Abstract
The purpose of this study was to determine the risk stratification of deep venous thrombosis (DVT) in patients undergoing gynecological surgery.
A retrospective study was conducted with a cohort of 739 consecutive female patients undergoing gynecological surgery between May 2008 and July 2013 in Beijing Chao-yang hospital. DVT of the leg was detected using complete compression and color Doppler ultrasound. Pulmonary embolism (PE) was diagnosed by computed tomography pulmonary angiogram (CTPA).
The overall incidence of DVT was 9.20% (68/739) in this patient population, including 16 (2.17%) symptomatic DVT and 52 (7.04%) silent DVT. A total of 66 (97.06%) DVT events were found within 7 days of surgery and 2 (2.94%) after 1 week. 94.82% thrombi were located in distal vein, and the rest 5.18% located in proximal and distal veins. Among the 68 patients with DVT, 46 patients with suspected PE received CTPA and 21 (45.65%) were confirmed with PE.
Six independent factors including varicose vein, bed rest time ≥48 h, length of operation ≥3 h, laparotomy surgery, hypertension, and age ≥50 years significantly increased the incidence of postoperative DVT on multivariate analysis. Patients with none risk factor are at low risk, with 1 or 2 risk factors are at moderate risk, and with ≥3 factors are at high risk of DVT.
The incidence of postoperative DVT and PE after gynecological surgery is high in patients with moderate or high-risk level. Noninvasive detection of DVT in 7 days after surgery is necessary because most patients showed no typical symptoms. Appropriate prophylaxis could be performed in patients at moderate or high risk of DVT.
INTRODUCTION
Venous thromboembolism (VTE), presenting as deep venous thrombosis (DVT) and pulmonary embolism (PE), remains a significant cause of morbidity and mortality in patients after operation. PE, mainly caused by DVT, is the leading cause for about approximately 10% hospital deaths and 40% deaths after gynecological operation.1,2 It is also potential preventable.
The incidence rate of DVT is about 10% to 40% in medical or general surgical patients without prophylaxis.1 With prophylaxis, the postoperative incidence of VTE was 1.14% in women with gynecological disease, and 0.7% in patients undergoing laparoscopic gynecological surgery, 0.3% in patients undergoing urogynecological surgery, and 4% in gynecological cancer patients, respectively.3–6 Most of published studies enrolled only symptomatic patients with DVT, whereas asymptomatic patients could be easily neglected under the absence of effective detection. In fact, approximately 50% of DVT patients are silent,7 so the actual incidence of postoperative DVT might be higher than reported. Our previous pilot prospective study found the postoperative incidence of DVT was up to 15.6%.8 The asymptomatic DVT has been confirmed to increase the development of post-thrombotic syndrome (PTS).9,10
Guidelines from American College of Chest Physicians (ACCP) and American College of Obstetricians and Gynecologists (ACOG) recommend appropriate prevention according to different risk level of postoperative VTE.1,11 Currently, few powerful evidence supports the risk stratification for patients undergoing gynecological surgery. Some reports determined several risk factors associated with postoperative VTE in patients with gynecological disease, such as body mass index (BMI) ≥30 or 40 kg/m2,12,13 operative time >180 minutes,13 cancer surgery, and blood transfusion ≥2000 mL.3 But they did not establish efficacious risk assessment based on these risk factors. While according to Caprini assessment, patients undergoing laparoscopic surgery for gynecological carcinoma are at high risk of VTE.7 But a multicenter study indicated that the prevalence of VTE after minimal invasive surgery for endometrial and cervical cancer was as low as 0.5%.14
So, we think these assessments of DVT may not be suitable for gynecological patients because of the existing differences in disease distribution and characteristics of patient population. Actually, in China, prophylaxis is not popular in most hospitals and only few selected patients with coronary heart disease or preoperative VTE receive prophylaxis. First, many doctors consider that the incidence of DVT is low in gynecological patients, but there are no relevant data. Second, many doctors do not use pharmacologic prophylaxis to avoid postoperative bleeding. Third, the mechanical prophylaxis is not popular in Chinese mainland because the equipment of IPC is relatively expensive for Chinese patients. Forth, prophylaxis increases the cost that will not be covered by medical insurance in China. Furthermore, we cannot directly follow the guideline from the United States or Europe because of the different characteristics of patient population. So, it is quite necessary to establish practical and feasible risk assessment for patients undergoing gynecological surgery. We embarked on this study to evaluate the incidence and potential risk factors of DVT and PE after gynecologic surgery, and establish the risk stratification of postoperative DVT.
MATERIAL AND METHODS
This retrospective study was approved by Ethics Committee of Beijing Chao-yang hospital. This retrospective study was approved by Ethics Committee of Beijing Chao-yang hospital. The medical records of a cohort of consecutive patients who underwent elective gynecological surgery without DVT protection from May 2008 to July 2013 were reviewed. All the patients underwent laparotomy or laparoscopic procedure for gynecologic disease and the duration of operation was >30 minutes. Meanwhile, they all received both lower extremity complete compression and color Doppler ultrasonography, the primary noninvasive test,2 within 7 days after operation, or within 28 days after operation when symptoms, such as leg pain, red and swollen or activity limitation, were complained.
Exclusion criteria were as follows: pregnant women; patients received any perioperative prophylaxis or anticoagulation with aspirin, warfarin, heparin, or low-molecular-weight heparin; patients had been confirmed with DVT or PE before surgery. All surgical procedures were performed by the members of gynecology department in our hospital.
With comprehensive review of the medical records, variables data were extracted: patients’ demographics including age, height, weight, and BMI; detailed information about medical history, diagnosis, comorbidities including hypertension, diabetes mellitus, and varicose vein were collected; information about the surgical procedure, length of operation, anesthesia method, volume of blood loss, and the length of postoperative immobility was extracted.
Ultrasound examination (American ACUSON12/XP or TCL-LD16000, 5.0–10.0 MHz) was performed by the designated experienced sonographers to assess proximal and distal venous thrombosis. The criteria of complete compression ultrasound followed this standard and the diagnosis of DVT was made when a vein is incompressible.15 Computed tomography pulmonary angiogram (CTPA), as a main diagnostic method for PE.16 The diagnosis of DVT and PE was made by 2 experienced radiologists.
Statistical Analysis
The Statistical Package for Social Science software (version 19.0, SPSS Inc, Chicago, IL) was used for all analyses. Continuous variables were expressed as mean and standard deviation. Student t test was used to evaluate the relationship between continuous variables. Pearson χ2 test or Fisher exact test was performed for categorical variables. Multivariate logistic regression was further performed among significant variables in univariate analysis. P value <0.05 indicates statistical significance.
RESULTS
Incidence and Characteristics of DVT
A total of 739 patients who met the criteria were enrolled in analysis including 148 malignant cases and 591 benign cases. There were 49 cases of ovarian cancer, 51 of endometrium cancer, 45 of cervical cancer, and 3 of vulva carcinoma. The indications of benign disease included benign ovarian tumor, myoma, endometriosis, adenomyosis et al. A total of 68 DVT events were identified out of 739 patients (9.20%).
The DVT incidence rate was significantly higher in malignant (40/148, 27.03%) than in benign disease (28/591, 4.73%, P < 0.001). Also, the DVT incidence was markedly higher in laparotomy group (50/286, 17.48%) than in laparoscopic group (18/453, 3.97%, P < 0.001).
Among the 68 postoperative DVT events, 97.06% occurred within 7 days of surgery. Thity-six (52.94%) events occurred within 3 days of surgery, 30 (44.12%) in 4 to 7 days, and 2 (2.94%) during 7 to 28 days. The median day of development of DVT was 3.8 ± 1.7 days (range 1–28 days).
Twenty-six cases (38.24%) were confined to the left leg, 25 (36.76%) were confined to the right leg, and 17 (25.0%) were confined to both lower limbs. 94.82% thrombi were located in distal vein, and the rest 5.18% in proximal (above the calf) and distal vein. In our study, the calf muscular vein was the most commonly involved vein.
Fifty-two (72.47%) DVT cases were asymptomatic, and 16 (23.53%) DVT patients had clinical manifestations including 12 patients experiencing leg swelling and 4 experiencing leg pain.
Incidence and Clinical Characteristics of PE
CTPAs were suggested to exclude PE in confirmed DVT or suspected PE patients. Forty-six patients underwent CTPA and 21 (45.65%) were diagnosed with PE finally. Among them, 9 of 25 (36.0%) were confirmed with PE in DVT patients with malignant tumor, and 12 of 21 (57.14%) were diagnosed with PE in patients with benign disease.
Fifteen (71.43%) PE patients showed no typical symptom, and 6 (28.57%) had clinical manifestations. Five of them presented with dyspnea and 1 presented with sudden syncope without leg symptoms in the first day after surgery. Then this patient received CTPA and confirmed acute PE and DVT.
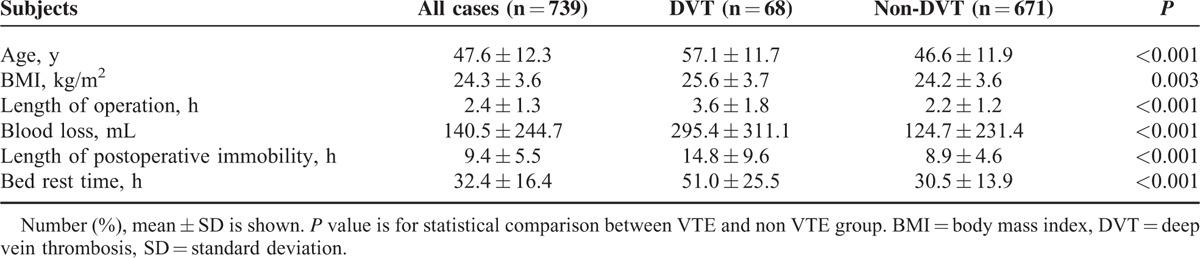
The demographic and the characteristic of this population were shown in Table 1. DVT group showed increasing age, higher BMI, prolonged duration of operation and anesthesia, more blood loss, and prolonged postoperative immobility.
TABLE 1.
Demographics and Clinical Characteristics of the Patients
Analysis of Potential Risk Factors for DVT
Thirteen potential risk factors were analyzed and the results of univariate analysis of risk factors associated with DVT were shown in Table 2. Preoperative variables including age ≥50 years (18.67% vs 2.13%, P < 0.001), BMI ≥25 kg/m2 (12.36% vs 7.32%, P = 0.022), varicose vein (29.17% vs 8.54%, P = 0.004), and hypertension (60.87% vs 6.63%, P < 0.001) were significant risk factors for DVT.
TABLE 2.
Evaluation of Potential Risk Factors Associated With DVT: Univariate Analysis
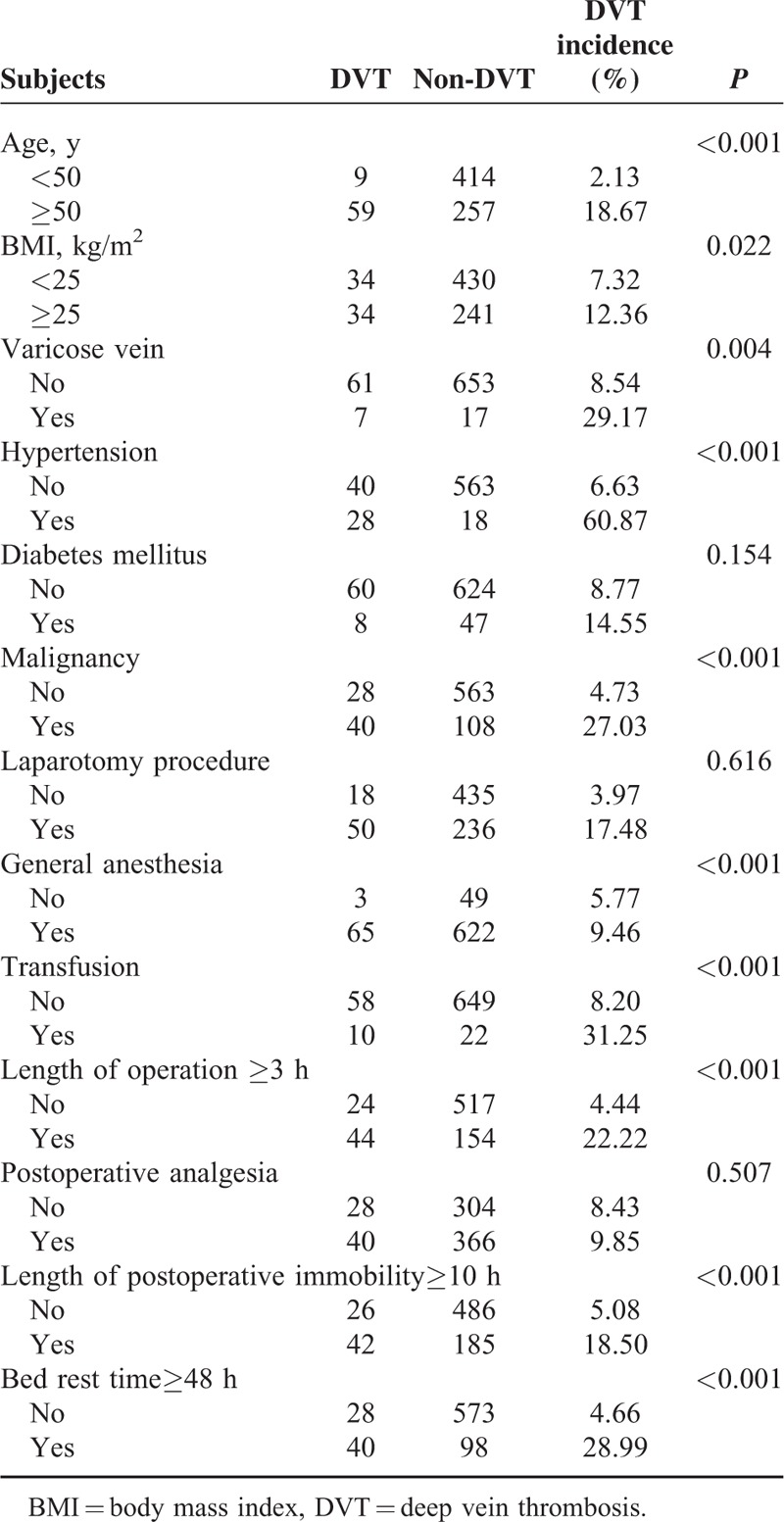
Six perioperative factors were also found to be significantly associated with DVT. They were malignancy (27.03% vs 4.73%, P < 0.001), laparotomy procedure (17.48% vs 3.97%, P < 0.001), length of operation ≥3 h (22.22% vs 4.44%, P < 0.001), transfusion (31.25% vs 8.20%, P < 0.001), length of postoperative immobility ≥10 h (18.5% vs 5.08%, P < 0.001), and bed rest for ≥48 h (28.99% vs 4.66%, P < 0.001).
But the 2 groups showed no significant differences in aspects such as history of diabetes mellitus (14.55% vs 8.77%, P = 0.154), having received general anesthesia or not (9.46% vs 5.77%, P = 0.616), and having received postoperative analgesia or not (9.85% vs 8.43%, P = 0.507).
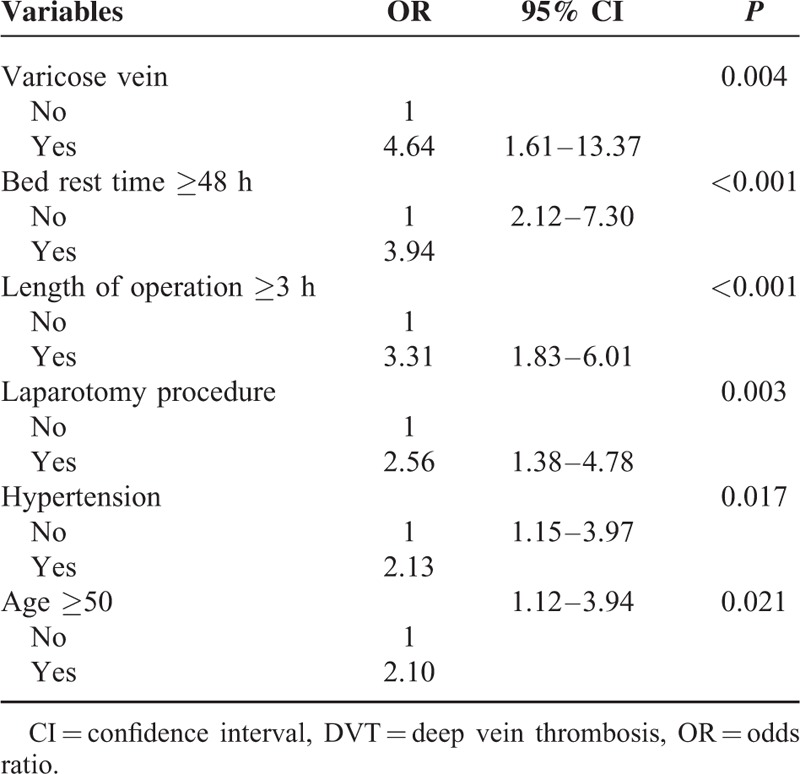
All the variables showing significant effect on DVT in univariate analysis were included in the multivariate forward stepwise logistic regression analysis. Table 3 provided the odds ratios and 95% confidence intervals. Varicose vein, bed rest for ≥48 h, length of operation ≥3 h, laparotomy surgery, hypertension, and age ≥50 years were independent risk factors for DVT.
TABLE 3.
Evaluation of Risk Factors for DVT: Multivariate Analysis Based on Logistic Regression
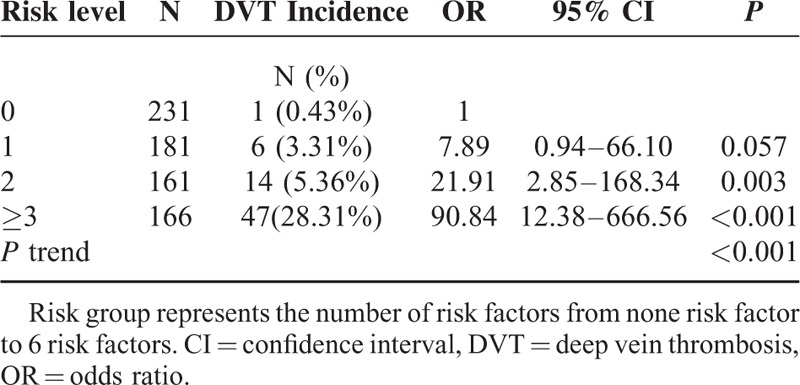
Based on the combination patterns of the above 6 independent risk factors for DVT, we proposed a predictive model to determine the risk of DVT (Table 5). In this model, patients with no risk factor had an incidence of DVT 0.43% (1/231). The incidence of DVT was 3.31% in single-risk-factor group, 5.36% in 2-factor group, 28.31% in ≥3-factor group. No mortality events were observed during the study.
TABLE 5.
Predictive Model of DVT Based on the Combination Patterns of the 5 Independent Risk Factors
DISCUSSION
In this study, we finally identified 68 (9.20%) cases with DVT in 739 patients with no thromboprophylaxis using ultrasonography. The incidence rate of symptomatic and asymptomatic DVT was 2.17% (16/739) and 7.04% (52/739), respectively.
The symptomatic postoperative DVT incidence was consistent with the previous study.17 We found that the DVT incidence is much higher than previous reports in patients with gynecological malignant tumor.6,18 Aside from different design of study, the patient population contributes to the discrepancy. Our study showed the incidence of silent DVT is over 3-fold of symptomatic DVT. The high detection rate of asymptomatic cases mainly benefited from the combination of compression and color Doppler ultrasound we employed, which may improve the reliability and precision of the results.
Unilateral or asymmetric swelling is the typical sign of DVT, which may persist for several weeks or months and may not disappear entirely.19 Other common symptoms and signs of DVT are leg edema, pain, erythema, fever, and leg warmth.18 Actually, most DVT patients are asymptomatic and only 9% to 17% of them show clinical manifestation.1 In this study, approximately one-fifth of DVT patients (23.53%) presented with lower limbs symptoms. Silent DVT accounts for a much higher proportion, which may result in secondary PE.
About 50% of patients with documented DVT are confirmed with PE and 70% PE patients are found to have asymptomatic venous thrombosis.20 Our study reconfirmed it, suggesting asymptomatic DVT should not be ignored.
The clinical features of PE include shock and hemodynamic instability,21 whereas for about one-fourth patients with PE, the initial clinical feature is sudden death.22 In a population-based study, 40.9% PE patients died within 7 days and 45.4% died within 30 days.23 PE, as a silent killer in hospital patients, should be identified as early as possible. Besides imaging examination, clinical features regarding signs or symptoms of DVT, cancer, dyspnea, increasing heart rate, and decreased blood oxygen saturation are important indicators.
On the time of development of postoperative DVT, our result is not consistent with previous report. Peedicayil et al6 found that 75% of VTE occurs 1 week after surgery and 36% after 4 weeks. Conversely, we observed that 97.06% DVT events occurred in the first week after surgery. Early DVT (days 0–7) is related with surgery, whereas late DVT (days 8–90) just reflects the period of recovery.6 Hence, we consider the first week after surgery is an important period to detect DVT.
Six independent risk factors associated with the development of DVT were identified: varicose vein, bed rest time ≥48 h, length of operation ≥3 h, laparotomy surgery, hypertension, and age ≥50 years. Based on our data, we believe that laparotomy surgery increases the risk of DVT through prolonging the length of bed rest time; yet laparosopic surgery is opposite. However, this is contrary to the assessment proposed by Caprini7, in which laparoscopic surgery increases the risk of DVT. Furthermore, we found that the risk of DVT increased with age (Table 4). Compared with patients younger than 50 years, the risk was 1.8-fold at 50 to 59 years and 3.0-fold at 60 years above.
TABLE 4.
Further Evaluation of Risk Factors Base on Age Group With Other Factors Unchanged
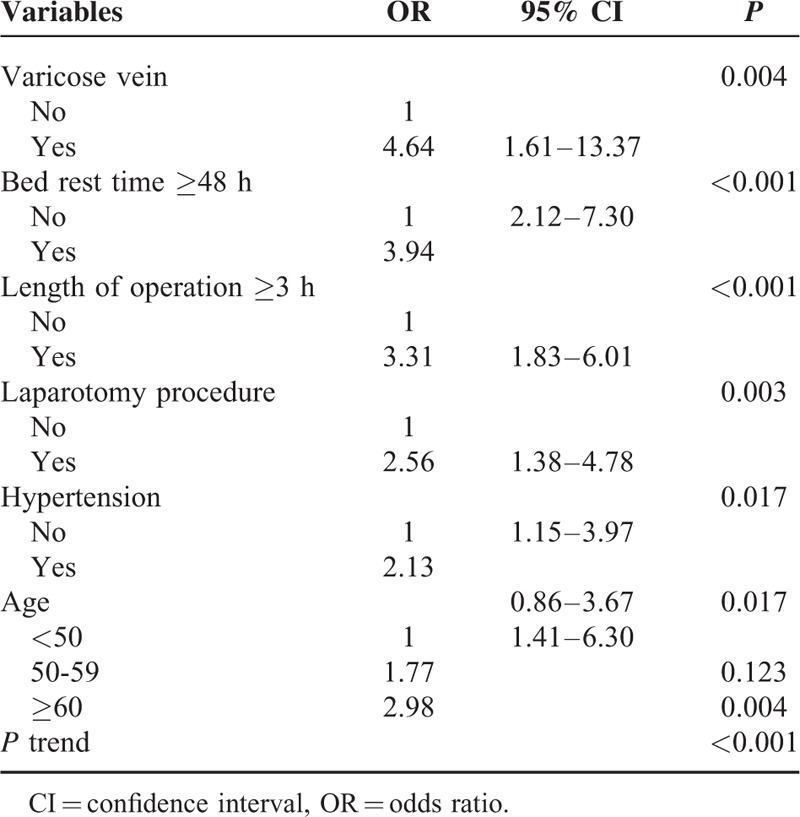
Interestingly, we found 4-fold risk of DVT among patients with varicose vein. As an independent risk factor, varicose vein also had been confirmed by Heit et al.24 In this population-based case-control study, they found that the VTE risk associated with varicose vein reduced with the increase of age, 4-fold at 45 years, 2-fold at 60 years, and 0.9 at 75 years. A study in orthopedic patients by Dua et al25 indicated a significant higher rate of DVT in patients with untreated previous varicose vein. Venous stasis and vessel damage resulted from valvular incompetency or abnormality of long saphenous vein may contribute to the development of DVT.25,26
For patients with varicose vein and additional risk factors, we should be alert for the occurrence of DVT, though controversies still exist regarding the fact that varicose vein increases the risk of VTE because of lack of evidence.
Cancer is deemed to be an independent risk factor for VTE, and the incidence of VTE increases 2 to 3 times in patients undergoing surgery for malignant tumor compared with those for benign disease.27–29 Our data showed the malignant group had significant higher incidence rate of postoperative VTE than benign group, but not an independent risk factor. Patients with malignant neoplasm usually experienced complicated surgery procedure, which directly leads to prolonged duration of operation and bed rest time. While the latter 2 directly increase the risk of postoperative DVT.
Based on the combination pattern of the 6 risk factors, we proposed the predictive model for postoperative DVT. Patients with none risk factor are at low risk of DVT, with 1 or 2 risk factors are at moderate risk, with ≥3 factors are at high risk. The incidence of postoperative PE increased markedly in patients with ≥3 risk factors. No PE event occurred in patients with none risk, 1 (1/181, 0.55%) in patients with single risk factor, 4 (4/161, 1.53%) in patients with 2 risk factors, and 16 (16/166, 9.64%) in patients with ≥3 risk factors. Patients at moderate or high-risk level of DVT need prophylaxis.
We evaluated the prevalence of DVT in this patient population based on the risk level of ACCP.1 The incidence of DVT in patients aged 40 to 60 years with no additional risk factor (moderate risk) and in patients aged 40 to 60 years with cancer (high risk) are 4.1% and 28.2%, respectively. It is consistent with our predictive model. But we consider more individual factors such as medical history and perioperative information such as hypertension, varicose vein, postoperative immobility, and bed rest time, which may obviously affect the development of DVT. In addition, compared with Caprini score,7 this model predicts the incidence of DVT more accurately. We determined a much lower incidence of DVT in patients undergoing laparoscopic surgery for malignancy compared with those undergoing laparotomy (21.1% vs 43.3%), whereas malignancy, major surgery, and laparoscopic surgery are identified as risk factors in Caprini assessment. However, another study also indicated low incidence in cancer patients undergoing laparoscopic surgery.14 Therefore, our predictive model may do better in gynecological patients. According to this risk stratification, we might be able to choose appropriate prophylaxis and suggest ultrasound scanning for DVT in moderate- or high-risk group.
Strengths and Limitations
Strengths of this study include the relative largest size of cohort in Chinese mainland female population and the focus on postoperative incidence of symptomatic and asymptomatic DVT and PE with no prophylaxis. However, this study has some limitations. Some incomplete medical records may limit the evaluation of potential risk factors of DVT and PE. In addition, not all patients with DVT received the test of CTPA, so the actual incidence of postoperative PE may be underestimated. Another potential limitation of this study is the short-term observation period, so that some DVT cases occurred later may be missed. A recent prospective research indicates that the risks for VTE still increase 7 to 12 weeks after surgery.30
Further research with long-term follow-up will be of greater clinical importance for assessing postoperative DVT and PE.
CONCLUSION
In conclusion, these results indicate that the postoperative DVT incidence is high in gynecological patients, and the proportion of silent DVT and PE is much higher than we expected. The first week after surgery is potential high-risk period of DVT.
Varicose vein, bed rest for ≥48 h, length of operation ≥3 h, laparotomy surgery, hypertension, and age ≥50 years are associated with postoperative DVT. Patients with none factor are at low risk, with 1 or 2 factors are at moderate risk, with ≥3 are at high risk. Prophylaxis should be implemented in patients with >2 risk factors.
Acknowledgement
We are very grateful to members of department of Obstetrics & Gynecology of Beijing Chao-yang hospital.
Footnotes
Abbreviations: ACCP = the American College of Chest Physicians, ACOG = American College of Obstetricians and Gynecologists, BMI = body mass index, 95%CI = 95%confidence interval, CTPA = computed tomography pulmonary angiogram, DVT = deep venous thrombosis, PE = pulmonary embolism, PTS = post-thrombotic syndrome, VTE = venous thromboembolism.
The authors declare no conflicts of interest.
REFERENCES
- 1.Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the seventh ACCP coference on antithrombotic and thromblytic therapy. Chest 2004; 33:8S–400S. [DOI] [PubMed] [Google Scholar]
- 2.Davis JD. Prevention, diagnosis, and treatment of venous thromboembolic complications of gynecologic surgery. Am J Obstet Gynecol 2001; 184:759–775. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki N, Yoshioka N, Ohara T, et al. Risk factors for perioperative venous thromboembolism: A retrospective study in Japanese women with gynecologic diseases. Thromb J 2010; 8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nick AM, Schmeler KM, Frumovitz MM, et al. Risk of thromboembolic disease in patients undergoing lapascopic gynecologic. Obstet Gynecol 2010; 116:1367–1374. [DOI] [PubMed] [Google Scholar]
- 5.Solomon ER, Frick AC, Paraiso MF, et al. Risk of deep venous thrombosis and pulmonary embolism in urogynecologic surgical patients. Am J Obstet Gynecol 2010; 203:510.e511–e514. [DOI] [PubMed] [Google Scholar]
- 6.Peedicayil A, Weaver A, Li X, et al. Incidence and timing of venous thromboembolism after surgery for gynecological cancer. Gynecol Oncol 2011; 121:64–69. [DOI] [PubMed] [Google Scholar]
- 7.Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon 2005; 51:70–78. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Zhang Z, Guo S, et al. Prospective investigation of postoperative lower extremity deep venous thrombosis in gynecological procedures. Chinese J Obstet Gynecol 2006; 41:107–110. [PubMed] [Google Scholar]
- 9.Wille-Jorgensen P, Jorgensen L, Crawford M. Asymptomatic postoperative deep vein thrombosis and the development of postthrombotic syndrome. A systematic review and meta-analysis. Thromb Haemost 2005; 93:236–241. [DOI] [PubMed] [Google Scholar]
- 10.Persson LM, Lapidus LJ, Larfars G, et al. Asymptomatic deep venous thrombosis is associated with a low risk of post-thrombotic syndrome. Eur J Vasc Endovasc Surg 2009; 38:229–233. [DOI] [PubMed] [Google Scholar]
- 11.Committee on Practice Bulletins—Gynecology, American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 84: prevention of deep vein thrombosis and pulmonary embolism. Obstet Gynecol 2007; 110:429–440. [DOI] [PubMed] [Google Scholar]
- 12.Abu Saadeh F, Norris L, O’Toole S, et al. Venous thromboembolism in ovarian cancer: incidence, risk factors and impact on survival. Eur J Obstet Gynecol Reprod Biol 2013; 170:214–218. [DOI] [PubMed] [Google Scholar]
- 13.Sabdadi S, Lee S, Walter A, et al. Incidence of venous thromboembolism after minimally invasive surgery in patients with newly diagnosed endometrial cancer. Obstet Gynecol 2012; 120:1077–1083. [DOI] [PubMed] [Google Scholar]
- 14.Kumar S, Al-Wahab Z, Sarangi S, et al. Risk of postoperative venous thromboembolism after minimally invasive surgery for endometrial and cervical cancer is low: a multi-institutional study. Gynecol Oncol 2013; 130:207–212. [DOI] [PubMed] [Google Scholar]
- 15.Schellong SM, Schwarz T, Halbritter K, et al. Complete compression ultrasonography of the leg veins as a single test for the diagnosis of deep venous thrombosis. Thromb Haemost 2003; 89:228–234. [PubMed] [Google Scholar]
- 16.Perrier A, Roy PM, Sanchez O, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med 2005; 352:1760–1768. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda M, Kan-no H. Hayashi M,et al. Predicting perioperative venous thromboembolism in Japanese gynecological patients. PloS One 2014; 9:e89206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santoso JT, Evans L, Lambrecht L, et al. Deep venous thrombosis in gynecological oncology: incidence and clinical symptoms study. Eur J Obstet Gynecol Reprod Biol 2009; 144:173–176. [DOI] [PubMed] [Google Scholar]
- 19.Line BR. Pathophysiology and diagnosis of deep venous thrombosis. Semin Nuclear Med 2001; 90–101. [DOI] [PubMed] [Google Scholar]
- 20.Hirsh J, Hoak J. Management of deep vein thrombosis and pulmonary embolism. A statement for healthcare professionals. Council on Thrombosis (in consulation with the Council on Cardiovascular Radiology), American Heart Association. Circulation 1996; 93:2212–2245. [DOI] [PubMed] [Google Scholar]
- 21.Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet 2012; 379:1835–1846. [DOI] [PubMed] [Google Scholar]
- 22.Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heit JA, Silverstein MD, Mohr DN, et al. Predictors of survival after deep vein thrombosis and pulmonary embolism a population-based cohort study. Arch Intern Med 1999; 159:445–453. [DOI] [PubMed] [Google Scholar]
- 24.Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism. Arch Intern Med 2000; 160:809. [DOI] [PubMed] [Google Scholar]
- 25.Dua A, Neiva S, Sutherland A. Does previous varicose vein surgery alter deep vein thrombosis risk after lower limb arthroplasty? Orthop Surg 2012; 4:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomson H. The surgical anatomy of the superficial and perforating veins of the lower limb. Ann R Coll Surg Engl 1979; 61:198–205. [PMC free article] [PubMed] [Google Scholar]
- 27.Fimognari FL, Repetto L, Moro L, et al. Age, cancer and the risk of venous thromboembolism. Crit Rev Oncol Hematol 2005; 55:207–212. [DOI] [PubMed] [Google Scholar]
- 28.Heit JA, Mohr DN, Silverstein MD, et al. Predictors of recurrence after deep vein thrombosis and pulmonary embolism. Arch Intern Med 2000; 160:761–768. [DOI] [PubMed] [Google Scholar]
- 29.Anderson FA, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107 (23 Suppl 1):I9–16. [DOI] [PubMed] [Google Scholar]
- 30.Sweetland S, Green J, Liu B, et al. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ 2009; 339:b4583. [DOI] [PMC free article] [PubMed] [Google Scholar]


