Abstract
Cyclophilin A (CyPA), an oxidative stress-induced factor, was found to play an important role in the aneurysm formation. Our working hypothesis was that the plasma level of CyPA in ruptured intracranial aneurysm could predict the neurological outcome.
From 2011 to 2013, a total of 36 patients with ruptured saccular intracranial aneurysm were recruited in our study. Before coil embolization, we draw blood samples at the orifice of a culprit aneurysm and in the remote peripheral vein for measurements of the CyPA levels. We utilized the modified Rankin scale 30 days after aneurysm rupture as the outcome measure. Generalized linear models were used to estimate the adjusted odds ratios of the poor neurological outcome given the presence of high plasma level of CyPA.
The aneurysmal and venous CyPA levels were significantly associated with the initial clinical severity (P = 0.004 and 0.03, respectively) and 30-day outcome (P = 0.01 and 0.02, respectively). The aneurysmal CyPA levels modestly correlated with age and high Fisher grade (ρ = 0.39 and 0.41; P = 0.02 and 0.01, respectively). The aneurysmal CyPA levels strongly correlated with the venous counterpart (ρ = 0.89; P < 0.001). Patients with high levels of aneurysmal CyPA were 15.66 times (95% CI, 1.48–166.24; P = 0.02) more likely to have worse neurological outcome than those with the low levels after adjustment of the age, gender, and the documented confounding factors.
High plasma level of CyPA is a significant prognostic biomarker for poor neurological outcome in patients with ruptured intracranial aneurysm.
INTRODUCTION
Subarachnoid hemorrhage (SAH) resulting from rupture of intracranial aneurysms is a highly fatal condition with a mortality rate as high as 50% in the 1st month.1–3 Although vasospasm was the main cause of mortality and morbidity after aneurysm rupture, the CONSCIOUS-2 trial showed no significant effect of clazosentan, an endothelin receptor antagonist, on preventing vasospasm-related sequelae.4,5 These disappointing results might indicate complex and uncertain pathophysiology of the cerebral vasospasm in patients with SAH. In addition to vasospasm, established predisposing factors for poor clinical outcome after aneurysmal SAH include older age, female gender, hypertension, smoking, and larger amount SAH on admission computed tomography (CT).6–9 In addition, manifestation of both local and systemic inflammation has been shown to associate with worse clinical outcome.10,11
Cyclophilin A (CyPA), a specific secreted oxidative stress-induced factor, is ubiquitously distributed and abundantly expressed in vascular smooth muscle cells.12–14 In addition to multiple intracellular functions, such as immunophilins and components of the cell cycle,15 CyPA has extracellular roles in inflammatory diseases such as rheumatoid arthritis and atherosclerosis.13,16 The characteristic pathological features of an aneurysm are intense oxidative stress, inflammation, and apoptosis of smooth muscle cells.17 In an animal study of abdominal aortic aneurysm, Satoh et al18 showed that intracellular and extracellular CyPA, derived from vascular smooth muscle cells, plays an important role in the formation of an aneurysm. Furthermore, Piechota-Polanczyk et al19 found decreased tissue levels of CyPA in simvastatin-treated patients with abdominal aortic aneurysm. However, to our knowledge, the clinical implication of CyPA in ruptured intracranial aneurysmal microenvironment has not been investigated.
The purpose of our study was to investigate the associations between the neurological outcome and CyPA levels, measured both at the orifice of ruptured saccular intracranial aneurysm and in the remote peripheral vein. Our hypothesis was that manifestation of high plasma CyPA is a prognostic biomarker for worsening clinical course.
METHODS
Patients
The approval from the institutional ethics review board of Changhua Christian Hospital, Changhua, Taiwan was received for this study, which was performed in compliance with the Helsinki Declaration. The patients or next of kin gave written informed consent for participation. From 2011 to 2013, patients with the diagnosis of ruptured saccular intracranial aneurysms were prospectively recruited and treated with endovascular embolization. The exclusion criteria included histories of brain tumor, head trauma, ischemic stroke, or hematological disorders.
We documented a battery of confounding risk factors associated with aneurysm rupture, including age, gender, smoking, as well as histories of hypertension and diabetes mellitus. The clinical severity of SAH was evaluated by a neurologist (CSL, 18 years of clinical experience in neurology) with the Glasgow coma scale and modified Rankin scale on admission (day 0) and day 30 recorded, respectively.
CT of the Brain
Noncontrast CT and CT angiography of the brain were performed with a dual source CT system (SOMATOM Definition FLASH, Siemens Healthcare, Forchheim, Germany) with standardized protocols for all patients. The CT an giography was performed with antecubital injection of nonionic contrast material (Ultravist 370, Bayer-Schering HealthCare, Berlin, Germany) at flow rate of 4 mL/second using a power injector and the following scanning parameters: 132 mAs at 100 and 140 kV, rotation time 0.33 seconds, pitch 0.9, and slice acquisition 64 × 0.6 mm. Images were reconstructed with a slice thickness of 0.75 mm each 0.5 mm with a 512 × 512 matrix and individually adapted the field of view (130–150 mm). The delay between the injection of contrast and the start of the scan was set to 40 seconds. The source images of CT angiography were then transferred to a stand-alone workstation (Wizard, Siemens, Forchheim, Germany) and reconstructed with the volume rendering method. An interventional neuroradiologist (KWL, 18 years of clinical experience in neurointerventions) weighed the Fisher grade20 on the noncontrast brain CT images. A culprit aneurysm was determined based on the SAH distribution and aneurysm location found on CT angiograms.
Endovascular Procedures
Patients were transferred to our neuroangiography suite once the vital signs were stable. We performed a focused 3-dimensional rotational digital subtraction angiography of every culprit aneurysm identified on the CT images with an Axiom Artis zee biplane (Siemens Medical Solution, Erlangen, Germany) neuroradiologic angiography system. The standard endovascular procedures and adjunctive therapies were detailed in our previous study.21 In addition to recording the aneurysmal location, number, size, and morphology, the neuroradiologist gauged the vasospasm grade, on a scale of 0 to 4.22
For each patient, blood samples were drawn from a microcatheter at the orifice of the ruptured culprit intracranial aneurysm and in a remote peripheral vein. We used commercially available enzyme-linked immunosorbent assay kits (USCN Life Science, Wuhan, China) to gauge the aneurysmal and venous CyPA levels.
Statistical Analyses
We dichotomized the day-0 Glasgow coma scale scores at 13 and the day-30 modified Rankin scale score at 3. The Fisher and vasospasm grades of 3 or higher were categorized as high-grade abnormalities. Mann–Whitney U and Fisher exact tests were used to exam the differences between groups for the continuous and categorical variables, respectively. The Pearson Chi-squared test was used to assess the difference of dichotomous clinical severities among patients with different locations of the culprit aneurysm. The correlations between the plasma level of aneurysmal CyPA and the confounding factors were evaluated with the Spearman rank correlation coefficients. Optimal cutoff values for the plasma CyPA levels within 3 days after aneurysm rupture to predict the neurological outcome on day 30 were determined by constructing the receiver operating characteristic curves.
Generalized linear models were used to estimate the association between the high CyPA levels and dichotomous 30-day outcome after adjusting for potential confounding factors, including age, gender, hypertension, smoking, multiple aneurysms, aneurysm size, vasospasm, and Fisher grades. The results are given in odds ratios with 95% confidence intervals. Significance was assigned at 2-sided P value < 0.05. IBM SPSS Statistics (version 20; IBM Corp., Armonk, NY) and MedCalc (version 14.10.2; MedCalc Software, Mariakerke, Belgium) were used for the statistical analyses.
RESULTS
A total of 39 consecutive patients with ruptured saccular intracranial aneurysm underwent endovascular treatments in our neuroangiography suite. Excluding 3 patients (7.7%) due to inadequate blood sampling, we included 36 patients with 39 aneurysms found. Among the 36 patients, the mean age was 55 years (range 32–82) and 52.8% were female. We secured all the ruptured intracranial aneurysms with platinum detachable coils within 1 day after admission. Three patients (8.3%) died during the follow-up. All of our patients had no history of diabetes mellitus. The ruptured aneurysms occurred most commonly in the anterior and posterior communicating arteries (14 and 13 patients, 53.8% and 50.0%, respectively). The less common locations were middle cerebral artery, internal carotid artery, and vertebral artery (4, 3, and 2 patients, 11.1%, 8.3%, and 5.5%, respectively). Patients with different locations of the ruptured aneurysm did not show significant different dichotomous clinical severities on admission and day 30 (P = 0.13 and 0.12, respectively).
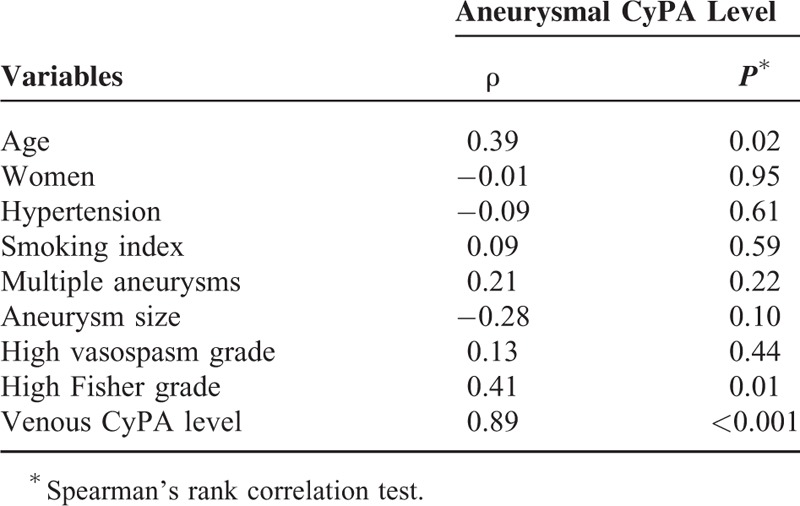
The ruptured intracranial aneurysms were generally small (median 5 mm; range 2–18). The median plasma CyPA levels were 62.5 ng/mL (range 26.0–147.0) and 77.1 ng/mL (range 15.7–182.0) at the orifices of the ruptured aneurysms and in the remote peripheral veins, respectively. Table 1 details the patient characteristics. The manifestation of higher aneurysmal and venous CyPA levels were significantly associated with worse initial clinical severity (P = 0.004 and 0.03, respectively) and 30-day outcome (P = 0.01 and 0.02, respectively). In the univariate analyses, the poor 30-day outcome was also significantly associated with high vasospasm grade and marginally associated with older age. Table 2 demonstrates a strong correlation between the aneurysmal CyPA level and the venous counterpart (ρ = 0.89; P < 0.001) as well as a moderate correlation between the aneurysmal CyPA level and age and high Fisher grade (ρ = 0.39 and 0.41; P = 0.02 and 0.01, respectively).
TABLE 1.
Patient Characteristics
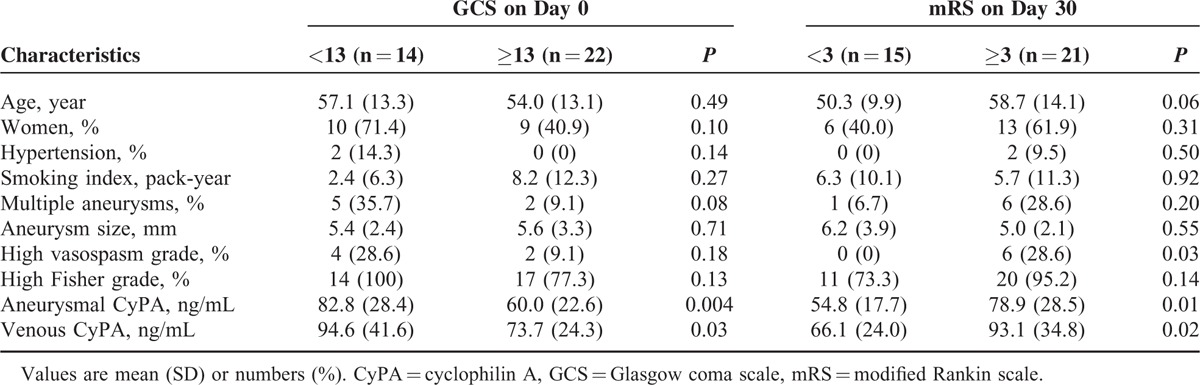
TABLE 2.
Correlations Between Aneurysmal cyclophilin A (CyPA) Level and Other Variables
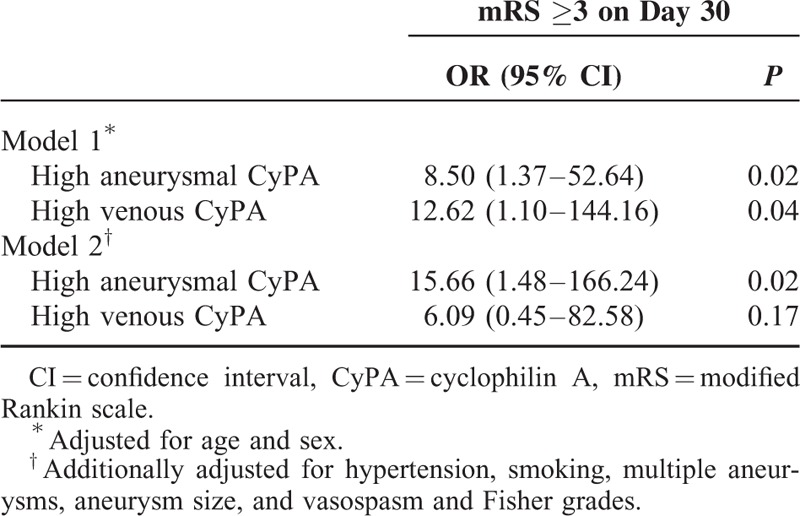
Receiver operating characteristic analyses showed cutoff values of 69.0 ng/mL (sensitivity 61.9%; specificity 86.7%; P = 0.002) and 85.8 ng/mL (sensitivity 52.4%; specificity 93.3%; P = 0.007) for the aneurysmal and venous CyPA levels, respectively, to predict poor 30-day outcome. In a generalized linear model, patients with high levels of aneurysmal CyPA were 15.66 times (95% confidence interval, 1.48–166.24; P = 0.02) more likely to have worse 30-day neurological outcome than those with the low levels after adjustment of the age, gender, and other confounding factors (Table 3).
TABLE 3.
Risk Factor-Adjusted Association Between High CyPA Levels and 30-Day Outcome
DISCUSSION
We discovered the association between high plasma levels of CyPA measured within 3 days after aneurysm rupture and worse 30-day neurological outcome. The aneurysmal CyPA levels showed a strong correlation with the corresponding venous CyPA levels while the venous levels were significantly higher than the aneurysmal counterpart. Moderate correlations were found between the aneurysmal CyPA and age and high Fisher grades.
The mechanisms involving the aneurysm formation and rupture include chronic inflammation and progressive destruction of the arterial wall, associated with premature senescence of vascular smooth muscle cells, oxidative stress, and increased local production of proinflammatory cytokines.23–25 In an animal study, Jin et al13 found that CyPA is secreted by vascular smooth muscle cells in response to reactive oxygen species, which feature cardiovascular disease states. Satoh et al18 demonstrated that the formation of abdominal aortic aneurysm in the angiotensin II-induced ApoE−/− mice model can be completely prevented against a CyPA−/− background. Furthermore, CyPA was found to associate with susceptibility of structural components of the aortic wall to aortic dissection in a mouse model.26 These findings approve the role of CyPA in the formation of an abdominal aortic aneurysm. However, the clinical implication of high CyPA level in the microenvironment of intracranial aneurysms has not been investigated in the English literature. Our results were the first to reveal the prognostic role of CyPA in patients with ruptured intracranial aneurysm.
In our study, the plasma level of CyPA was positively associated with age, a finding consistent with the results in a cohort of patients with diabetes mellitus and in a cohort with coronary artery disease.27,28 Furthermore, CyPA expression was found significantly higher in elderly rats than in young ones.29 Although not measured with the same laboratory testing kit nor in the same patient age group, the plasma level of CyPA (median 77.1 ng/mL) in our patients (mean age 55 years) with ruptured intracranial aneurysm was apparently higher than the levels in the cohort of coronary artery disease (4th quartile CyPA 17.5–50.9 ng/mL; median age 68 years).28 In addition, we found that patients with high Fisher grades had higher plasma level of CyPA than those with low grades. These findings may suggest an elevation of plasma CyPA after SAH although our patients were not recruited before rupture of the aneurysm and the baseline CyPA levels were not available. Histologically, ruptured intracranial aneurysms manifest significant endothelial damage and inflammatory cell invasion, which may exist before aneurysm rupture.30 Hence, the accumulation of plasma CyPA may be explained by increased excretion from activated inflammatory cells, such as diseased vascular smooth muscle cells, macrophage, and lymphocyte, either in the aneurysmal lumen or in the subarachnoid space.
In our results, the aneurysmal CyPA level was lower than the venous counterpart. Although it is conceivable to measure higher plasma levels of CyPA in patients with larger intracranial aneurysms, the association was not evident in our patient group. The mismatch might be contributed partly by the small aneurysm sizes in our patients and partly by the overwhelming inflammatory reaction in the relative large subarachnoid space. As a result, the plasma CyPA levels might more likely reflect the local and systemic inflammation than the aneurysm size.
This study's main strength was the simultaneous measurements of the plasma level of CyPA at the orifice of the ruptured saccular aneurysm and the venous counterpart to reveal the association between the inflammatory biomarker in the early phase of aneurysm rupture and neurological outcome. Our study had limitations. First, the patients in our study were relatively small in number, and the results were from a single medical center. Second, we excluded patients undergoing surgical clipping in order to eliminate a variety of surgical confounding factors. Third, we measured the plasma CyPA levels once within 3 days after aneurysm rupture and targeted to find early biomarkers to predict clinical course. Furthermore, we did not routinely assess imaging dynamics of the vasospasm and associated delayed cerebral ischemia. However, a large series showed that the occurrence of delayed cerebral ischemia does not contribute to worse outcomes.31
In conclusion, the early manifestation of focal and systemic inflammation, reflected by plasma CyPA levels, is associated with poor neurological outcome in patients with ruptured intracranial aneurysm. The result delineates a prognostic role for CyPA in the ruptured intracranial aneurysm and suggests CyPA as a potential target in treating aneurysmal SAH.
ACKNOWLEDGMENTS
The authors thank the patients who participated the study. This study was supported by Changhua Christian Hospital, Changhua, Taiwan (grants 102-CCH-IRP-065 and 103-CCH-IRP-064).
Footnotes
Abbreviations: CT = computed tomography, CyPA = cyclophilin A, SAH = subarachnoid hemorrhage.
C-SL and K-WL contributed equally to this work.
This study was supported by Changhua Christian Hospital, Changhua, Taiwan (grants 102-CCH-IRP-065 and 103-CCH-IRP-064).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Broderick JP, Brott T, Tomsick T, et al. The risk of subarachnoid and intracerebral hemorrhages in blacks as compared with whites. N Engl J Med 1992; 326:733–736. [DOI] [PubMed] [Google Scholar]
- 2.Brown RD, Whisnant JP, Sicks JD, et al. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke 1996; 27:373–380. [PubMed] [Google Scholar]
- 3.Kirkpatrick PJ. Subarachnoid haemorrhage and intracranial aneurysms: what neurologists need to know. J Neurol Neurosurg Psychiatry 2002; 73 Suppl 1:i28–i33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macdonald RL, Higashida RT, Keller E, et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol 2011; 10:618–625. [DOI] [PubMed] [Google Scholar]
- 5.Macdonald RL, Higashida RT, Keller E, et al. Randomised trial of clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid hemorrhage undergoing surgical clipping (CONSCIOUS-2). Acta Neurochir Suppl 2013; 115:27–31. [DOI] [PubMed] [Google Scholar]
- 6.Rose MJ. Aneurysmal subarachnoid hemorrhage: an update on the medical complications and treatments strategies seen in these patients. Curr Opin Anaesthesiol 2011; 24:500–507. [DOI] [PubMed] [Google Scholar]
- 7.Rosengart AJ, Schultheiss KE, Tolentino J, et al. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke 2007; 38:2315–2321. [DOI] [PubMed] [Google Scholar]
- 8.Juvela S. Prehemorrhage risk factors for fatal intracranial aneurysm rupture. Stroke 2003; 34:1852–1857. [DOI] [PubMed] [Google Scholar]
- 9.Rosenlrn J, Eskesen V, Schmidt K. Clinical features and outcome in females and males with ruptured intracranial saccular aneurysms. Br J Neurosurg 1993; 7:287–290. [DOI] [PubMed] [Google Scholar]
- 10.Dumont AS, Dumont RJ, Chow MM, et al. Cerebral vasospasm after subarachnoid hemorrhage: putative role of inflammation. Neurosurgery 2003; 53:123–133.discussion 133–125. [DOI] [PubMed] [Google Scholar]
- 11.Dhar R, Diringer MN. The burden of the systemic inflammatory response predicts vasospasm and outcome after subarachnoid hemorrhage. Neurocrit Care 2008; 8:404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Handschumacher RE, Harding MW, Rice J, et al. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science 1984; 226:544–547. [DOI] [PubMed] [Google Scholar]
- 13.Jin ZG, Melaragno MG, Liao DF, et al. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res 2000; 87:789–796. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki J, Jin ZG, Meoli DF, et al. Cyclophilin A is secreted by a vesicular pathway in vascular smooth muscle cells. Circ Res 2006; 98:811–817. [DOI] [PubMed] [Google Scholar]
- 15.Marks AR. Cellular functions of immunophilins. Physiol Rev 1996; 76:631–649. [DOI] [PubMed] [Google Scholar]
- 16.Jin ZG, Lungu AO, Xie L, et al. Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler Thromb Vasc Biol 2004; 24:1186–1191. [DOI] [PubMed] [Google Scholar]
- 17.Laaksamo E, Tulamo R, Liiman A, et al. Oxidative stress is associated with cell death, wall degradation, and increased risk of rupture of the intracranial aneurysm wall. Neurosurgery 2013; 72:109–117. [DOI] [PubMed] [Google Scholar]
- 18.Satoh K, Nigro P, Matoba T, et al. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat Med 2009; 15:649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piechota-Polanczyk A, Demyanets S, Nykonenko O, et al. Decreased tissue levels of cyclophilin A, a cyclosporine a target and phospho-ERK1/2 in simvastatin patients with abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2013; 45:682–688. [DOI] [PubMed] [Google Scholar]
- 20.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 1980; 6:1–9. [DOI] [PubMed] [Google Scholar]
- 21.Kao HW, Lee KW, Kuo CL, et al. Interleukin-6 as a Prognostic Biomarker in Ruptured Intracranial Aneurysms. PLoS One 2015; 10:e0132115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yalamanchili K, Rosenwasser RH, Thomas JE, et al. Frequency of cerebral vasospasm in patients treated with endovascular occlusion of intracranial aneurysms. AJNR Am J Neuroradiol 1998; 19:553–558. [PMC free article] [PubMed] [Google Scholar]
- 23.Kunieda T, Minamino T, Nishi J, et al. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation 2006; 114:953–960. [DOI] [PubMed] [Google Scholar]
- 24.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part II: animal and human studies. Circulation 2003; 108:2034–2040. [DOI] [PubMed] [Google Scholar]
- 25.Jones KG, Brull DJ, Brown LC, et al. Interleukin-6 (IL-6) and the prognosis of abdominal aortic aneurysms. Circulation 2001; 103:2260–2265. [DOI] [PubMed] [Google Scholar]
- 26.Fan LM, Douglas G, Bendall JK, et al. Endothelial cell-specific reactive oxygen species production increases susceptibility to aortic dissection. Circulation 2014; 129:2661–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramachandran S, Venugopal A, Kutty VR, et al. Plasma level of cyclophilin A is increased in patients with type 2 diabetes mellitus and suggests presence of vascular disease. Cardiovasc Diabetol 2014; 13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satoh K, Fukumoto Y, Sugimura K, et al. Plasma cyclophilin A is a novel biomarker for coronary artery disease. Circ J 2013; 77:447–455. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Rider DA, Ruan R. Identification of valid housekeeping genes and antioxidant enzyme gene expression change in the aging rat liver. J Gerontol A Biol Sci Med Sci 2006; 61:20–27. [DOI] [PubMed] [Google Scholar]
- 30.Kataoka K, Taneda M, Asai T, et al. Structural fragility and inflammatory response of ruptured cerebral aneurysms. A comparative study between ruptured and unruptured cerebral aneurysms. Stroke 1999; 30:1396–1401. [DOI] [PubMed] [Google Scholar]
- 31.Dorhout Mees SM, Molyneux AJ, Kerr RS, et al. Timing of aneurysm treatment after subarachnoid hemorrhage: relationship with delayed cerebral ischemia and poor outcome. Stroke 2012; 43:2126–2129. [DOI] [PubMed] [Google Scholar]


