Abstract
It remains difficult to accurately predicate short-term mortality of acute-on-chronic hepatitis B liver failure (ACHBLF). Tumor necrosis factor-α-induced protein 8-like 2 (TIPE2) is a novel identified negative regulator of immune response and we have previously demonstrated TIPE2 play an essential role in the pathogenesis of ACHBLF. We therefore aimed to evaluate the diagnosis value of TIPE2 mRNA in peripheral blood mononuclear cells (PBMCs) for predicting 3-month mortality of ACHBLF patients.
This prospective study consisted of 108 ACHBLF patients from March 2009 to May 2013 as training cohort and 63 ACHBLF patients from June 2013 to December 2014 as validation cohort. Forty-two patients with chronic hepatitis B (CHB) and 22 healthy volunteers were also included as controls. The mRNA level of TIPE2 in PBMCs was determined using quantitative real-time polymerase chain reaction. Univariate analysis and Cox proportional hazard regression analysis were performed to identify independent risk factors to 3-month mortality. Area under the receptor operating characteristic curve (AUROC) was performed to assess diagnostic value of TIPE2 mRNA in training and validation cohort.
The level of TIPE2 mRNA was significantly higher in ACHBLF patients (median (interquartile): 6.5 [3.7, 9.6]) compared with CHB (2.3 [1.6, 3.7]) and healthy controls (0.4 [0.3, 0.6]; both P < 0.05). Cox proportional hazards regression analyses showed 5 independent risk factors associated with 3-month mortality of ACHBLF: white blood cells (HR = 1.058, 95% CI: 1.023–1.095), spontaneous bacterial peritonitis (HR = 2.541, 95% CI: 1.378–4.686), hepatic encephalopathy (HR = 1.848, 95% CI: 1.028–3.321), model for end-stage liver diseases (MELD) score (HR = 1.062, 95% CI: 1.009–1.118), and TIPE2 mRNA (HR = 1.081, 95% CI: 1.009–1.159). An optimal cut-off point 6.54 of TIPE2 mRNA showed sensitivity of 74.63%, specificity of 90.24%, positive predictive value of 92.5%, and negative predictive value of 67.3% for predicting 3-month mortality in training cohort. Furthermore, TIPE2 mRNA plus MELD performed better than MELD alone for predicting 3-month mortality in training (AUROC, 0.853 vs 0.722, P < 0.05) and validation cohort (AUROC, 0.909 vs 0.717, P < 0.001).
TIPE2 mRNA level might be a novel biomarker in predicting 3-month mortality of ACHBLF. Combination of TIPE2 mRNA and MELD would improve the diagnostic value of MELD alone in predicting 3-month mortality of patients with ACHBLF.
INTRODUCTION
Acute-on-chronic liver failure (ACLF) is a severe life-threatening clinical syndrome, which is characterized by acute deterioration of liver function during the progression of diagnosed or undiagnosed chronic liver disease.1 In China, hepatitis B virus (HBV) associated ACLF, also termed as acute-on-chronic hepatitis B liver failure (ACHBLF), accounts for over 80% of ACLF.2 ACHBLF has a rapidly progressive course and may lead to a high short-term mortality.3 However, there are no specific and powerful regimes for the treatment of ACHBLF. Liver transplantation is the definitive treatment to lower mortality rate, but there is a great imbalance between donation and potential recipients.4 Agreeable consensus is that early identification of patients with high mortality and timely intervention would be effective for the treatment of ACHBLF.4,5 Several independent risk factors for mortality of ACLF have been identified in consecutive cohorts, including serum total bilirubin (TBIL), international normalized ratio (INR), model for end-stage liver disease (MELD), and pharmaceutical treatment regimen. MELD was the most common used predictor for the severity of ACLF. Nevertheless, most of the predictive models are not HBV-specific to liver failure and usually have poor predictive value for ACHBLF.6 Therefore, new potential biomarkers are urgently needed to predict accurately the short-term prognosis of patients at the early stage of ACHBLF patients.
Tumor necrosis factor-α-induced protein 8-like 2 (TIPE2) is a newly identified negative regulatory molecular of immune response, and governs immune homeostasis by negatively regulating signaling through T cell receptors and Toll-like receptors.7 In recent years, accumulating studies reported that TIPE2 might play an important role in the development of chronic inflammatory diseases, autoimmune disorders, stroke, diabetic nephropathy, carcinoma, and atherosclerosis.8–17 Zhang et al reported the aberrant expression of TIPE2 in hepatitis B patients and revealed that TIPE2 might regulate HBV-specific CD8(+) T cells function and contribute to the pathogenesis of hepatitis B.18 In the patients with ACHBLF, we also previously reported the elevation of TIPE2 mRNA and speculated that TIPE2 might negatively regulate cell-mediating immunity to abnormal liver function in a preliminary study.19 Therefore, we forward the hypothesis that TIPE2 mRNA might be a useful biomarker for predicting the outcome of ACHBLF patients.
To test this hypothesis, we designed this prospective cohort to identify whether TIPE2 mRNA could be a useful and effective biomarker in predicting short-term mortality of ACHBLF patients. This prospective study consisted of 108 ACHBLF patients from March 2009 to May 2013 as training cohort and 63 ACHBLF patients from June 2013 to December 2014 as validation cohort. Using these dataset of the 2 separate cohorts, we firstly determined the diagnostic value of TIPE2 mRNA in predicating the 3-month mortality of ACHBLF patients, and then we also analyzed the potential for combination of TIPE2 mRNA and MELD scores in training and validation cohorts.
MATERIALS AND METHODS
Study Protocol
In our study, a total of 171 patients with ACHBLF were prospectively collected in the Department of Hepatology, Qilu Hospital of Shandong University from March 2009 to December 2014. There were also 42 patients with chronic hepatitis B (CHB) and 22 healthy volunteers as control. All the ACHBLF patients were divided into 2 cohorts: the training cohort consisted of 108 consecutive ACHBLF patients who were hospitalized from March 2009 to May 2013; the validation cohort consisted of 63 consecutive ACHBLF patients who were hospitalized from June 2013 to December 2014. There were no differences in age, sex, and survival rate between the 2 cohorts. All participants gave written informed consents under protocols approved by the local research and ethic committee at Qilu Hospital of Shandong University, in accordance with the guidelines of the 1975 Declaration of Helsinki.20
The start date of follow up was set as the onset of diagnosis of ACHBLF. All ACHBLF patients were followed up for at least 6 months. The outcome of each patient with ACHBLF was recorded as survival or death. In this present study, there were no patients died after 3 months to the end of 6 months follow-up period. Therefore, we used 3-month mortality as the prognosis of ACHBLF patients.
Definition of Patients
CHB patients were identified as the presence of positive hepatitis B surface antigen (HBsAg) for at least 6 months before the beginning of this study. According to the guideline of the Asian Pacific Association for the Study of the Liver (APASL), ACHBLF was identified as the following issues: history of CHB; progressive jaundice (TBIL ≥85 μmol/L); increasing INR ≥1.5 or decreasing prothrombin activity (PTA) ≤40%; onset of ascites and/or hepatic encephalopathy (HE) within 4 weeks.1 Exclusive criteria including: co-infection with human immunodeficiency virus; hepatitis C virus; hepatitis D virus; autoimmune liver diseases; metabolic liver disease; severe alcohol abuse; pregnancy; and liver tumors.
The treatment and medical care for ACHBLF patients were provided according to the APASL recommendations.1,21 The following managements for ACHBLF patients included anti-HBV treatment, bed rest, supplement of energy and vitamins, albumin infusion, maintenance of water, electrolyte and acid–base balance, treatment and prevention of clinical complication, etc. In this present study, there was no patient receiving liver transplantation.
RNA and cDNA Preparation From PBMC
Five milliliters of citrate-anticoagulated peripheral blood was obtained from all subjects. After Ficoll-Paque Plus (GE Healthcare, Uppsala, Sweden) density gradient centrifugation, peripheral blood mononuclear cells (PBMCs) from the interface were collected and washed 3 times with phosphate-buffered saline. Total RNA of PBMCs was extracted by TRIzol (Invitrogen, Carlsbad, CA). Two micrograms of RNA were reverse transcribed into cDNA using first-strand cDNA synthesis kit (Fermentas, Vilnius, Lithuania).
Quantitative Real-Time PCR
The expression of TIPE2 mRNA was determined by real-time PCR. Primers for TIPE2 were the forward 5′-GGAACATCCAAGGCAAGACTG-3′ and the reverse 5′-AGCACCTCACTGCTTGTCTCATC-3′. Primers for β-actin were the forward 5′-ATGGGTCAGAAGGATTCCTATGTG-3′ and the reverse 5′-CTTCATGAGGTAGTCAGTCAGGTC-3′. β-actin was used as the endogenous control. Real-time PCR was conducted with Lightcycler 480 (Roche Diagnostics, Mannheim, Germany). Real-time PCR was performed using an SYBR Premix Ex Taq™ (Takara, Shiga, Japan) according to the manufacturer's instructions. The reaction of PCR was according to the following thermal profile: denaturation at 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec, 60°C for 30 sec, and 72°C for 30 sec. Each sample was carried out in triplicate. Data analysis was performed with the LightCycler 480 Software (Roche Diagnostics, Mannheim, Germany), and results were determined using the comparative (2−ΔΔCt) method.
Clinic Pathological Data Collection
Blood samples were taken from each participant. The serum biochemical markers (COBAS integra 800, Roche Diagnostics, Mannheim, Germany) included aspartate aminotransferase (AST), alanine aminotransferase (ALT), TBIL, albumin (ALB), and creatinine (Cr). Hemostasis markers (ACL TOP 700, Instrument Laboratory, Brea, CA, USA) included INR and PTA. Hematological markers (Sysmex XE-2100, Sysmex Corporation, Kobe, Japan) included white blood cell (WBC), hemoglobin (HGB), and platelet (PLT). α-fetoprotein (AFP) was measured by an automatic analyzer (COBAS e 601, Roche Diagnostics, Mannheim, Germany). Hepatitis B virus serologies, including HBsAg, hepatitis B e antigen (HBeAg), and anti-HBe were measured by an automatic analyzer (cobas 6000 analyzer series, Roche Diagnostics, Rotkreuz, Switzerland). These markers were measured using standard laboratory methods in Department of Medicine Laboratory, Qilu Hospital, Shandong University. Serum viral load of HBV-DNA was quantified by a PCR System (ABI 7300, Applied Biosystems, Foster City, CA), with a detection sensitivity of 500 IU/mL.
Model for end-stage liver disease (MELD) scores were calculated according to the malinchoc formula: MELD score = 9.57 × loge (creatinine [mg/dL]) + 3.78 × loge (bilirubin [mg/dL]) + 11.2 × loge (INR) + 6.43 × (etiology: 0 if cholestatic or alcoholic, 1 otherwise).
Statistical Analysis
The Kolmogorov–Smirnov test was performed to determine whether data were a normal distribution population. Quantitative variables were expressed as median (centile 25; centile 75). Categorical variables were expressed as number (percentage). Kruskal–Wallis test was used to compare the nonparametric quantitative variables within multiple groups. The chi-squared test was used to compare the categorical data. The correlation between variables was evaluated using the Spearman rank correlation test. Univariate Cox proportional hazards regression analysis was done for determining the association of clinical parameters with mortality and survival time. Covariables with a P value <0.05 as each univariate regression analyses were entered into a forward conditional step-wise Cox proportional hazards regression model to identify independent risk factors for the outcome of ACHBLF patients. The area under the receiver operating characteristic (ROC) curve was used to assess diagnostic accuracy. Survival rate was estimated by the Kaplan–Meier method and compared by the log-rank test. Differences were considered significant at a 2-tailed P < 0.05. All statistical analyses were performed using the IBM SPSS 19.0 software (SPSS, Inc., Chicago, IL)
RESULTS
General Characteristics
The flowchart for the inclusion of all the patients has been shown in Figure 1. A total of 171 patients with ACHBLF, 42 patients with CHB and 22 healthy controls (HCs) were enrolled in this present study from March 2009 to December 2014. Of all the 171 ACHBLF patients, 108 ACHBLF patients hospitalized from March 2009 to May 2013 were set as training cohort; meanwhile, 63 ACHBLF patients hospitalized from June 2013 to December 2014 were set as validating cohort. The demographic and clinical characteristics of patients with ACHBLF in training cohort, patients with CHB, and HC were summarized in Table 1. In the training cohort, 67 patients died at the end of 3-month follow up and the mortality was 62%. Cirrhosis, hepatic encephalopathy (HE), ascites, and spontaneous bacterial peritonitis were found in 59 (54.6%), 39 (36.1%), 50 (46.3%), and 40 (37%) patients with ACHBLF, respectively.
FIGURE 1.

Flowchart for the inclusive procession of all the subjects in this present study.
TABLE 1.
Baseline Characteristics of the Enrolled Participants

The general characteristics of ACHBLF patients in training and validation cohort have been listed in Table 2. There were 35 patients died in validation cohort with mortality of 55.6%, which was similar to the mortality (62%) in training cohort (P > 0.05). We did not found significant differences on ALT, AST, ALB, HBsAg, HBeAg, HBV-DNA, Cr, INR, PTA, MELD score, AFP, WBC, HGB, PLT, cirrhosis, spontaneous bacterial peritonitis (SBP), ascites, and HE in training and validation cohorts (all P > 0.05, respectively).
TABLE 2.
Characteristics of ACHBLF Patients, Stratified by Different Cohorts
Table 3 shows the general characteristics of ACHBLF patients stratified by different cohorts. In training cohort, survivals had a lower MELD score (22 [19.7–26.6] vs 26.9 [23.1–30.5], P < 0.001), ascites (29.3% vs 56.7%, P < 0.05), cirrhosis (39% vs 64.2%, P < 0.05), SBP (14.6% vs 50.7%, P < 0.001), and HE (14.6% vs 49.3%, P < 0.001) than nonsurvivals. Significant differences of ALB (32.8 [30.5–37] vs 31.2 [28.5–33.7], P < 0.05), INR (2.4 [1.9–3.0] vs 3.0 [2.4–3.6], P < 0.05), and WBC (5.9 [4.0–8.7] vs 7.4 [5.6–11.9], P = 0.001) were found between survivals and nonsurvivals. There were no significant differences between survivals and nonsurvivals with respect to HBeAg, HBV-DNA, ALT, AST, Cr, HBG, and PLT. In the validation cohort, survivals were also found to have a lower MELD score (23.5 [19.5–27.7] vs 28.4 [25.3–32.9], P < 0.05), INR (2.3 [1.7–3.0] vs 3.1 [2.9–3.7], P < 0.05), WBC (6.1 [3.7–8.8] vs 8.1 [5.9–12.0], P < 0.05), ascites (32.1% vs 60.0%, P < 0.05), cirrhosis (39.3% vs 65.7%, P < 0.05), SBP (17.9% vs 45.7%, P < 0.05), and HE (14.3% vs 48.6%, P < 0.05), whereas there was no significant differences about HBeAg, HBV-DNA, ALT, AST, HBG, Cr, and PLT.
TABLE 3.
Characteristics of ACHBLF Patients, Stratified by Different Cohorts

Expression of TIPE2 mRNA From Peripheral Blood Mononuclear Cells and Associations With Clinical Characteristics in ACHBLF Patients
We firstly investigated the expression of TIPE2 mRNA in PBMCs in ACHBLF patients. Figure 2A shows that the relative expression of TIPE2 mRNA in ACHBLF patients (6.5 [3.7, 9.6]) was significant higher compared with CHB patients (2.3 [1.6, 3.7]) and HCs (0.4 [0.3, 0.6]) (both P < 0.001), indicating TIPE2 might participate in the progression of ACHBLF. A total of 108 ACHBLF patients in training cohort were divided into 41 survivals and 67 nonsurvivals according to 3-month follow up. Figure 2B shows that nonsurvivals had significant higher TIPE2 mRNA expression than survivals (8.7 [6.5, 12.3] vs 3.9 [2.3, 5.5], P < 0.001).
FIGURE 2.
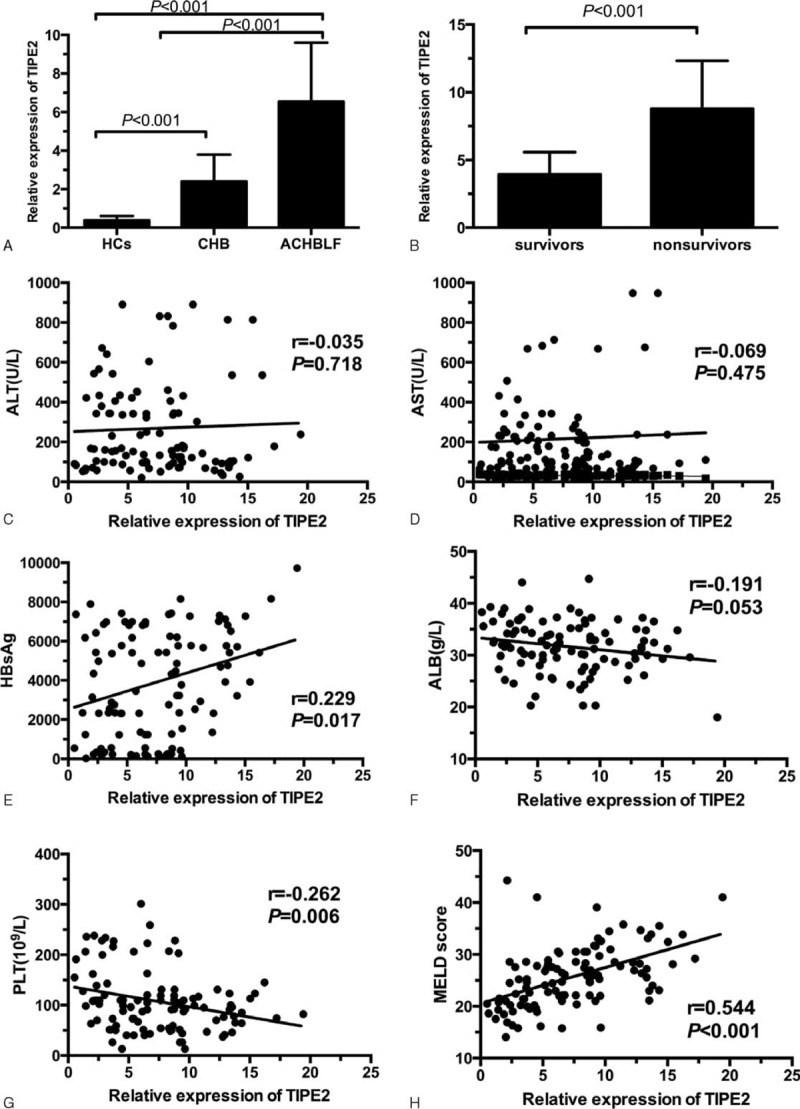
Expression of TIPE2 mRNA from peripheral blood mononuclear cells and associations with clinical characteristics in acute-on-chronic hepatitis B liver failure (ACHBLF) patients. (A) TIPE2 mRNA levels in ACHBLF, chronic hepatitis B (CHB), and healthy control (HC) group; (B) TIPE2 mRNA level in survivors and nonsurvivors of patients with ACHBLF (P < 0.001); (C) correlation between TIPE2 mRNA level and ALT level (r = −0.035, P = 0.718); (D) correlation TIPE2 mRNA level and AST level (r = −0.069, P = 0.475); (E) correlation between TIPE2 mRNA level and HBsAg (r = 0.229, P = 0.017); (F) correlation between TIPE2 mRNA level and ALB level (r = −0.191, P = 0.053); (G) correlation between TIPE2 mRNA level and PLT (r = −0.262, P = 0.006); and (H) correlation between TIPE2 mRNA level and MELD score (r = 0.544, P < 0.001). ALB = albumin, ALT = alanine aminotransferase, AST = aspartate aminotransferase, HBsAg = hepatitis B surface antigen, PLT = platelet, TIPE2 = tumor necrosis factor-α-induced protein 8-like 2.
Then, we analyzed the correlation between TIPE2 mRNA levels and clinical parameters using spearman analysis. As shown in Figure 2C to F, the expression of TIPE2 mRNA was significantly positively correlated with MELD score (r = 0.544, P < 0.001), HBsAg (r = 0.229, P < 0.05), and negatively correlated with PLT (r = −0.262, P < 0.05). No significant correlation has been observed between TIPE2 mRNA and age (r = 0.141, P = 0.146), ALT (r = −0.035, P = 0.718), AST (r = −0.069, P = 0.475), ALB (r = −0.191, P = 0.053), WBC (r = 0.185, P = 0.055), and HGB (r = 0.033, P = 0.734).
Univariate and Multivariate Analysis for the Risk Factors to 3-Month Mortality of ACHBLF Patients
Univariate Cox proportional hazards regression analysis was done for determining the association of clinical parameters with mortality and survival time. Table 4 shows that ALB (HR = 0.948, 95% CI: 0.906–0.993, P < 0.05), WBC (HR = 1.045, 95% CI: 1.015–1.076, P = 0.003), cirrhosis (HR = 1.977, 95% CI: 1.199–3.263, P = 0.008), SBP (HR = 3.052, 95% CI: 1.875–4.967, P < 0.001), ascites (HR = 1.855, 95% CI: 1.141–3.017, P < 0.05), HE (HR = 3.353, 95% CI: 2.062–5.453, P < 0.001), MELD score (HR = 1.093, 95% CI: 1.052–1.136, P < 0.001), and TIPE2 mRNA (HR = 1.202, 95% CI: 1.140–1.267, P < 0.001) were significantly associated with mortality in the training cohort.
TABLE 4.
Uni- and Multivariate Cox Regression Analysis of Prognosis Factors Associated With Survival in Patients With ACHBLF

All the above-mentioned variables were entered into a forward conditional step-wise Cox proportional hazards regression model to identify independent risk factors for the prognosis of ACHBLF patients. Cox proportional hazards regression analyses showed 5 independent risk factors associated with 3-month mortality of ACHBLF: WBC (HR = 1.058, 95% CI: 1.023–1.095, P = 0.001), SBP (HR = 2.541, 95% CI: 1.378–4.686, P = 0.003), HE (HR = 1.848, 95% CI: 1.028–3.321, P = 0.040), MELD score (HR = 1.062, 95% CI: 1.009–1.118, P = 0.021), and TIPE2 mRNA (HR = 1.081, 95% CI: 1.009–1.159, P = 0.027).
TIPE2 mRNA in PBMCs as a Predictor for 3-Month Mortality of ACHBLF Patients in Training Cohort
The predictive values of TIPE2 mRNA and MELD score in predicting 3-month mortality for ACHBLF patients were assessed by area under the receiver operating characteristic curve (AUROC). The AUROC was 0.849 (SE: 0.0360, 95% CI: 0.767–0.911) for TIPE2 mRNA and 0.722 (SE: 0.0484, 95% CI: 0.628–0.804) for MELD score (P < 0.005), indicating TIPE2 mRNA might be a better predictor than MELD for prognosis of ACHBLF (Figure 3A). An optimal cut-off value of 6.54 for the level of TIPE2 mRNA was identified to discriminate survival or death in ACHBLF with sensitivity of 74.63%, specificity of 90.24%, positive predictive value (PPV) of 92.5%, and negative predictive value (NPV) of 67.3%. An MELD score of 24.64 were identified as the best cut-off value with sensitivity of 71.64%, specificity of 68.29%, PPV of 78.7 %, and NPV of 59.6%. According to their expression of TIPE2 above 6.54 or not, we further divided the patients with ACHBLF into 2 groups and the Kaplan–Meier test was used to compare the survival rate. Log-rank analysis showed that the group with a high TIPE2 level >6.54 and MELD score >24.64 had a significantly lower survival rate, indicating that TIPE2 might be an independent predictor of 3-month mortality of ACHBLF patients (Figure 3B and D).
FIGURE 3.

TIPE2 mRNA in PBMCs as a predictor for 3-month mortality of ACHBLF patients in training cohort. (A) The receiver operating characteristic (ROC) curve for TIPE2 was 0.849 (SE: 0.0360, 95% CI: 0.767–0.911) and for MELD score 0.722 (SE: 0.0484, 95% CI: 0.628–0.804). There was a significant different between them (P < 0.05). (B) Kaplan–Meier graph showing the survival probability in ACHBLF patients with different TIPE2 mRNA. Patients with ACHBLF who had TIPE2 mRNA >6.54 showed significantly poorer survival than those had TIPE2 mRNA ≤6.54 (P < 0.001, log-rank test); (C) ROC curves of TIPE2 mRNA level plus MELD score and MELD score alone; (D) Kaplan–Meier graph showing patients with ACHBLF who had MELD score >24.64 showed significantly poorer survival than those had MELD score ≤24.64 (P < 0.001, log-rank test). ACHBLF = acute-on-chronic hepatitis B liver failure, CI = confidence interval, MELD = model for end-stage liver diseases, PBMC = peripheral blood mononuclear cell, SE = standard error, TIPE2 = tumor necrosis factor-α-induced protein 8-like 2.
To assess the combined influence of TIPE2 level and MELD score on the prediction of 3-month mortality, a new variable predicted probability (P) was established according to an equation obtained by binary logistic regression:
Variables (TIPE2 plus MELD) = 0.041 × MELD + 0.415 × TIPE2 − 3.181.
ROC analysis was used to evaluate the diagnostic value of TIPE2 plus MELD score for 3-month mortality of ACHBLF. Figure 3C shows that the TIPE2 plus MELD score had an AUROC of 0.853 (SE: 0.0357, 95% CI: 0.772–0.914), which was significantly higher than that of MELD score (P < 0.05).
Validation for TIPE2 mRNA as a Predictor for 3-Month Mortality of ACHBLF Patients
To validate the finding from training cohort, 63 consecutive patients with ACHBLF were set as a prospective validation cohort. Similar to the training cohort, AUROC for TIPE2 mRNA (0.912, SE: 0.0369, 95% CI: 0.814–0.969) was significantly higher than MELD score (0.717, SE: 0.0640, 95% CI: 0.590–0.824; P = 0.001) (Figure 4A). Figure 4B and D shows that ACHBLF patients with baseline TIPE2 > 6.54 and MELD > 24.64 had a significantly short survival time compared with those with TIPE2 of more than 6.54 and MELD score of more than 24.64 (log-rank test, P < 0.001, respectively). ROC analysis was used to evaluate the diagnostic value of TIPE2 plus MELD score for 3-month mortality of ACHBLF in the validation cohort. Figure 4C shows that TIPE2 mRNA plus MELD score had an AUROC of 0.909 (SE: 0.0375, 95% CI: 0.810–0.967), which was significantly higher than that of MELD score (P = 0.0004).
FIGURE 4.
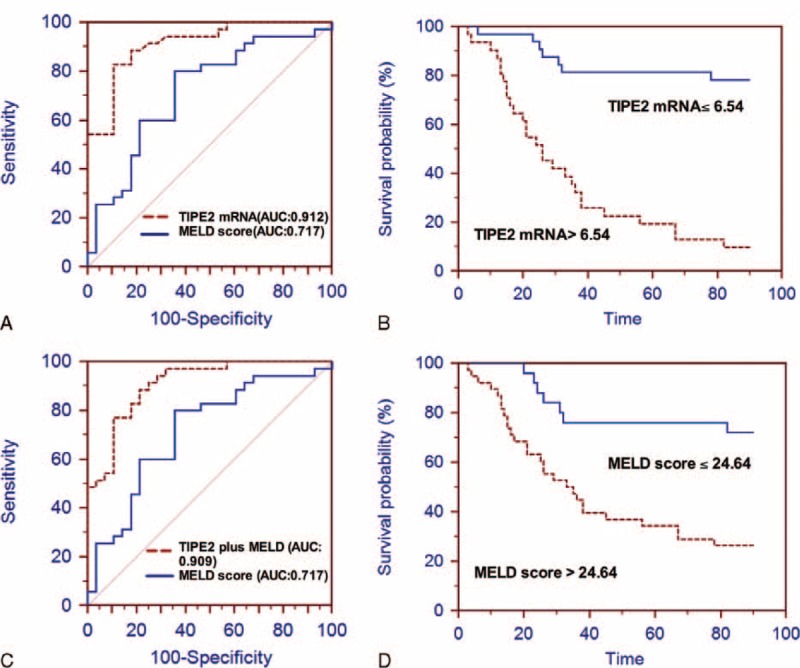
Validation for TIPE2 mRNA as a predictor for 3-month mortality of ACHBLF patients. (A) ROC analysis of the predictive accuracy of TIPE2 mRNA to predict 3-month mortality of ACHBLF in validation cohort. ROC curve for TIPE2 mRNA was 0.912 (SE: 0.0369, 95% CI: 0.814–0.969) and for MELD score 0.717 (SE: 0.0640, 95% CI: 0.590–0.824; P = 0.001). (B) Patients with ACHBLF in validation cohort who had TIPE2 mRNA >6.54 showed significantly poorer survival than those had TIPE2 mRNA ≤6.54 (P < 0.001, log-rank test); (C) ROC curves of TIPE2 mRNA level plus MELD score and MELD score alone; (D) Kaplan–Meier graph showing patients with ACHBLF who had MELD score >24.64 showed significantly poorer survival than those had MELD score ≤24.64 (P < 0.001, log-rank test). ACHBLF = acute-on-chronic hepatitis B liver failure, CI = confidence interval, MELD = model for end-stage liver diseases, ROC = receiver operating characteristic, SE = standard error, TIPE2 = tumor necrosis factor-α-induced protein 8-like 2.
DISCUSSION
Up to date, exploring effective and accurate noninvasive biomarker for the prognosis of ACHBLF is still a hot spot in this research field. TIPE2 is a novel identified negative regulator of immune response and we have previously demonstrated TIPE2 play an essential role in the pathogenesis of ACHBLF. Therefore, we hypothesized that TIPE2 mRNA might be a useful biomarker for predicting the outcome of ACHBLF patient. In this present study, we firstly reported the significant elevation of TIPE2 mRNA in PBMCs from ACHBLF patients compared with CHB and HCs. Spearman analysis showed TIPE2 mRNA was significantly correlated with MELD score, HBsAg and PLT level. These results strongly indicated that TIPE2 might contribute to the pathogenesis of ACHBLF. Furthermore, we mainly focused on investigating the predicative value of TIPE2 mRNA in the prognosis of ACHBLF. Univariate and multiple Cox regression analysis revealed that TIPE2 mRNA was an independent risk factor for 3-month mortality. This present study was the first report to demonstrate that TIPE2 mRNA could serve as a promising predictor in 3-month mortality in training and validation cohorts. In addition, ROC analysis illustrated that TIPE2 mRNA performed better than MELD score in predicting 3-month mortality in both training and validation cohorts. Finally, we demonstrated that combination of TIPE2 mRNA plus MELD score would definitely improve the diagnostic value of MELD alone in predicting 3-month mortality of patients with ACHBLF.
We supposed that TIPE2 mRNA might contribute to the disease pathogenesis of ACHBLF basing on the following aspects: First, TIPE2 mRNA expression in patients with ACHBLF was significantly elevated and positively correlated with MELD score, which often serve as markers of diseases severity in patients with ACHBLF. Second, TIPE2 mRNA expression of nonsurvivors was significantly higher than survivors and we also observed the dynamic increase of TIPE2 mRNA in nonsurvivors. Third, the multivariate Cox proportional hazards regression analyses revealed TIPE2 mRNA was the independent risk factors for predicting poor short-term mortality of ACHBLF. Overall, the increased TIPE2 mRNA level could provide timely knowledge for the outcomes of ACHBLF. TIPE2 is an newly identified negative regulator in innate and adaptive immune response and functions complex roles in human disease.22 Previous reported demonstrated that TIPE2 might regulate HBV-specific CD8(+) T cells function and contribute to the pathogenesis of hepatitis B.18 A similar degree of cellular immune depression like severe sepsis was regarded as a common characteristic of liver failure, resulting in high mortality associated with variceal bleeding and multi-organ failure.23,24 Despite little known about the pathophysiology of ACHBLF, abrupt strength of immune response is demonstrated to contribute to the pathogenesis of ACHBLF.25 In this present study, the expression of TIPE2 mRNA in patients with ACHBLF was significantly positively correlated with MELD score, HBsAg and negatively correlated with PLT. We also found the increased TIPE2 mRNA in nonsurvivors and these results might be explained by the role of TIPE2 in inhibiting the pathway function via T cell receptors and Toll-like receptors and then leading to infection and mortality.7 However, the exact mechanism for the function of TIPE2 in ACHBLF should be extensively studied in the future studies.
ACHBLF was defined as acute exacerbation of liver function as progressive jaundice and coagulopathy, complicated by the syndromes with ascites and/or encephalopathy during the progression of CHB.1 Therefore, the occurrence of ascites and HE would definitely contribute to the high mortality of ACHBLF.26,27 In this present study, we identified that white blood cells, SBP, HE, MELD score, and TIPE2 mRNA were independent risk factors for the 3-month mortality of ACHBLF. The finding also supports the importance of ascites and HE in the progression of ACHBLF. In fact, HE is the most leading cause for death in ACHBLF patients. SBP is an infection of ascites caused by translocation of bacteria from the intestinal lumen into the systemic circulation and its mortality reduced to approximately 20% with early diagnosis and treatment.28 Gram-negative bacteria are common pathogens in patients with culture-positive SBP.28 Of note, we revealed that TIPE2 mRNA was also an independent risk factor for the outcome of ACHBLF, which strongly suggested the potential role of TIPE2 mRNA in the pathogenesis of ACHBLF and to be a possible biomarker for the predicting of ACHBLF prognosis.
MELD score is widely accepted and used as a relatively accurate model to evaluate short-term mortality risk in patients with cirrhosis.29 Despite the many advantages of the MELD score, there are approximately 15% to 20% of patients whose survival cannot be accurately predicted by MELD score.30 Recently, persistent ascites and low serum sodium identify patients with cirrhosis with high mortality risk despite low MELD score.31 When a cirrhotic patient experiences an acute event, such as an infection, their pre-event MELD does not accurately predict their mortality risk. These results indicated that diagnosis value of MELD scores to discriminate the progression of ACHBLF is not well robust. So, an accurate and simple prognostic factor in patients with ACLF is urgent need. In present study, ROC analysis was used to evaluate the diagnostic value of TIPE2 score for 3-month mortality of ACHBLF. The result showed that TIPE2 mRNA level had a better ROC level than MELD score, which is a well established and commonly used scoring system for predicting prognosis and survival in liver disease. A cut-off value of 6.54 for the level of TIPE2 mRNA was used to discriminate survival or death with ACHBLF with sensitivity of 74.63%, specificity of 90.24%, PPV of 92.5%, and NPV of 67.3%. A MELD score of 24.64 were identified as the best cut-off value with sensitivity of 71.64%, specificity of 68.29%, PPV of 78.7%, and NPV of 59.6%. Moreover, measurement of TIPE2 and MELD together significantly improved the diagnostic value of MELD alone. The performance of the TIPE2 mRNA level was significantly better than that of MELD in both the training and validation cohorts. Thus, TIPE2 mRNA level might be a simple and promising marker for short-term mortality in patients with ACHBLF.
There are some limitations in our study. Firstly, the expression of TIPE2 mRNA was built and tested on a single-center cohort. It could be argued that data originating from other centers might lead to different conclusions. Thus, multicenter, prospective studies of a large dataset are all needed in the validation analysis for our further research. Meanwhile, the molecular mechanism about how TIPE2 was involved in the progress of ACHBLF remained unclear and might also be studied in our further study. However, the present study was the first report to demonstrate that TIPE2 mRNA could serve as a promising predictor in 3-month mortality using training and validation cohorts in prospective study. The findings would provide a meaningful clue to improve the diagnosis accuracy of MELD score using immune negative regulatory molecule as noninvasive biomarker with great clinic significance.
In summary, TIPE2 mRNA level might be a novel biomarker in predicting severity of ACHBLF. Combination of TIPE2 mRNA and MELD would improve the diagnostic value of MELD alone in predicting 3-month mortality of patients with ACHBLF.
Footnotes
Abbreviations: ACHBLF = acute-on-chronic hepatitis B liver failure, AFP = α-fetoprotein, ALB = albumin, ALT = alanine aminotransferase, APASL = the Asian Pacific Association for the Study of the Liver, AST = aspartate aminotransferase, AUROC = area under the receiver operating characteristic curve, CHB = chronic hepatitis B, CI = confidence interval, Cr = creatinine, HBeAg = hepatitis B e antigen, HBsAg = hepatitis B surface antigen, HC = healthy control, HE = hepatic encephalopathy, HGB = hemoglobin, HR = hazard ratio, INR = international normalized ratio, LC = liver cirrhosis, MELD = model for end-stage liver diseases scores, NPV = negative predictive value, PBMCs = peripheral blood mononuclear cells, PLT = platelet, PPV = positive predictive value, PTA = prothrombin activity, SBP = spontaneous bacterial peritonitis, SE = standard error, TIPE2 = tumor necrosis factor-α-induced protein 8-like 2, WBC = white blood cell.
Y-CF and NW contributed equally to this work.
Author contributions: Y-CF, NW, and Y-YS contributed to the majority of the experiments and data collection. Y-CF, NW, and X-YX contributed to data analysis. Y-CF, NW, and KW contributed to the study design and drafted the manuscript. Y-CF and KW conceived the study. KW was responsible for study supervision and obtaining funding.
This work is supported by the grants from National Natural Science Foundation of China (81201287, 81171579, and 81371832), Key Project of Chinese Ministry of Science and Technology (2012ZX10002007and 2013ZX10002001), and Science and Technology Development Plan of Shandong Province (2014GSF118068).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Sarin SK, Kumar A, Almeida JA, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int 2009; 3:269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jia J-D, Zhuang H. A winning war against hepatitis B virus infection in China. Chin Med J 2007; 120:2157–2158. [PubMed] [Google Scholar]
- 3.Nava LEZ, Valadez JA, Chavez-Tapia NC, et al. Acute-on-chronic liver failure: a review. Ther Clin Risk Manag 2014; 10:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H, Xia Q, Zeng B, et al. Submassive hepatic necrosis distinguishes HBV-associated acute on chronic liver failure from cirrhotic patients with acute decompensation. J Hepatol 2015; 63:50–59. [DOI] [PubMed] [Google Scholar]
- 5.Chan AC, Fan ST, Lo CM, et al. Liver transplantation for acute-on-chronic liver failure. Hepatol Int 2009; 3:571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu FL, Shi KQ, Chen YP, et al. Scoring systems predict the prognosis of acute-on-chronic hepatitis B liver failure: an evidence-based review. Exp Rev Gastroenterol Hepatol 2014; 8:623–632. [DOI] [PubMed] [Google Scholar]
- 7.Sun H, Gong S, Carmody RJ, et al. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell 2008; 133:415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xi WJ, Hu YJ, Liu YG, et al. Roles of TIPE2 in hepatitis B virus-induced hepatic inflammation in humans and mice. Mol Immunol 2011; 48:1203–1208. [DOI] [PubMed] [Google Scholar]
- 9.Kong L, Liu K, Zhang YZ, et al. Downregulation of TIPE2 mRNA expression in peripheral blood mononuclear cells from patients with chronic hepatitis C. Hepatol Int 2013; 7:844–849. [DOI] [PubMed] [Google Scholar]
- 10.Li D, Song LJ, Fan YC, et al. Down-regulation of TIPE2 mRNA expression in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Clin Immunol 2009; 133:422–427. [DOI] [PubMed] [Google Scholar]
- 11.Lou YW, Sun HH, Morrissey S, et al. Critical roles of TIPE2 protein in murine experimental colitis. J Immunol 2014; 193:1064–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Wei XB, Liu LX, et al. TIPE2, a novel regulator of immunity, protects against experimental stroke. J Biol Chem 2012; 287:32546–32555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang SY, Zhang Y, Wei XB, et al. Expression and regulation of a novel identified TNFAIP8 family is associated with diabetic nephropathy. BBA-Mol Basis Dis 2010; 1802:1078–1086. [DOI] [PubMed] [Google Scholar]
- 14.Cao XL, Zhang L, Shi YY, et al. Human tumor necrosis factor (TNF)-alpha-induced protein 8-like 2 suppresses hepatocellular carcinoma metastasis through inhibiting Rac1. Mol Cancer 2013; 12:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Q, Zhao M, Dong T, et al. Tumor necrosis factor-alpha-induced protein-8 like-2 (TIPE2) upregulates p27 to decrease gastic cancer cell proliferation. J Cell Biochem 2015; 116:1121–1129. [DOI] [PubMed] [Google Scholar]
- 16.Zhang G, Zhao L, Wang Y, et al. TIPE2 protein prevents injury-induced restenosis in mice. Biochim Biophys Acta 2015; 1852:1574–1584. [DOI] [PubMed] [Google Scholar]
- 17.Lou Y, Liu S, Zhang C, et al. Enhanced atherosclerosis in TIPE2-deficient mice is associated with increased macrophage responses to oxidized low-density lipoprotein. J Immunol 2013; 191:4849–4857. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Zhang J, Zhao L, et al. TIPE2 protein negatively regulates HBV-specific CD8(+) T lymphocyte functions in humans. Mol Immunol 2015; 64:204–209. [DOI] [PubMed] [Google Scholar]
- 19.Wang LY, Fan YC, Zhao J, et al. Elevated expression of tumour necrosis factor-alpha-induced protein 8 (TNFAIP8)-like 2 mRNA in peripheral blood mononuclear cells is associated with disease progression of acute-on-chronic hepatitis B liver failure. J Viral Hepat 2014; 21:64–73. [DOI] [PubMed] [Google Scholar]
- 20.Hellmann F, Verdi M, Schlemper BR, Jr, et al. 50th anniversary of the Declaration of Helsinki: the double standard was introduced. Arch Med Res 2014; 45:600–601. [DOI] [PubMed] [Google Scholar]
- 21.Sarin SK, Kedarisetty CK, Abbas Z, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int 2014; 8:453–471. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Shi Y, Wang Y, et al. The unique expression profile of human TIPE2 suggests new functions beyond its role in immune regulation. Mol Immunol 2011; 48:1209–1215. [DOI] [PubMed] [Google Scholar]
- 23.Wasmuth HE, Kunz D, Yagmur E, et al. Patients with acute on chronic liver failure display “sepsis-like” immune paralysis. J Hepatol 2005; 42:195–201. [DOI] [PubMed] [Google Scholar]
- 24.Arvaniti V, D’Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 2010; 139:1246–1256.1256.e1241–1245. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Zhang H, Wan G, et al. Neutrophil-lymphocyte ratio: a novel predictor for short-term prognosis in acute-on-chronic hepatitis B liver failure. J Viral Hepat 2014; 21:499–507. [DOI] [PubMed] [Google Scholar]
- 26.Zheng MH, Shi KQ, Lin XF, et al. A model to predict 3-month mortality risk of acute-on-chronic hepatitis B liver failure using artificial neural network. J Viral Hepat 2013; 20:248–255. [DOI] [PubMed] [Google Scholar]
- 27.Stadlbauer V, Davies NA, Sen S, et al. Artificial liver support systems in the management of complications of cirrhosis. Semin Liver Dis 2008; 28:96–109. [DOI] [PubMed] [Google Scholar]
- 28.European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 2010; 53:397–417. [DOI] [PubMed] [Google Scholar]
- 29.Northup PG, Intagliata NM, Shah NL, et al. Excess mortality on the liver transplant waiting list: unintended policy consequences and model for end-stage liver disease (MELD) inflation. Hepatology 2015; 61:285–291. [DOI] [PubMed] [Google Scholar]
- 30.Kamath PS, Kim WR. The model for end-stage liver disease (MELD). Hepatology 2007; 45:797–805. [DOI] [PubMed] [Google Scholar]
- 31.Heuman DM, Abou-Assi SG, Habib A, et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology 2004; 40:802–810. [DOI] [PubMed] [Google Scholar]


