Abstract
Many epidemiologic studies indicate a potential association between fruit and vegetable intake and various cancers. The purpose of this meta-analysis is to investigate the association between citrus fruit intake and esophageal cancer risk. The authors conducted a comprehensive search on PubMed, EMBASE, and the Cochrane Library from inception until July 2014. Studies presenting information about citrus intake and esophageal cancer were analyzed. The authors extracted the categories of citrus intake, study-specific odds ratio or relative risk, and the P value and associated 95% confidence intervals for the highest versus lowest dietary intake of citrus fruit level. The association was quantified using meta-analysis of standard errors with a random-effects model. Thirteen case–control studies and 6 cohort studies were eligible for inclusion. Citrus intake may significantly reduce risk of esophageal cancer (summary odds ratio = 0.63; 95% confidence interval = 0.52–0.75; P = 0), without notable publication bias (intercept = −0.79, P = 0.288) and with significant heterogeneity across studies (I2 = 52%). The results from epidemiologic studies suggest an inverse association between citrus fruit intake and esophageal cancer risk. The significant effect is consistent between case–control and cohort studies. Larger prospective studies with rigorous methodology should be considered to validate the association between citrus fruits and esophageal cancer.
INTRODUCTION
Esophageal cancer, including squamous cell carcinoma (SCC) and esophageal adenocarcinoma (EAC), is a serious malignancy with a poor prognosis in the majority of cases.1,2 SCC is the predominant form of esophageal carcinoma worldwide, but a shift in epidemiology has been seen in some countries and regions like Australia, UK, USA, and western Europe, where the incidence of EAC has exceeded that of SCC.3 Every year, >450,000 people worldwide are diagnosed with esophageal cancer and the incidence is rapidly increasing.3,4 It is the eighth most common cancer, and the sixth most common cause of cancer-related deaths worldwide with developing nations making up >80% of total cases and deaths.5,6 The mortality from these cancers is high and the response to treatments during advanced stages is poor, so effectively reducing the chances of exposure to relative risk factors will have an important impact on the incidence of esophageal cancer.
Cigarettes, red meat, alcohol, hot tea, pickled vegetables, low intake of fresh fruits and vegetables, and low socioeconomic status are associated with a higher risk of SCC.7–10 Barrett esophagus is clearly recognized as a risk factor for EAC, with other factors including gastroesophageal reflux disease, acid-suppressive medication use, obesity, tobacco use, and processed meat.11–14 Some foods can reduce the incidence of esophageal cancer.9,15–18 Many researchers conducted meta-analyses on diet and esophageal cancer. The study by Coleman et al19 suggested that dietary fiber may protect against esophageal carcinogenesis, especially esophageal adenocarcinoma. Zhu et al20 found that meat consumption is associated with the risk of esophageal cancer. The intake of red meat is likely to increase the esophageal SCC risk and the processed meat may increase esophageal adenocarcinoma risk; however, the consumption of fish may not be associated with esophageal cancer incidence. This phenomenon may be explained by the effects of various micronutrients such as folate, B vitamins, antioxidants, lutein, and carotenoids.21–24
Citrus fruits include oranges, tangerines, grapefruits, lemons, and limes. They include several components, including flavonoids, folate, carotenoids, and vitamin C,25-26 which have protective effects against cancer. Previous studies have suggested that citrus intake may improve the incidence of various cancers including pancreatic, breast, and prostate cancers.27–29 Consequently, we hypothesize that citrus intake is associated with a reduced risk of esophageal cancer. Epidemiologic evidence from cohort and case–control studies on this association has not yet been summarized. Therefore, we conducted a meta-analysis to explore this hypothesis.
STUDY CHARACTERISTICS
Search Strategy
A computerized search of the English language literature on citrus fruits and esophageal cancer yielded no relevant publications from inception to July 2014. We, therefore, decided to use the key words “fruit” and “citrus.” The search terms were ([esophagus] OR [esophageal]) AND ([cancer] OR [tumor] OR [carcinoma]) AND (‘citrus’itrus OR ‘fruit’ruits). We limited the search to human adults without language restrictions. We searched the 3 major electronic databases: PubMed, EMBASE, and The Cochrane Library. Additionally, we reviewed the references from retrieved articles for additional studies. Furthermore, ethical approval was not necessary because our article is a review.
Study Selection
The included studies29 had to be epidemiologic studies such as case–control and cohort studies. The studies concerning human that addressed the association between citrus intake and incidence of esophageal cancer were collected; however, if the study provides no original data or insufficient information on the odds ratio (OR) or relative risk (RR), and their corresponding 95% confidence intervals (CIs), we excluded it. The studies not measuring the intake of citrus fruits or citrus juice at the individual level are not eligible. The instrument of assessment of citrus intake is questionnaire. Two independent reviewers read the abstracts or full-text articles to assess the eligibility of studies in a standardized manner. We resolved the disagreement by consensus.
Data Abstraction
We extracted important information from all eligible studies. They included study design, country of origin, years of publication, origin of control, number of cases and control, sex distribution, types of citrus fruits, types of cancer, comparison of exposure level, and potential confounding variables adjusted. The estimates of OR/RR, their associated 95% CIs, and P values were also extracted by us. If separate researches based on the same population were published, we selected the article containing more complete information for inclusion.
Statistical Analyses
We extracted the study specific OR/RR and 95% CIs for highest versus lowest intake of citrus fruits from every study. And we calculated the standard error (SE) of the log OR/RR by using the following equation: SE = (ln[OR/RR_upper − ln OR/RR_lower]) ÷ 3.92. Then, we summarized the overall OR and CI by using general variance-based methods30 of RevMan 5.0. For studies that provided OR/RR by cancer subtypes,15,31 we used a random-effects model to obtain a pooled estimate from the individual study (Table 1). We adopted the Newcastle-Ottawa Scale to evaluate research quality and defined them as high, middle, and low quality by score 7 to 9, 4 to 6, 1 to 3, respectively. The Grades of Recommendation, Assessment, Development, and Evaluation working group system of rating quality of evidence also were used to evaluate the research quality.
TABLE 1.
Logarithmic OR or RR (Log[OR/RR]) and Its SE for the Meta-Analysis
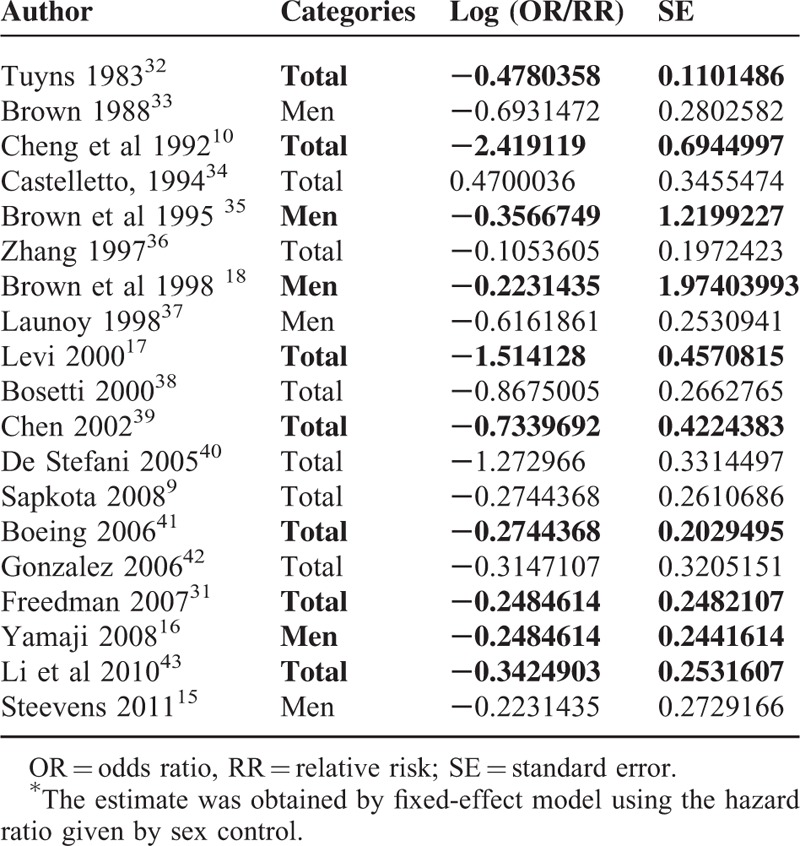
The value of I2 was used to evaluate the extent of heterogeneity derived from study differences rather than chance.44 The smaller value I2 suggested less obvious heterogeneity. We used the random-effects model to calculate the summary OR and its 95% CI45 with suspecting heterogeneity. We evaluated the impact of the changes on pooled ORs by study design, cancer subtypes, geographical location, source of controls, research quality, and some adjusted confounders such as alcohol and body mass index as prior hypotheses to explain heterogeneity through subgroup analyses and meta-regression analyses. Sensitivity analyses were conducted by removing 1 study from all studies to evaluate the impact on the pooled ORs and heterogeneity. We can, therefore, evaluate whether the results are stable. In an attempt to detect publication bias, we visually examined asymmetry in a funnel plot. We conducted Begg and Egger test to assess whether there is an obvious publication. We considered the funnel plot to be asymmetrical if the intercept of the regression line deviated from zero with P < 0.10. If the test suggests an obvious publication bias, we would conduct the trim and fill analysis to further verify.
We used the Cochrane Collaboration software (Oxford, UK) to analyze the extracted data with fixed or random-effects model analysis.46 STATA (StataCorp, College Station, TX) was used to conduct the Egger and Begg regression asymmetry test by using the metabias command.47 We conducted the trim and fill analysis to observe whether the results are stable and evaluate the publication bias.
RESULTS
Search Results
The computerized search yielded 433 references, of which 112 were included after abstract review. Citation search identified another 715 articles. Of the 827 articles that were obtained for full-text review, we excluded 808 articles based on the exclusion criteria. In particular, the result of Tuyns et al48 published in 1987 was replaced by Tuyns et al32 published in 1983, as it shared the same database. The result of De Stefani et al49 published in 2003 was replaced by De Stefani et al40 published in 2005, as the latter expanded the sample size based on the former population.
A total of 19 articles were included in the meta-analysis, including 6 cohort studies15,16,61,41-43 and 13 case–control studies9,10,17,18,32-39 (Figure 1).
FIGURE 1.
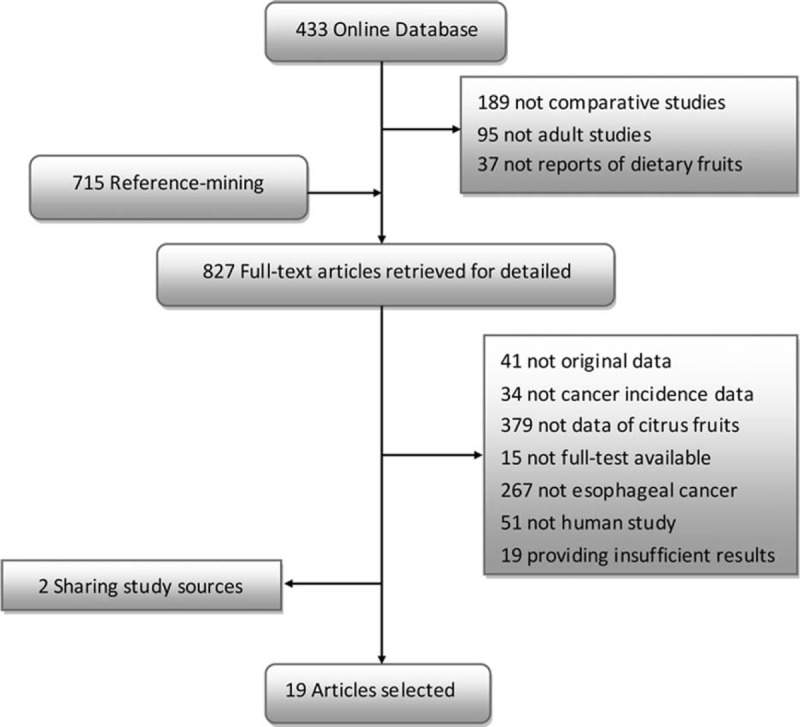
Flowchart of the searching and review of literatures.
Study Characteristics
Some details of the selected studies are shown in Tables 2 and 3. All articles were published in English. Six studies were conducted among residents of the United States,18,31,33,35,36,39 1 in Italy,38 2 in Japan,16,43 2 in France,32,37 3 in Europe,9,41,42 and the remaining 5 in China,10 Argentina,34 Switzerland,17 Uruguay,32 and the Netherlands.15 Two of the studies recruited participants in the 1980s, 5 in the 1990s, and 12 between 2000 and 2011.
TABLE 2.
Summary of Case–Control Studies Included in the Meta-Analysis
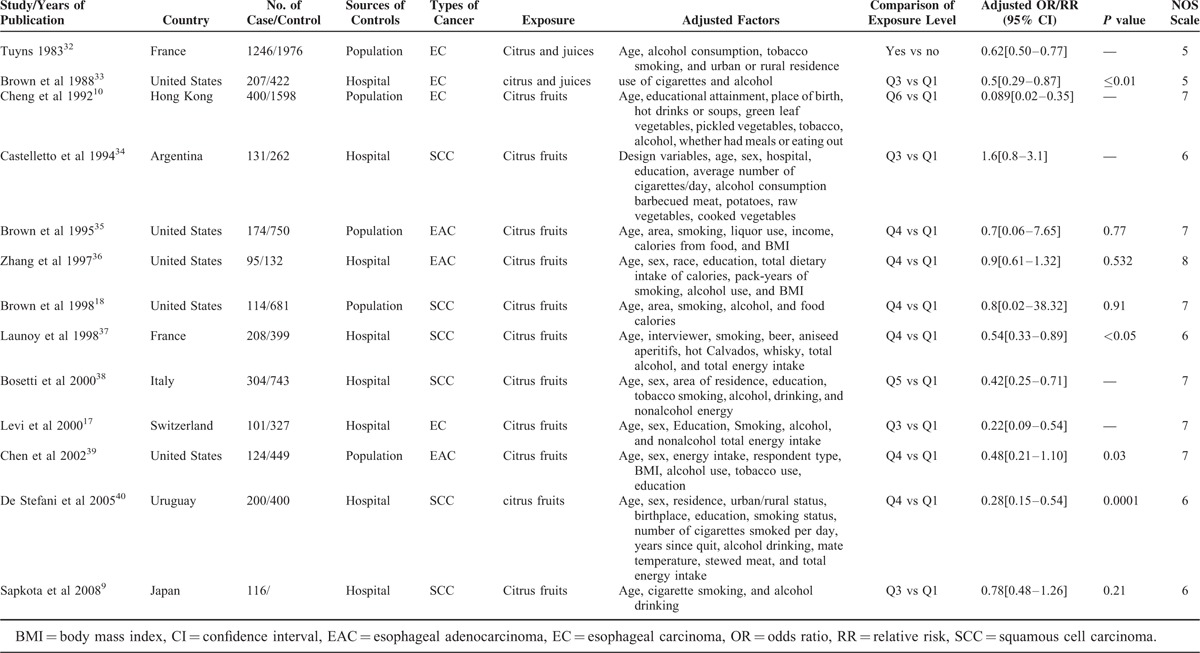
TABLE 3.
Summary of Cohort Studies Included in the Meta-Analysis
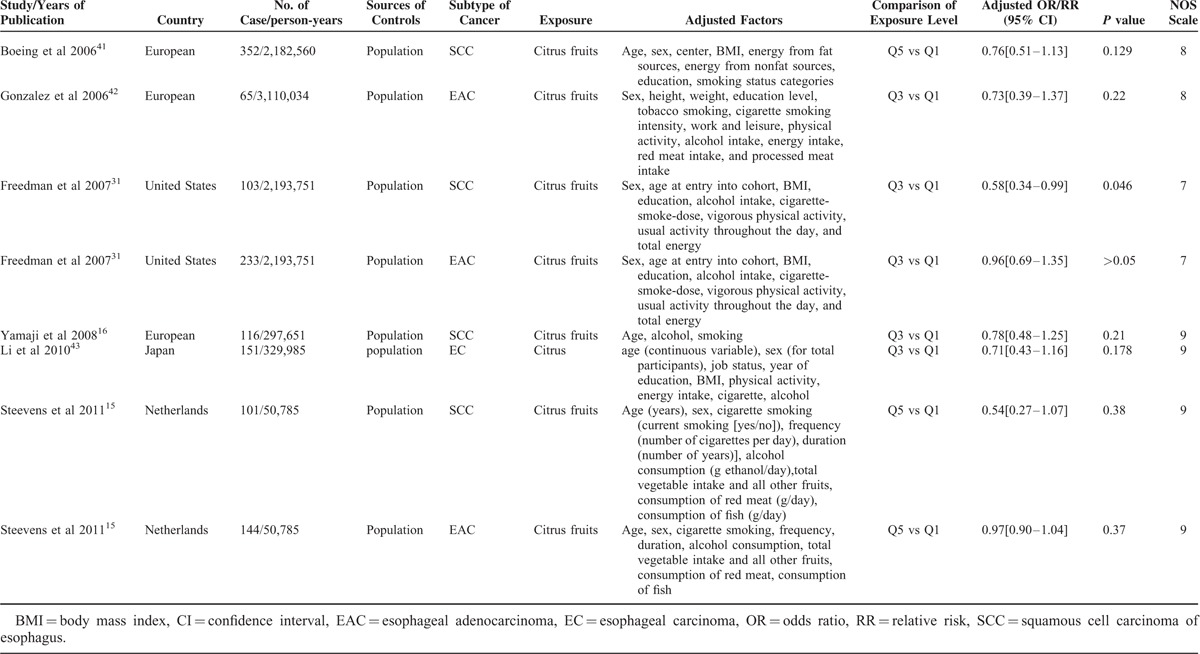
The factor of age was adjusted in all of the studies except Brown et al50 The confounding variables that were adjusted in different studies were presented in detail in Tables 2 and 3. For all of the studies, the relationship between intake of citrus fruits and esophageal cancer was not primary hypothesis and the citrus fruits were often included in a broader dietary evaluation. The ranges of adjusted ORs/RRs were from 0.089 to 1.6 and only 5 studies31,32,33,37,39 reached the usual threshold of P = 0.05 in the association between citrus fruits and esophageal cancer.
Heterogeneity and Pooled Results
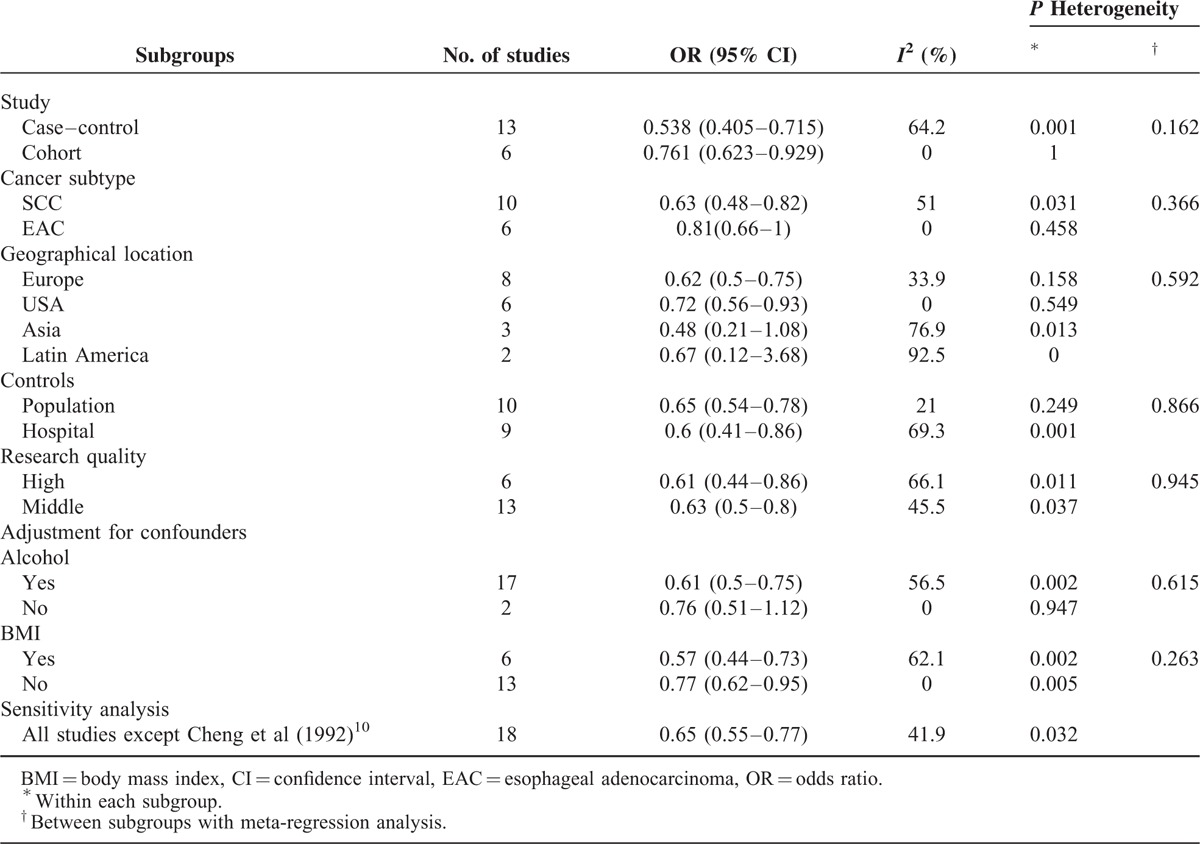
There was no significant heterogeneity among the study results (I2 = 52%; P = 0.005). Overall summary OR using the random-effects model showed a 37%, statistically significant reduction in risk of esophageal cancer associated with citrus fruits intake (summary OR = 0.63; 95% CI = 0.52–0.75). The subgroup of case–control studies (summary OR = 0.54; 95% CI = 0.4–0.72; I2 = 64.2%; P = 0.001) and the subgroup of cohort studies (summary OR = 0.76; 95% CI = 0.62–0.93; I2 = 0%; P = 1) showed a respective 46% and 24% statistically significant reduction in risk of esophageal cancer associated with citrus fruits intake (Figure 2). In subgroup analyses defined by study type, cancer subtype, geographical location, source of controls, research quality, and adjusted confounders, citrus intake was inversely associated with risk of esophageal cancer in most subgroups, with no evidence of significant heterogeneity between subgroups with meta-regression analyses. (Table 4).
FIGURE 2.
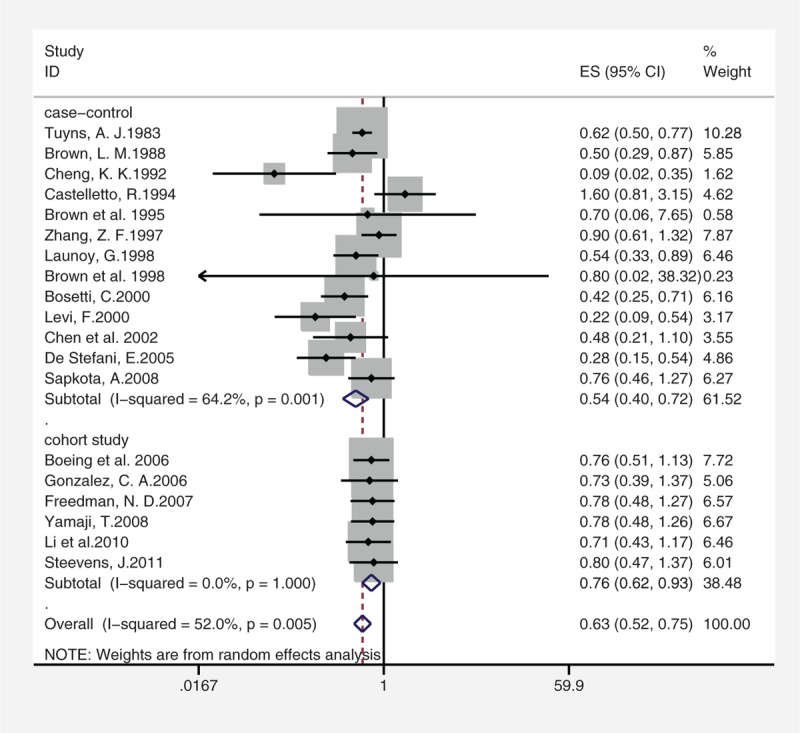
Summary estimates of the association between citrus intake and esophageal cancer risks sorted by effect estimate. CI = confidence interval; df = degree of freedom; chi2 = chi-square statistic; I2 = the percentage of total variation across studies that is due to heterogeneity rather than change; fixed = using fixed-effect model.
TABLE 4.
Subgroup Analyses of Citrus Intake and Risk of Esophageal Cancer, Sensitivity Analysis, Meta-Regression Analysis
Publication Bias
No publication bias was observed in the selected studies. Visualization of Begg funnel plot was symmetrical (Figure 3). Formal testing using the Egger method supports the notion that there was no publication bias (intercept = −0.79, P = 0.288); however, the result of Begg test suggested an obvious publication bias (P = 0.046). And the outcome of trim and fill analysis demonstrated that there was no publication bias.
FIGURE 3.
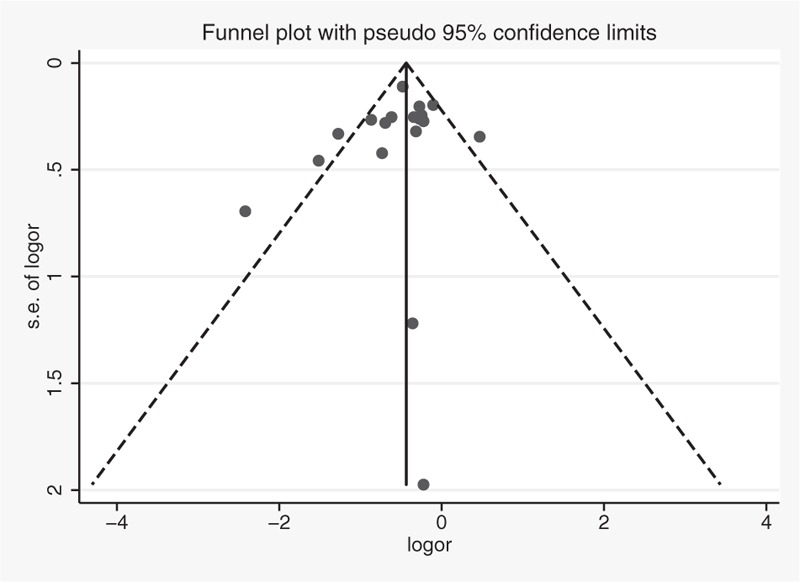
Funnel plot of studies evaluating the association between citrus fruit intake and esophageal cancer risks. Dot lines are 95% pseudo-confidence intervals. SE = standard error; OR = odds ratio.
DISCUSSION
The overall summary OR in our study presents an inverse association between citrus fruits and esophageal cancer (summary OR = 0.63; 95% CI = 0.52–0.75; P = 0). The result is supported by the strengths of our review, which includes a systematic literature search, strict selection criteria, comprehensive data abstraction, and rigorous statistical analysis. Additionally, the results of similar reviews about the association between citrus fruits and other cancers26–29 are encouraging.
Nevertheless, some limitations of our study should be taken into consideration. First, the considerable variables within observational studies made the outcome more likely to be suspicious.50 Furthermore, the included studies were evaluated to be low quality using the Grades of Recommendation, Assessment, Development and Evaluation system.51 These inherent drawbacks of observational study make outcomes more conservative.52 In addition, most of the included studies were not originally designed to evaluate citrus fruits and esophageal cancer, which possibly turns the pooled result into a simple summary.50 Measurement errors resulting from citrus intake should also be considered because of diversity of consumption patterns. Therefore, in the process of food intake measurement, various confounding factors made it difficult to obtain accurate dietary exposure information.27 Most case–control studies on diet are based on recent estimates of dietary intake,15 whereas the development of cancer after exposure to even a potent risk factor takes several decades.53,54
We found heterogeneity among the included studies, which decreases the quality of evidence to very low quality.51 Although rigorous criteria would make selective studies homogeneous, these could give rise to an inclusion bias.55 We excluded 2 studies48,49 sharing the same population, because the inclusion of duplicated data may lead to overestimation of exposure effects.29,56 Cancer deaths reflect failure of treatment as well as the occurrence of the cancer.57 Therefore, incidence rates are preferable as an early indicator of the impact of a risk factor. After careful screening of the eligible studies without CI or original data,18,35 we calculated the corresponding SE by the ORs and exact P values.58 The most appropriate way of handling the selection of studies is to perform sensitivity analyses with regard to the different possible entry criteria.55 Considering that the wide confidence internal of studies18,35 may obviously affect our outcome, we conducted sensitivity analysis.29 The analytic result showed that the studies have no apparent impact on the overall outcome. We also omitted 1 study59 that provided no citrus intake measurement. Methodology is a significant source of heterogeneity,50 so we performed subgroup analysis to verify the effect of study designs on heterogeneity. Figure 2 shows that study design causes heterogeneity. In the 6 cohort studies, the I2 of summary OR was 0%, because prospective studies can avoid recall and selection biases. In the 13 case–control studies, the I2 of summary OR was 64.2%. Both study designs demonstrate that citrus intake could reduce the incidence of esophageal cancer with summary OR 0.57 (CI 0.4–0.72), 0.76 (CI 0.62–0.93) for the case–control study and cohort study, respectively. The discrepancies between study results can be explained by recall and selection biases in case–control studies and by imprecise dietary measurements and limited variability of dietary intake in cohort studies.27,60 To further explore the source of heterogeneity, we conducted subgroups analyses and meta-regression analyses by many factors such as cancer subtypes, geographical location, source of controls, research quality, and adjusted confounders.
The pathogenesis and risk factors for different types of esophageal cancer18,21 vary widely, so exploring the impacts of citrus intake on these cancers is essential. Four of the included studies did not describe the specific cancer subtypes or included both subtypes. Table 4 shows the association between citrus and SCC (summary OR 0.63; CI 0.48–0.82) and EAC (summary OR 0.81; CI 0.66–1). The lack of overlapping confidence internal could partially explain the study heterogeneity. The forest plot (Figure 2) demonstrates that there is no overlap in CIs between 3 studies10 and the summary OR. Repeat meta-analysis of a new model excluded the study10 from all 19 selected articles was conducted.61 The level of heterogeneity decreased from high (I2 = 52%) to low (I2 = 0).
Citrus fruits include many bioactive components.25,62 Dietary antioxidants are emerging as potentially modifiable risk factors for EAC. High intake of beta-carotene may be associated with decreased risk of dysplastic Barrett esophagus, which is regarded as the precursor of EAC.63 Some studies64,65 showed that carotenoids may be responsible nutritional factors (as nutritional scavengers) in the development of different malignant diseases including esophageal cancer.66,67 Carotenoids may intervene in cancer-related molecular pathways and the expression proteins involved in cell proliferation, differentiation, apoptosis and angiogenesis, carcinogen detoxification, DNA damage, and repair.68 A related study indicates69 that a high intake of vitamin C is associated with a reduced risk of EAC and reflux esophagitis. Antioxidants may also play a role in the pathogenesis of reflux esophagitis and EAC and may be more important in terms of progression rather than initiation of the disease process69; however, low intake of vitamin C and E correlates significantly with the development of SCC as well as EAC in males.70,71 Regarding the mechanism, researchers think that vitamin C could enhance the EGCG- and TF3-induced apoptosis in SPC-A-1 and Eca-109 cells via MAPK pathways.72 Additionally, folate and other dietary methyl group factors are implicated in the etiology of EAC and its precursors. Folate is implicated in carcinogenesis via effects on DNA synthesis, repair, and methylation.21,22 Some studies indicate that flavanone intake is inversely associated with SCC risk and may account for the protective effect of fruit, especially citrus fruits, on esophageal cancer.73,74 Because citrus fruits account for 90% of flavanone intake, the findings of Rossi et al73 suggest that flavanones may play a role in the protective effect of citrus fruits on esophageal cancer. Therefore, the basic research of mechanisms flavanones protect against esophageal cancer are worth studying. Although the results are exciting, we have to taken in account the interaction between medicines and fruits. The research by Bailey et al75 suggested that there exist adverse reactions when grape is combined with some drugs.
Our review demonstrates that citrus fruit intake could reduce the incidence of esophageal cancer by 37% based on published results of epidemiologic studies. The trends are consistent between case–control studies and cohort studies; however, considering the drawbacks mentioned above, our conclusions should be taken cautiously. There are no relevant studies that provide explicit evidence for the inconsistency between SCC and EAC. The low quantity of EAC cases and the limitations of meta-analysis are responsible for the results. Therefore, larger studies with rigorous and prospective methodology should be considered to validate the association between citrus fruits and esophageal cancer. It is still unknown which components in citrus fruits have an effect on esophageal cancer prevention. Our conclusion may encourage researchers to further explore the protective elements and potential mechanisms, which may contribute to reducing the esophageal cancer risk. We hope further research will explore this issue.
Footnotes
Abbreviations: CIs = confidence intervals, EAC = esophageal adenocarcinoma, OR = odds ratio, RR = relative risk, SCC = squamous cell carcinoma, SEs = standard errors.
Drs Anqiang Wang, Chengpei Zhu, and Lilan Fu contributed equally to the writing of this article.
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Esophageal cancer: epidemiology, pathogenesis and, prevention. Nat Clin Pract Gastroenterol Hepatol 2008; 5:517–526.doi: 10.1038/ncpgasthep1223. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol 2013; 19:5598–5606.doi: 10.3748/wjg.v19.i34.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013; 381:400–412.doi: 10.1016/s0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Yu C, Li Y. Physical activity and risks of esophageal and gastric cancers: a meta-analysis. PLoS One 2014; 9:e88082.doi: 10.1371/journal.pone.0088082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Napier KJ, Scheerer M, Misra S. Esophageal cancer: a review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol 2014; 6:112–120.doi: 10.4251/wjgo.v6.i5.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herszenyi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci 2010; 14:249–258. [PubMed] [Google Scholar]
- 7.Lindblad M, Rodriguez LA, Lagergren J. Body mass, tobacco and alcohol and risk of esophageal, gastric cardia, and gastric non-cardia adenocarcinoma among men and women in a nested case-control study. Cancer Causes Control 2005; 16:285–294.doi: 10.1007/s10552-004-3485-7. [DOI] [PubMed] [Google Scholar]
- 8.Choi Y, Song S, Song Y, et al. Consumption of red and processed meat and esophageal cancer risk: meta-analysis. World J Gastroenterol 2013; 19:1020–1029.doi: 10.3748/wjg.v19.i7.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sapkota A, Hsu CC, Zaridze D, et al. Dietary risk factors for squamous cell carcinoma of the upper aerodigestive tract in central and eastern Europe. Cancer Causes Control 2008; 19:1161–1170.doi: 10.1007/s10552-008-9183-0. [DOI] [PubMed] [Google Scholar]
- 10.Cheng KK, Day NE, Duffy SW, et al. Pickled vegetables in the aetiology of oesophageal cancer in Hong Kong Chinese. Lancet 1992; 339:1314–1318. [DOI] [PubMed] [Google Scholar]
- 11.Carr JS, Zafar SF, Saba N, et al. Risk factors for rising incidence of esophageal and gastric cardia adenocarcinoma. J Gastrointest Cancer 2013; 44:143–151.doi: 10.1007/s12029-013-9480-z. [DOI] [PubMed] [Google Scholar]
- 12.Lepage C, Drouillard A, Jouve JL, et al. Epidemiology and risk factors for oesophageal adenocarcinoma. Dig Liver Dis 2013; 45:625–629.doi: 10.1016/j.dld.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Salehi M, Moradi-Lakeh M, Salehi MH, et al. Meat, fish, and esophageal cancer risk: a systematic review and dose-response meta-analysis. Nutr Rev 2013; 71:257–267.doi: 10.1111/nure.12028. [DOI] [PubMed] [Google Scholar]
- 14.Spechler SJ. Barrett esophagus and risk of esophageal cancer: a clinical review. JAMA 2013; 310:627–636.doi: 10.1001/jama.2013.226450. [DOI] [PubMed] [Google Scholar]
- 15.Steevens J, Schouten LJ, Goldbohm RA, et al. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Int J Cancer 2011; 129:2681–2693.doi: 10.1002/ijc.25928. [DOI] [PubMed] [Google Scholar]
- 16.Yamaji T, Inoue M, Sasazuki S, et al. Fruit and vegetable consumption and squamous cell carcinoma of the esophagus in Japan: the JPHC study. Int J Cancer 2008; 123:1935–1940.doi: 10.1002/ijc.23744. [DOI] [PubMed] [Google Scholar]
- 17.Levi F, Pasche C, Lucchini F, et al. Food groups and oesophageal cancer risk in Vaud, Switzerland. Eur J Cancer Prev 2000; 9:257–263. [DOI] [PubMed] [Google Scholar]
- 18.Brown LM, Swanson CA, Gridley G, et al. Dietary factors and the risk of squamous cell esophageal cancer among black and white men in the United States. Cancer Causes Control 1998; 9:467–474. [DOI] [PubMed] [Google Scholar]
- 19.Coleman HG, Murray LJ, Hicks B, et al. Dietary fiber and the risk of precancerous lesions and cancer of the esophagus: a systematic review and meta-analysis. Nutr Rev 2013; 71:474–482. [DOI] [PubMed] [Google Scholar]
- 20.Zhu HC, Yang X, Xu LP, et al. Meat consumption is associated with esophageal cancer risk in a meat- and cancer-histological-type dependent manner. Dig Dis Sci 2014; 59:664–673. [DOI] [PubMed] [Google Scholar]
- 21.Sharp L, Carsin AE, Cantwell MM, et al. Intakes of dietary folate and other B vitamins are associated with risks of esophageal adenocarcinoma, Barrett's esophagus, and reflux esophagitis. J Nutr 2013; 143:1966–1973.doi: 10.3945/jn.113.174664. [DOI] [PubMed] [Google Scholar]
- 22.Jiao L, Kramer JR, Rugge M, et al. Dietary intake of vegetables, folate, and antioxidants and the risk of Barrett's esophagus. Cancer Causes Control 2013; 24:1005–1014.doi: 10.1007/s10552-013-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ge XX, Xing MY, Yu LF, et al. Carotenoid intake and esophageal cancer risk: a meta-analysis. Asian Pac J Cancer Prev 2013; 14:1911–1918. [DOI] [PubMed] [Google Scholar]
- 24.Abnet CC, Qiao YL, Dawsey SM, et al. Prospective study of serum retinol, beta-carotene, beta-cryptoxanthin, and lutein/zeaxanthin and esophageal and gastric cancers in China. Cancer Causes Control 2003; 14:645–655. [DOI] [PubMed] [Google Scholar]
- 25.Silalahi J. Anticancer and health protective properties of citrus fruit components. Asia Pac J Clin Nutr 2002; 11:79–84. [DOI] [PubMed] [Google Scholar]
- 26.Fountoulakis A, Martin IG, White KL, et al. Plasma and esophageal mucosal levels of vitamin C: role in the pathogenesis and neoplastic progression of Barrett's esophagus. Dig Dis Sci 2004; 49:914–919. [DOI] [PubMed] [Google Scholar]
- 27.Bae JM, Lee EJ, Guyatt G. Citrus fruit intake and pancreatic cancer risk: a quantitative systematic review. Pancreas 2009; 38:168–174.doi: 10.1097/MPA.0b013e318188c497. [DOI] [PubMed] [Google Scholar]
- 28.Bae JM, Lee EJ, Guyatt G. Citrus fruits intake and prostate cancer risk: a quantitative systematic review. J Prev Med Public Health 2008; 41:159–164. [DOI] [PubMed] [Google Scholar]
- 29.Bae JM, Lee EJ, Guyatt G. Citrus fruit intake and stomach cancer risk: a quantitative systematic review. Gastric Cancer 2008; 11:23–32.doi: 10.1007/s10120-007-0447-2. [DOI] [PubMed] [Google Scholar]
- 30.Deeks J, Altman DG, Bradburn MJ. Egger M, Smith DG, Altman DG. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. Systematic Reviews in Health Care 2nd edLondon, England: BMJ Books; 2007. 285–312. [Google Scholar]
- 31.Freedman ND, Park Y, Subar AF, et al. Fruit and vegetable intake and esophageal cancer in a large prospective cohort study. Int J Cancer 2007; 121:2753–2760.doi: 10.1002/ijc.22993. [DOI] [PubMed] [Google Scholar]
- 32.Tuyns AJ. Protective effect of citrus fruit on esophageal cancer. Nutr Cancer 1983; 5:195–200.doi: 10.1080/01635588309513796. [DOI] [PubMed] [Google Scholar]
- 33.Brown LM, Blot WJ, Schuman SH, et al. Environmental factors and high risk of esophageal cancer among men in coastal South Carolina. J Natl Cancer Inst 1988; 80:1620–1625. [DOI] [PubMed] [Google Scholar]
- 34.Castelletto R, Castellsague X, Munoz N, et al. Alcohol, tobacco, diet, mate drinking, and esophageal cancer in Argentina. Cancer Epidemiol Biomarkers Prev 1994; 3:557–564. [PubMed] [Google Scholar]
- 35.Brown LM, Swanson CA, Gridley G, et al. Adenocarcinoma of the esophagus: role of obesity and diet. J Natl Cancer Inst 1995; 87:104–109. [DOI] [PubMed] [Google Scholar]
- 36.Zhang ZF, Kurtz RC, Yu GP, et al. Adenocarcinomas of the esophagus and gastric cardia: the role of diet. Nutr Cancer 1997; 27:298–309.doi: 10.1080/01635589709514541. [DOI] [PubMed] [Google Scholar]
- 37.Launoy G, Milan C, Day NE, et al. Diet and squamous-cell cancer of the oesophagus: a French multicentre case-control study. Int J Cancer 1998; 76:7–12. [DOI] [PubMed] [Google Scholar]
- 38.Bosetti C, La Vecchia C, Talamini R, et al. Food groups and risk of squamous cell esophageal cancer in northern Italy. Int J Cancer 2000; 87:289–294. [PubMed] [Google Scholar]
- 39.Chen H, Ward MH, Graubard BI, et al. Dietary patterns and adenocarcinoma of the esophagus and distal stomach. Am J Clin Nutr 2002; 75:137–144. [DOI] [PubMed] [Google Scholar]
- 40.De Stefani E, Boffetta P, Deneo-Pellegrini H, et al. The role of vegetable and fruit consumption in the aetiology of squamous cell carcinoma of the oesophagus: a case-control study in Uruguay. Int J Cancer 2005; 116:130–135.doi: 10.1002/ijc.20950. [DOI] [PubMed] [Google Scholar]
- 41.Boeing H, Dietrich T, Hoffmann K, et al. Intake of fruits and vegetables and risk of cancer of the upper aero-digestive tract: the prospective EPIC-study. Cancer Causes Control 2006; 17:957–969.doi: 10.1007/s10552-006-0036-4. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez CA, Pera G, Agudo A, et al. Fruit and vegetable intake and the risk of stomach and oesophagus adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Int J Cancer 2006; 118:2559–2566.doi: 10.1002/ijc.21678. [DOI] [PubMed] [Google Scholar]
- 43.Li WQ, Kuriyama S, Li Q, et al. Citrus consumption and cancer incidence: the Ohsaki cohort study. Int J Cancer 2010; 127:1913–1922.doi: 10.1002/ijc.25203. [DOI] [PubMed] [Google Scholar]
- 44.Higgins JPT, Thompson SG. Quantifying heterogeneity in a metaanalysis. Stat Med 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 45.Fleiss JL, Gross AJ. Meta-analysis in epidemiology, with special reference to studies of the association between exposure to environmental tobacco smoke and lung cancer: a critique. J Clin Epidemiol 1991; 44:127–139. [DOI] [PubMed] [Google Scholar]
- 46.The Cochrane Collaboration. Review Manager Version 4.2 for Windows. Copenhagen, Denmark: The Nordic Cochrane Centre; 2003. [Google Scholar]
- 47.Stata Corporation. Stata Statistical Software. Special Edition. 8.2 for Windows. College Station, TX: Stata Corporation; 2004. [Google Scholar]
- 48.Tuyns AJ, Riboli E, Doornbos G, et al. Diet and esophageal cancer in Calvados (France). Nutr Cancer 1987; 9:81–92.doi: 10.1080/01635588709513915. [DOI] [PubMed] [Google Scholar]
- 49.De Stefani E, Deneo-Pellegrini H, Ronco AL, et al. Food groups and risk of squamous cell carcinoma of the oesophagus: a case-control study in Uruguay. Br J Cancer 2003; 89:1209–1214.doi: 10.1038/sj.bjc.6601239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 51.Schunemann HJ, Jaeschke R, Cook DJ, et al. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. Am J Respir Crit Care Med 2006; 174:605–614.doi: 10.1164/rccm.200602-197ST. [DOI] [PubMed] [Google Scholar]
- 52.Easterbrook PJ, Berlin JA, Gopalan R, et al. Publication bias in clinical research. Lancet 1991; 337:867–872. [DOI] [PubMed] [Google Scholar]
- 53.Pou SA, Niclis C, Aballay LR, et al. Cancer and its association with dietary patterns in Cordoba (Argentina). Nutr Hosp 2014; 29:618–628.doi: 10.3305/nh.2014.29.3.7192. [DOI] [PubMed] [Google Scholar]
- 54.Michaud DS, Skinner HG, Wu K, et al. Dietary patterns and pancreatic cancer risk in men and women. J Natl Cancer Inst 2005; 97:518–524.doi: 10.1093/jnci/dji094. [DOI] [PubMed] [Google Scholar]
- 55.Egger M, Smith GD. Bias in location and selection of studies. BMJ 1998; 316:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ioannidis JP, Trikalinos TA, Zintzaras E. Extreme between-study homogeneity in meta-analyses could offer useful insights. J Clin Epidemiol 2006; 59:1023–1032.doi: 10.1016/j.jclinepi.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 57.Ezzat A. Book review: national cancer control programmes, policies and managerial guidelines. Ann Saudi Med 1996; 16:358.17372517 [Google Scholar]
- 58.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987; 9:1–30. [DOI] [PubMed] [Google Scholar]
- 59.Navarro Silvera SA, Mayne ST, Risch H, et al. Food group intake and risk of subtypes of esophageal and gastric cancer. Int J Cancer 2008; 123:852–860.doi: 10.1002/ijc.23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bai Y, Yuan H, Li J, et al. Relationship between bladder cancer and total fluid intake: a meta-analysis of epidemiological evidence. World J Surg Oncol 2014; 12:223.doi: 10.1186/1477-7819-12-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560.doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parmar HS, Dixit Y, Kar A. Fruit and vegetable peels: paving the way towards the development of new generation therapeutics. Drug Discov Ther 2010; 4:314–325. [PubMed] [Google Scholar]
- 63.Ibiebele TI, Hughes MC, Nagle CM, et al. Dietary antioxidants and risk of Barrett's esophagus and adenocarcinoma of the esophagus in an Australian population. Int J Cancer 2013; 133:214–224.doi: 10.1002/ijc.28016. [DOI] [PubMed] [Google Scholar]
- 64.Stice CP, Wang XD. Carotenoids and alcoholic liver disease. Hepatobiliary Surg Nutr 2013; 2:244–247.doi: 10.3978/j.issn.2304-3881.2013.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peng HC, Chen YL, Yang SY, et al. The antiapoptotic effects of different doses of beta-carotene in chronic ethanol-fed rats. Hepatobiliary Surg Nutr 2013; 2:132–141.doi: 10.3978/j.issn.2304-3881.2013.06.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rumi G, Jr, Matus Z, Toth G, et al. Changes of serum carotenoids in patients with esophageal, gastric, hepatocellular, pancreatic and colorectal cancer. J Physiol, Paris 2001; 95:239–242. [DOI] [PubMed] [Google Scholar]
- 67.Hammerich L, Tacke F. Eat more carrots? Dampening cell death in ethanol-induced liver fibrosis by beta-carotene. Hepatobiliary Surg Nutr 2013; 2:248–251.doi: 10.3978/j.issn.2304-3881.2013.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palozza P. Carotenoids and modulation of cancer: molecular targets. Curr Pharmacogenomics 2004; 2:35–45. [Google Scholar]
- 69.Murphy SJ, Anderson LA, Ferguson HR, et al. Dietary antioxidant and mineral intake in humans is associated with reduced risk of esophageal adenocarcinoma but not reflux esophagitis or Barrett's esophagus. J Nutr 2010; 140:1757–1763.doi: 10.3945/jn.110.124362. [DOI] [PubMed] [Google Scholar]
- 70.Bollschweiler E, Wolfgarten E, Nowroth T, et al. Vitamin intake and risk of subtypes of esophageal cancer in Germany. J Cancer Res Clin Oncol 2002; 128:575–580.doi: 10.1007/s00432-002-0380-z. [DOI] [PubMed] [Google Scholar]
- 71.Malekshah AF, Kimiagar M, Pourshams A, et al. Vitamin deficiency in Golestan Province, northern Iran: a high-risk area for esophageal cancer. Arch Iran Med 2010; 13:391–394.doi: 010135/aim.005. [PubMed] [Google Scholar]
- 72.Gao Y, Li W, Jia L, et al. Enhancement of (-)-epigallocatechin-3-gallate and theaflavin-3-3’-digallate induced apoptosis by ascorbic acid in human lung adenocarcinoma SPC-A-1 cells and esophageal carcinoma Eca-109 cells via MAPK pathways. Biochem Biophys Res Commun 2013; 438:370–374.doi: 10.1016/j.bbrc.2013.07.078. [DOI] [PubMed] [Google Scholar]
- 73.Rossi M, Garavello W, Talamini R, et al. Flavonoids and risk of squamous cell esophageal cancer. Int J Cancer 2007; 120:1560–1564.doi: 10.1002/ijc.22499. [DOI] [PubMed] [Google Scholar]
- 74.Woo HD, Kim J. Dietary flavonoid intake and smoking-related cancer risk: a meta-analysis. PLoS One 2013; 8:e75604.doi: 10.1371/journal.pone.0075604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bailey DG, Dresser G, Arnold JM. Grapefruit-medication interactions: forbidden fruit or avoidable consequences? CMAJ 2013; 185:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]


