertiles.
TABLE 2.
Association Between Serum BMP 4 Tertile and Clinical Characteristics
Association Between Serum BMP-4 Concentration and CAD Severity
Table 3 shows that in males, age (odds ratio [OR], 1.038; 95% confidence interval [CI], 1.020–1.056, P < 0.01), DM (OR, 1.716; 95% CI, 1.210 –2.435, P < 0.01), and serum BMP-4 level (OR, 0.991; 95% CI, 0.985–0.997; P < 0.01) were predictors for MDV in univariate analysis. A high serum BMP-4 level was an independent predictor for a decreasing risk of MVD after adjusting for age, DM, hypertension, smoking, and LDL (OR, 0.992; 95% CI, 0.985–0.998; P = 0.01). Patients with a lower serum BMP-4 tertile showed a higher risk of MVD compared with upper tertile patients (OR, 1.556; 95% CI, 1.024–2.364; P = 0.03, Figure 2). However, there was no association between serum BMP-4 and CAD severity in females (data not shown).
TABLE 3.
Logistic Regression Analysis to Predict Multivessel Disease in Male
FIGURE 2.
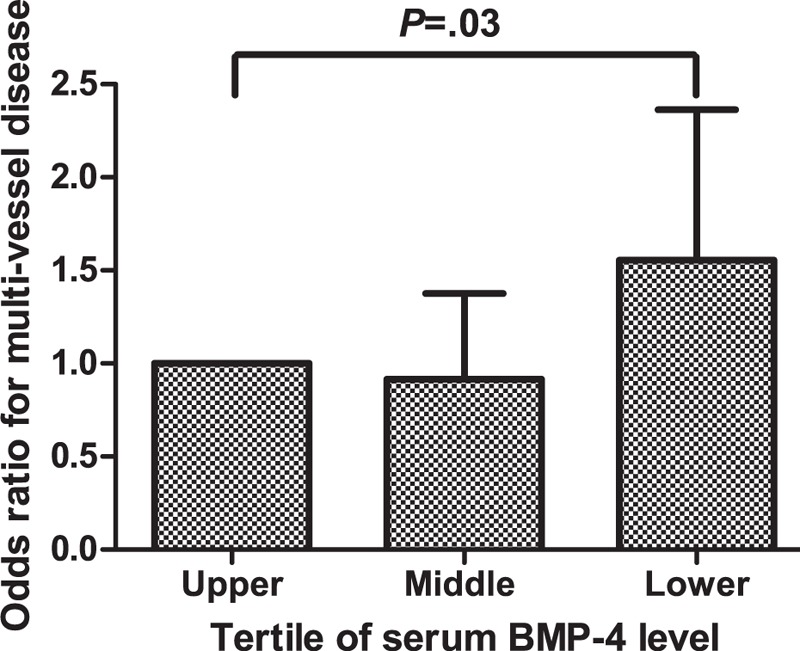
Adjusted odds ratios for multivessel disease by serum BMP-4 tertile level. The upper BMP-4 tertile level is the reference, and I bars represent the 95% confidence interval. BMP-4 = bone morphogenic protein-4.
Contribution of Serum BMP-4 Level in Discriminating CAD Severity in Males
Receiver-operating characteristic analysis determined that the serum BMP-4 level had a 56.5% AUC (95% CI, 51.9–61.0%, P < 0.01) with a 54% sensitivity and 54% specificity for predicting MVD (Figure 3A). In contrast, the model consisting of conventional risk factors such as age, DM, hypertension, smoking, and LDL showed a 63.6% AUC (95% CI, 59.1–68.0%, P < 0.01) with a 62% sensitivity and 60% specificity (Figure 3B). A combined model consisting of serum BMP-4 level and conventional risk factors showed a 64.9% AUC (95% CI, 60.4–69.3%, P < 0.01) with a 63% sensitivity and 61% specificity (Figure 3C). According to the likelihood ratio test, the combined model was a better-fit for predicting MVD compared with the model consisting of conventional risk factors only (likelihood ratio χ2 = 6.20, P = 0.01).
FIGURE 3.
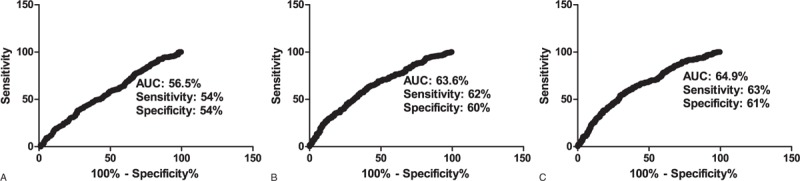
Receiver-operating characteristic curves of BMP-4, conventional risk factors, and combined model for predicting multivessel disease. BMP-4 = bone morphogenic protein-4.
DISCUSSION
In the present study, we evaluated a potential link between serum BMP-4 level and CAD severity. To our knowledge, this is the first study to demonstrate an association between serum BMP-4 level and CAD severity in a relatively large human study population. We found that the serum BMP-4 concentration significantly decreased in the MVD group compared with the SVD group. On multivariate analysis, the lower serum BMP-4 level was associated with MVD independently of traditional cardiovascular risk factors in males, while no association was observed in females.
Although BMP-4 signaling has been associated with endothelial dysfunction,7–9,12 monocyte adhesion,7 and foam cell formation,17 which is an early step in atherogenesis, it is unclear whether the atherogenic effect of BMP-4 signaling is maintained and actively involved in the progression of atherosclerosis. Kim et al18 recently reported that knockdown of the BMP receptor II (BMPR II) markedly elevated monocyte adhesion independently of BMP-4, and that BMPR II was down-regulated by pro-atherogenic risk factors such as disturbed flow or hypercholesterolemia. These results suggested a limited role for BMP-4 in atherogenesis.
The decrease in serum BMP-4 levels following progression of atherosclerosis observed in the current study can be explained in several ways. First, as Kim et al18 reported, BMP-4 is primarily involved in early atherogenesis, and the progression to advanced atherosclerosis might be dependent on other mechanisms. Second, irrespective of the atherogenic effect of BMP-4, its serum level might simply decrease as a result of BMP-4 deposition into the atherosclerotic vascular tissue as demonstrated in previous studies.8,10 Third, it also might be due to a possible inhibitory mechanism by BMP-4 antagonists such as gremlin, matrix Gla protein, follistatin, and noggin.6,11,19,20 Although we do not know the exact mechanisms underlying the decrease in serum BMP-4 during CAD progression, this might suggest that the active atherogenic role of BMP-4 in the progression to advanced CAD is not important.
We found that a model incorporating the serum BMP-4 level produced a better fit in the prediction of MVD compared with the model consisting only of established cardiovascular risk factors. The capability to predict MVD estimated by the AUC was also modestly improved from 63.6 to 64.9% by the addition of the serum BMP-4 level to the model consisting of conventional cardiovascular risk factors. Even though this improvement is modest, patients in the lower serum BMP-4 tertile were 1.55-fold more likely to have MVD compared with upper tertile patients.
In our study, there was no correlation between serum BMP-4 concentration and CAD severity in females. As a regulator of BMP-4 expression, estrogen facilitates osteoblast differentiation by up-regulating BMP-4 signaling21 and prevents cardiac hypertrophy by inhibiting BMP-4 expression in cardiomyocytes.22 Hence, in females, BMP-4 expression might be more influenced by this hormonal change than by the development of atherosclerosis. This sex-based difference in BMP-4 expression should be considered in future studies.
This study has several limitations. First, although we created a model to discriminate CAD according to severity, we could not validate our model in a different test set. Further studies are warranted in larger study populations. Second, because there were no data on the subjects without CAD, we could not estimate the role of serum BMP-4 level to predict CAD. Because previous human studies13,14,23 reported contradictory data in different clinical settings, it would be interesting to evaluate subjects with and without CAD. Third, no association was seen between serum BMP-4 level and CAD severity in females in the present study. However, there could be an association in menopausal females if this lack of an association is due to the effect of estrogen. We did not analyze postmenopausal females in this study due to the lack of information about menopausal status and the small number of females included.
In conclusion, here we demonstrated that serum BMP-4 concentration is independently associated with CAD severity and contributes to its discrimination.
Acknowledgment
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2012R1A1A207098) and the Grant from Daewoong Pharmaceutical Company (2014-21).
Footnotes
Abbreviations: AUC = area under the curve, BMP-4 = bone morphogenic protein 4, CAD = coronary artery disease, CI = confidence interval, DM = diabetes mellitus, HDL = high-density lipoprotein, LDL = low-density lipoprotein, MVD = multivessel disease, OR = odds ratio, PCI = percutaneous coronary intervention, SVD = single-vessel disease.
Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2012R1A1A207098) and the Grant from Daewoong Pharmaceutical Company (2014-21).
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2012R1A1A207098) and the Grant from Daewoong Pharmaceutical Company (2014–21). The granting agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
REFERENCES
- 1.Libby P. Inflammation in atherosclerosis. Nature 2002; 420:868–874. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med 1999; 340:115–126. [DOI] [PubMed] [Google Scholar]
- 3.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics: 2011 update: a report from the American Heart Association. Circulation 2011; 123:e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol 2004; 24:12–22. [DOI] [PubMed] [Google Scholar]
- 5.Zarins CK, Giddens DP, Bharadvaj BK, et al. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res Oct 1983; 53:502–514. [DOI] [PubMed] [Google Scholar]
- 6.Chang K, Weiss D, Suo J, et al. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries: role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation 2007; 116:1258–1266. [DOI] [PubMed] [Google Scholar]
- 7.Sorescu GP, Song H, Tressel SL, et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res 2004; 95:773–779. [DOI] [PubMed] [Google Scholar]
- 8.Sorescu GP, Sykes M, Weiss D, et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem 2003; 278:31128–31135. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Liu J, Tian XY, et al. Inhibition of bone morphogenic protein 4 restores endothelial function in db/db diabetic mice. Arterioscler Thromb Vasc Biol 2014; 34:152–159. [DOI] [PubMed] [Google Scholar]
- 10.Dhore CR, Cleutjens JP, Lutgens E, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol 2001; 21:1998–2003. [DOI] [PubMed] [Google Scholar]
- 11.Bostrom KI, Jumabay M, Matveyenko A, et al. Activation of vascular bone morphogenetic protein signaling in diabetes mellitus. Circ Res 2011; 108:446–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong WT, Tian XY, Chen Y, et al. Bone morphogenic protein-4 impairs endothelial function through oxidative stress-dependent cyclooxygenase-2 upregulation: implications on hypertension. Circ Res 2010; 107:984–991. [DOI] [PubMed] [Google Scholar]
- 13.Son JW, Kim MK, Park YM, et al. Association of serum bone morphogenetic protein 4 levels with obesity and metabolic syndrome in non-diabetic individuals. Endocr J 2011; 58:39–46. [DOI] [PubMed] [Google Scholar]
- 14.Son JW, Jang EH, Kim MK, et al. Serum BMP-4 levels in relation to arterial stiffness and carotid atherosclerosis in patients with Type 2 diabetes. Biomark Med 2011; 5:827–835. [DOI] [PubMed] [Google Scholar]
- 15.Stahls PF3rd, Lightell DJ, Jr, Moss SC, et al. Elevated serum bone morphogenetic protein 4 in patients with chronic kidney disease and coronary artery disease. J Cardiovasc Transl Res 2013; 6:232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics: 2014 update: a report from the American Heart Association. Circulation Jan 2014; 129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng J, Gao J, Li Y, et al. BMP4 enhances foam cell formation by BMPR-2/Smad1/5/8 signaling. Int J Mol Sci 2014; 15:5536–5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim CW, Song H, Kumar S, et al. Anti-inflammatory and antiatherogenic role of BMP receptor II in endothelial cells. Arterioscler Thromb Vasc Biol 2013; 33:1350–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun J, Zhuang FF, Mullersman JE, et al. BMP4 activation and secretion are negatively regulated by an intracellular gremlin-BMP4 interaction. J Biol Chem 2006; 281:29349–29356. [DOI] [PubMed] [Google Scholar]
- 20.Sweatt A, Sane DC, Hutson SM, et al. Matrix Gla protein (MGP) and bone morphogenetic protein-2 in aortic calcified lesions of aging rats. J Thromb Haemost 2003; 1:178–185. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto Y, Otsuka F, Takano-Narazaki M, et al. Estrogen facilitates osteoblast differentiation by upregulating bone morphogenetic protein-4 signaling. Steroids 2013; 78:513–520. [DOI] [PubMed] [Google Scholar]
- 22.Wang YC, Xiao XL, Li N, et al. Oestrogen inhibits BMP4-induced BMP4 expression in cardiomyocytes: a potential mechanism of oestrogen-mediated protection against cardiac hypertrophy. Br J Pharmacol 2014; doi: 10.1111/bph.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stahls PF3rd, Lightell DJ, Jr, Moss SC, et al. Elevated serum bone morphogenetic protein 4 in patients with chronic kidney disease and coronary artery disease. J Cardiovasc Transl Res 2013; 6:232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]


