Abstract
Immune cells contribute to determining the prognosis of gastric cancer. However, their exact role is less clear.
We determined the prognostic significance of different immune cells in intratumoral tissue (T), stromal tissue (S), and adjacent normal tissue (N) of 166 gastric cancer cases and their interactions, including CD3+, CD4+, CD8+, CD57+, CD68+, CD66b+, and Foxp3+ cells, and established an effective prognostic nomogram based on the immune reactions.
We found high densities of TCD3+, TCD4+, TCD8+, SCD3+, SCD4+, SCD57+, SCD66b+, and NFoxp3+ cells, as well as high TCD8+/SCD8+ ratio, TCD68+/SCD68+ ratio, TCD3+/TFoxp3+ ratio, TCD4+/TFoxp3+ ratio, TCD8+/TFoxp3+ ratio, SCD3+/SFoxp3+ ratio, and SCD4+/SCD8+ ratio were associated with better survival, whereas high densities of TCD66b+, TFoxp3+, SFoxp3+ and NCD66b+ cells as well as high TCD57+/SCD57+ ratio, TCD66b+/SCD66b+ ratio, SCD8+/SFoxp3+ ratio, and TFoxp3+/NFoxp3+ ratio were associated with significantly worse outcome. Multivariate analysis indicated that tumor size, longitudinal tumor location, N stage, TCD68+/SCD68+ ratio, TCD8+/TFoxp3+ ratio, density of TFoxp3+ cells, and TCD66b+/SCD66b+ ratio were independent prognostic factors, which were all selected into the nomogram. The calibration curve for likelihood of survival demonstrated favorable consistency between predictive value of the nomogram and actual observation. The C-index (0.83, 95% CI: 0.78 to 0.87) of our nomogram for predicting prognosis was significantly higher than that of TNM staging system (0.70).
Collectively, high TCD68+/SCD68+ ratio and TCD8+/TFoxp3+ ratio were associated with improved overall survival, whereas high density of TFoxp3+ cells and TCD66b+/SCD66b+ ratio demonstrated poor overall survival, which are promising independent predictors for overall survival in gastric cancer.
INTRODUCTION
Gastric cancer is one of the common malignancies with high incidence in the world, especially in East Asian countries.1 Currently, the main treatment of gastric cancer consists of surgical resection plus standard D2 lymphadenectomy, adjuvant chemotherapy, and some molecular targeting therapy.2–4 Although our cognitions on gastric cancer have been significantly developed in recent years, the prognosis was still undesirable yet. In addition, it is very common that gastric cancer patients with the same TNM stage have the different long-term survival. Therefore, in order to improve the long-term survival, it is important to better understand the mechanisms of disease progression and find new effective predictive prognostic factors as the targets of interventions. Although many predictive factors have been evaluated, such as clinicopathologic factors, biomarkers, genes, and microsatellite instability,5–7 their prognostic accuracies are controversial and an ideal factors has not yet been found. Recently, it became clearer that there is a positive correlation between the presence of tumor-infiltrating inflammatory cells (TLCs) and survival of patients with malignancies.8–14 The types, density, and location of immune cells are even more accurate in predicting prognosis than the currently used the TNM stage for colon cancer,8 which suggests that evaluation of the TLCs might be more useful for further comprehension of tumor development, prediction of prognosis, and immunotherapy.
Recent studies have highlighted several types of TLCs, such as CD3+ T cells, CD8+ T cells, regulatory T cells (Tregs), natural killer cells (NKC), neutrophils or macrophages cells (MAC), are associated with disease outcomes for various human cancers.8–15 For gastric cancer, it was reported that the combination of high numbers of intratumoral macrophage and Tregs was associated with improved survival.15 However, others showed the Tregs played a role of immunosuppression and tumor progression in patients with gastric and esophageal cancers and led to a poorer prognosis.16 Intratumoral high Tregs /CD8+ T cells ratio was an independent predictor for the worse prognosis of gastric cancer.17 However, CD4+ and CD8+ TLCs were not associated with overall survival.17 It was also found that tumor-infiltrating neutrophils were significantly associated with higher survival rates in gastric cancer,18 but the presence of intratumoral neutrophil was an independent factor of poor prognosis for patients with other cancers.12
Therefore, the above results provide strong evidence that immune cells contribute to determining the prognosis of gastric cancer. However, the exact role of immune cells in gastric cancer is less clear. On the other hand, whether immune cells play a protecting or promoting role only can be interpreted after understanding the definite functions of each cell phenotype in this process.19 The aims of the present study were to determine the prognostic significance of different immune cells and their interactions in gastric cancer, including CD3+ (Marker of T cells), CD4+ (Marker of T helper cells), CD8+ (Marker of cytotoxic T cells), CD57+ (Marker of natural killer cells), CD68+ (Marker of macrophage), CD66b+ (Marker of neutrophil), and Foxp3+ (Marker of Tregs) cells. This study also aimed to establish an effective prognostic nomogram based on the immune cells infiltration. To our limited knowledge, this is the first report demonstrating prognostic values of various kinds of immune cells and their combined effects between cells. In addition, this is also the first time that the tumor compartments were considered separately by intratumoral tissue (T), stromal tissue (S), and adjacent normal tissue (N) simultaneously.
MATERIALS AND METHODS
Patients and Specimens
Formalin-fixed, paraffin-embedded specimens were obtained from 166 patients who under surgical resection for gastric adenocarcinoma in West China Hospital, Sichuan University between 2006 and 2009. Clinicopathological and follow-up data of these patients were collected from our prospective database of gastric cancer. Clinicopathological data including demographic parameters, tumor size (cm), Borrmann types, T, N, M, stage, and degree of tumor differentiation (well differentiated, moderate, poor, signet-ring cell, and mucinous type) were reviewed. Clinicopathological terminology was based on the Japanese classification of gastric carcinoma (3rd English version).20 The West China Hospital research ethics committee approved retrospective analysis of anonymous data.
Follow-up
Overall survival was calculated from the time of surgery until death or the last observation for surviving patients. Follow-up assessments were performed every 3 to 6 months for the first 2 years, every 6 to 12 months for 3 to 5 years after surgery and then annually.21 The postoperative follow-up was carried out by regular out-patient visits and telephone interviews. Follow-up information was updated to December, 2014. Reasons for those patients lost to follow-up were mainly due to cancellation of out-patient visit or change of telephone number and address. The overall follow-up rate was 90.36% (150/166). Sixteen patients were lost to follow-up.
Immunohistochemistry (IHC)
Formalin-fixed paraffin-embedded tissue specimens were consecutively sliced into 4 μm-thick sections. Primary polyclonal antibodies including anti-CD3 clone SP7 (dilution 1:250; Thermo Scientific, Fremont, CA), anti-CD4 clone 113 (dilution 1:200; Sino Biological, BDA, Beijing, PR China), anti-CD8 clone SP16 (dilution 1:150; Thermo Scientific, Fremont, CA), anti-CD57 clone NK1 (dilution 1:100; Thermo Scientific, Fremont, CA), anti-CD66b clone 80H3 (dilution 1:100; LifeSpan Biosciences, Seattle, WA), anti-CD68 clone KP1 (dilution 1:800; eBioscience, San Diego, CA), and anti-Foxp3 clone 236A/E7 (dilution 1:100; eBioscience, San Diego, CA) were used. A 2-step protocol (Novolink Polymer Detection System, Novocastra, Newcastle, UK) was performed on the paraffin sections for the immunohistochemistry. According to the manufacturer's instructions, paraffin sections were deparaffinaged in xylene and received gradient elution in ethanol. Then the slides were incubated in 0.3% H2O2 to block the endogenous peroxidase activity. Antigen retrieval was carried out by immersing the slides in the hot water of 95 centigrade degree for ∼45 min. Incubation with primary antibodies was performed followed by washing with phosphate-buffered saline and then incubation with secondary antibodies using GTVisionTM III Detection System/Mo&Rb (Gene Tech, Shanghai, PR China). The sections were pigmentized in 3, 3-diamiobenzidine (DAB) solution (dilution 1:50; Gene Tech, Shanghai, PR China) for ∼5 s under the monitoring of microscopic observation. Then all the sections were counterstained with haematoxylin. Negative control sections without primary antibodies were all performed in every series.
Evaluation of Immunohistochemical Variables
The number of immune cells was determined separately in the following compartment: (I) within the intratumoral tissue; (II) within the tumor stromal tissue at the invasive border; and (III) within the peripheral normal tissue (normal tissue with distance >1 microscope field at ×200 magnification from invasive border). Each section was evaluated for immune cells by microscopic examination (×400; BX51; Olympus, Tokyo, Japan). Five noncontiguous microscopic areas that represent the densest immune cells were randomly selected for each compartment on each sample in order to ensure representativeness and homogeneity. The numbers of immune cells in the 5 fields were accumulated and then averaged to calculate the mean number for 1 computerized 400× microscopic field (0.1590 mm2/field). The photographs were captured with a light microscope (BX51; Olympus, Tokyo, Japan) that connected with a personal computer and displayed on a high-resolution color 14-inch monitor. The evaluation of cells was performed by 2 independent pathologists that were blinded to clinicopathologic data. Variations in the enumeration, within a range of 5%, were re-evaluated and a consensus decision was made.
Statistical Analysis
Statistical analysis was performed using SPSS 19.0 (SPSS®, Chicago, IL). Variables of normality were tested, and if conforming to the normal distribution, data were expressed as mean ± standard deviation. Two independent t tests for quantitative data and chi-square test or Fisher's exact test for categorical data were performed; data were expressed as medians with a range taking the Spearman test into consideration. For all immunohistochemical variables, the median was used as the cutoff point for division of subgroups.11–13 Survival curves were derived from Kaplan–-Meier estimates, and the curves were compared by log-rank tests. Significant factors were identified by univariate analysis, and further examined by multivariate analysis. The multivariate regression was performed using the Cox proportion hazards model. A nomogram was formulated according to the results of multivariate analysis with R project (http://www.r-project.org/). The nomogram's predictive accuracy was measured via a concordance index (C-index) (the larger the C-index, the more accurate the prediction) and assessed by comparing prediction by nomogram and actual observed survival. A calibration curve showed as the plot of predicted probabilities from the nomogram versus the actual probabilities was generated. Comparisons between the nomogram and TNM staging systems20 were performed in R and were evaluated by the C-index. Two-sided P value <0.05 was considered as statistical significance.
RESULTS
Patient Characteristics
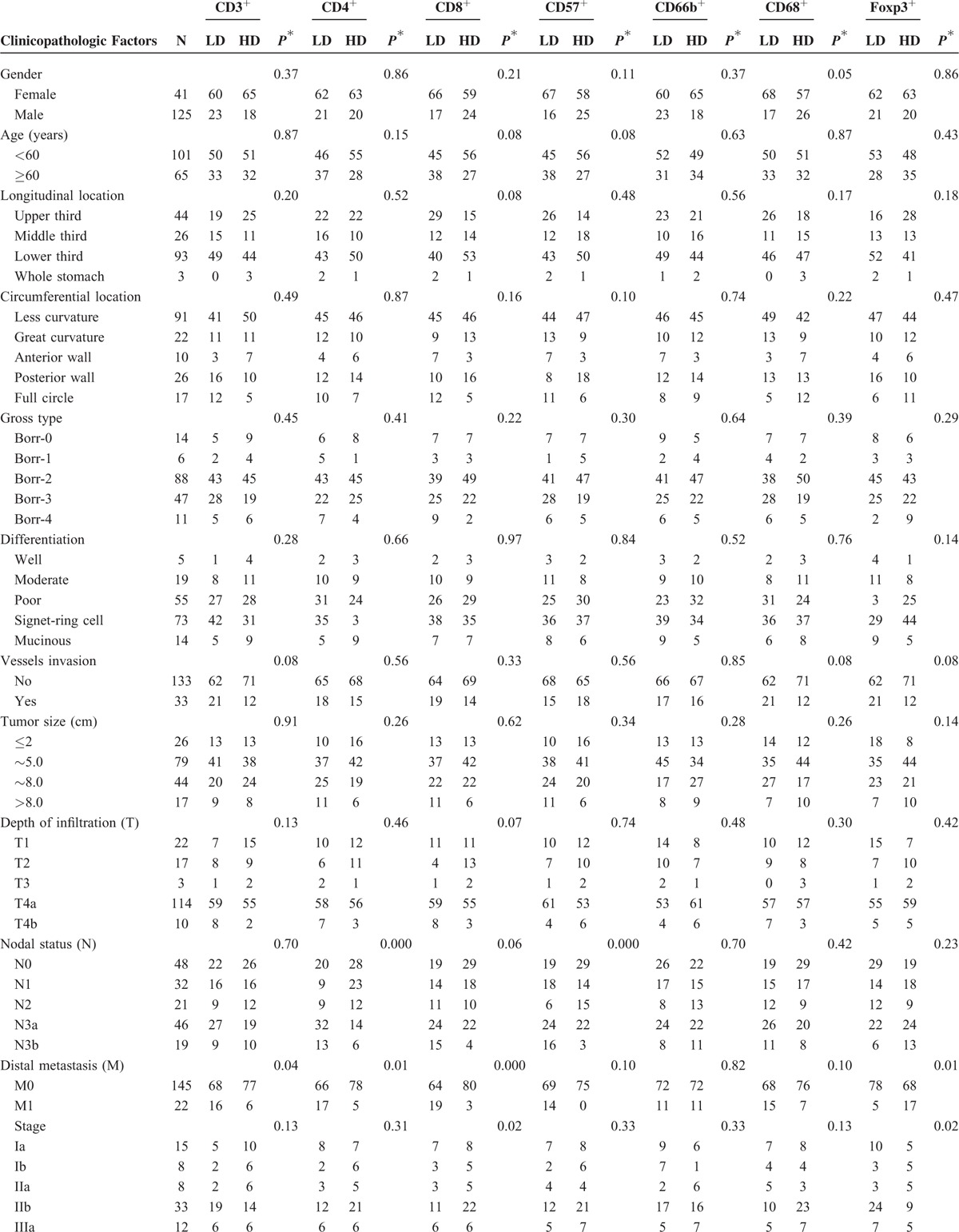
The characteristics of patients are presented in Table 1 . The mean age was 55.30 ± 11.87 years (range, 19–79 years), and 75.3% of patients were men. Only 22 patients (13.3%) had early gastric cancer, and 142 patients (85.5%) had poor differentiation. Eighty eight (53.0%) patients had postoperative chemotherapy. The median number of harvested lymph nodes was 25.5 (11–69) in this study. Seventy-nine (47.6%) patients had died at the end of follow-up. The median duration of follow-up for patients was 65.88 months. The median survival for all patients was 79.57 months (95% confidence interval [CI]: 49.06–110.07 months). The 5-year survival for the study population was 52.0%.
TABLE 1.
Characteristics of Patients and Association of Intratumoral Infiltrating Cells With Clinicopathologic Factors
Immune Cells in Gastric Cancer and Correlations of Different Immune Cells
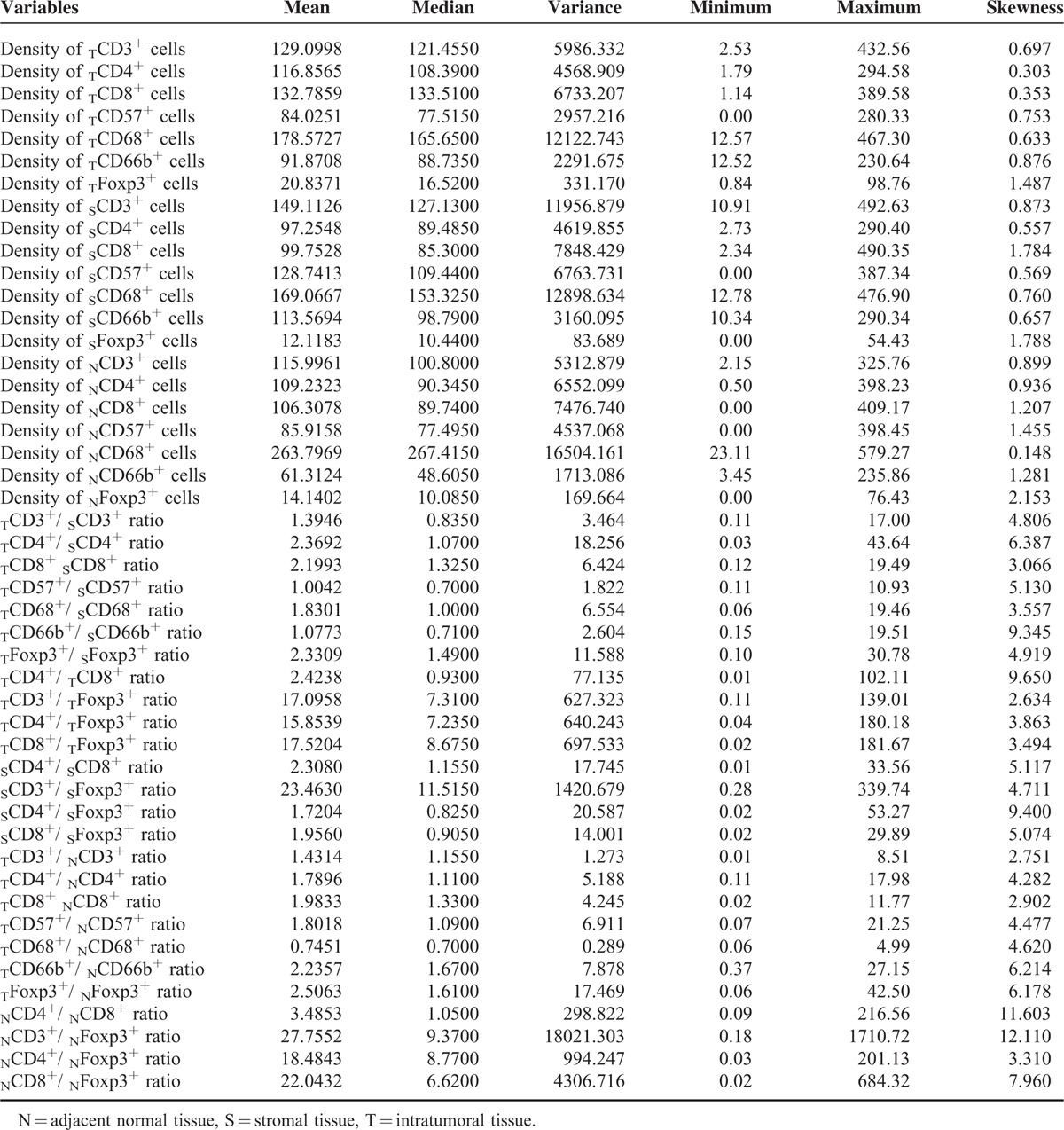
CD3+, CD4+, CD8+, CD57+, CD68+, and CD66b+ cells showed cell membrane staining, whereas Foxp3+ cells exhibited distinct nuclear staining. The distribution and density of positive cells varied substantially among samples. Representative images are shown in Figure 1. The densities of intratumoral CD8+ T cells (TCD8+) and TFoxp3+ cells were highest compared to stromal tissue and adjacent normal tissue, whereas the densities of CD3+ T cells, CD57+ cells, and CD66b+ cells were the highest in stromal tissue. The density of CD68+ cells was the highest in adjacent normal tissue. The density of CD4+ cells was significantly higher in intratumoral tissue than that of stromal tissue, but higher without significance compared to adjacent normal tissue. The average counts of immune cells are shown in Table 2. The densities of CD3+, CD4+, and CD8+ T cells were strongly associated with each other in intratumoral tissues. The densities of CD8+ and CD57+ cells, as well as CD4+ and CD68+ cells, were also significantly correlated in intratumoral tissues. The density of Foxp3+ cells was negatively correlated with CD3+, CD8+, CD57+, CD4+, and CD68+ cells in intratumoral tissues with significant difference. Other correlations between the immune cells are listed in Table 3.
FIGURE 1.

Representative pictures of CD8, CD68, CD66b, and Foxp3 immunostainings. (A) CD8+ (x200); (B) CD68+ (x200); (C) CD66b+ (x400); and (D) Foxp3+ (x400).
TABLE 1 (Continued).
Characteristics of Patients and Association of Intratumoral Infiltrating Cells With Clinicopathologic Factors
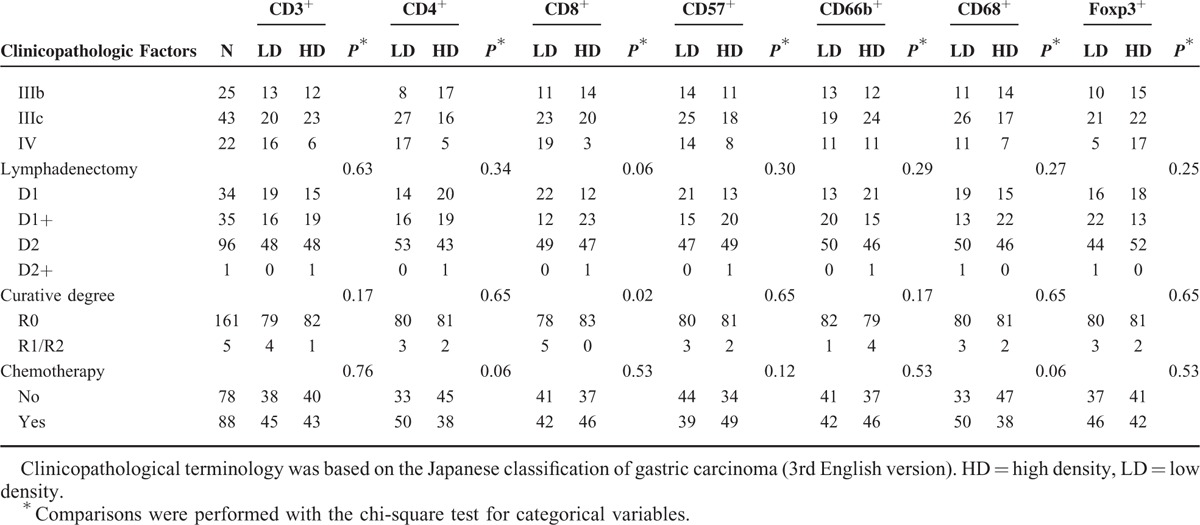
TABLE 2.
Descriptive Statistics of Immunohistochemical Variables
Association of TLCs with Clinicopathologic Factors
Associations between the densities of TLCs and clinicopathologic factors are listed in Table 1 . The densities of TCD3+, TCD4+, and TCD8+ cells were associated with M1 significantly. Tumors with more lymph nodes metastasis were found to have lower densities of TCD4+ and TCD57+ cells. The density of TCD8+ cells was significantly lower in tumors showing more advanced stages and therefore more palliative resections. Significant association was observed between the density of TCD68+ cells and gender. However, the density of TCD66b+ cells was not associated with either of these features. As expected, TFoxp3+ cell density was higher in tumors reported as M1 or more advanced stages. In terms of lymphadenectomy and chemotherapy, no differences were observed between patients with lower densities of TLCs and those with higher densities of TLCs.
Univariate Analysis
Table 4 shows the results of univariate survival analysis for the clinicopathologic features and for immunohistopathologic variables. Clinical factors statistically associated with overall survival were age, longitudinal tumor location, tumor size, Borrmann type, T, N, distant metastasis (M), stage, resection type, and lymphadenectomy. Densities of TCD3+, TCD4+, TCD8+, TCD66b+, TFoxp3+, SCD3+, SCD4+, SCD57+, SCD66b+, SFoxp3+, NCD66b+, and NFoxp3+ cells were associated with overall survival. Neither TCD68+ cells, sCD68+ cells, nor NCD68+ cells were associated with survival. High densities of TCD3+, TCD4+, TCD8+, SCD3+, SCD4+, SCD57+, SCD66b+, and NFoxp3+ cells were associated with better survival, whereas high densities of TCD66b+, TFoxp3+, SFoxp3+, and NCD66b+ cells were associated with significantly worse outcome. Patients with low density of TFoxp3+ cells had longer overall survival (median, 74.4 months) than did those with high density (median, 34.98 months). Due to the existence of synergistic or antagonistic effects between different kinds of immune cells and between the different locations of immune cells, the combined influences were also evaluated. Our results suggest the subgroup of patients with high TCD57+/SCD57+ ratio, TCD66b+/SCD66b+ ratio, SCD8+/SFoxp3+ ratio, and TFoxp3+/NFoxp3+ ratio demonstrated worse survival. The survival for patients with high TCD8+/SCD8+ ratio, TCD68+/SCD68+ ratio, TCD3+/TFoxp3+ ratio, TCD4+/TFoxp3+ ratio, TCD8+/TFoxp3+ ratio, SCD3+/SFoxp3+ ratio, and SCD4+/SCD8+ ratio were significantly improved.
TABLE 3.
Correlation Between Different Immune Cells
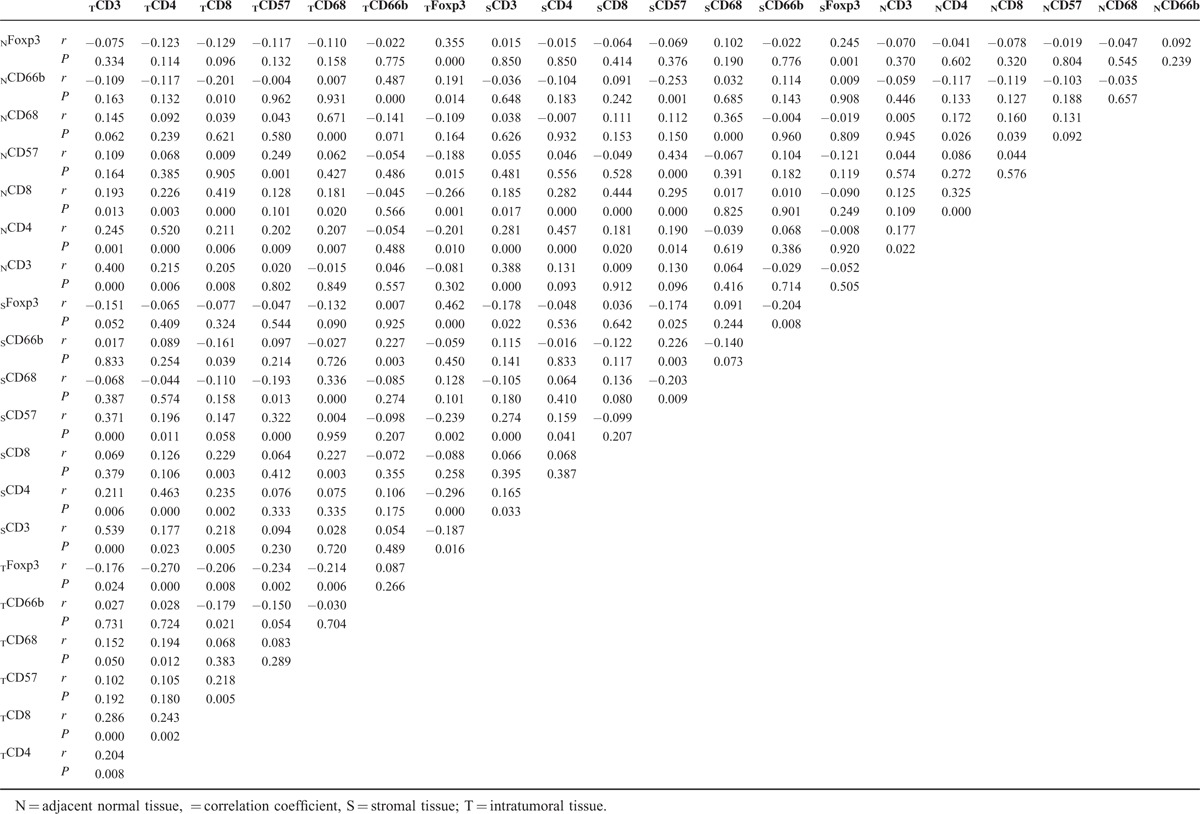
TABLE 4 (Continued).
Univariate Analyses of Factors Associated With Survival
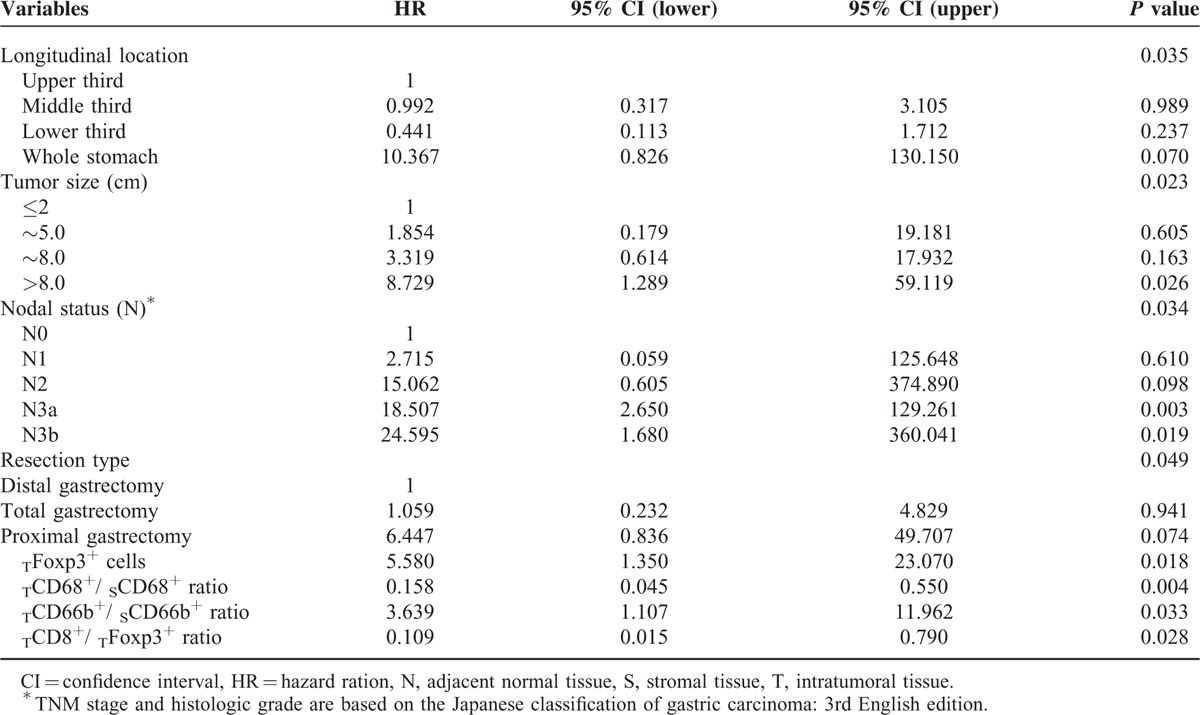
Multivariate Analysis
Clinicopathologic features and immunohistopathologic variables showing significances by univariate analysis were adopted as covariates when multivariate Cox proportional hazards analysis was performed. Details of the results are presented in Table 5. The analysis revealed that tumor size, longitudinal tumor location, and N stage were factors to show independent prognostic significances. High TCD68+/SCD68+ ratio and TCD8+/TFoxp3+ ratio were associated with improved overall survival, whereas high density of TFoxp3+ cells and TCD66b+/SCD66b+ ratio demonstrated a significant association with poor survival (Figure 2).
TABLE 4.
Univariate Analyses of Factors Associated With Survival
FIGURE 2.
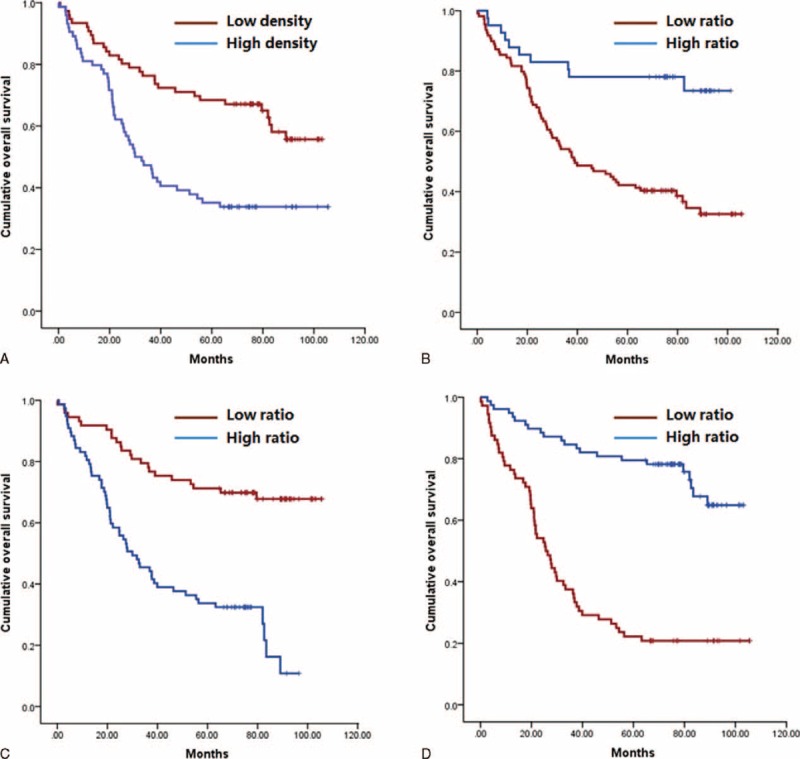
Kaplan–Meier analysis of overall survival. (A) High density of TFoxp3+ cells versus low density of TFoxp3+ cells; (B) high TCD68+/SCD68+ ratio versus low TCD68+/SCD68+ ratio; (C) high TCD66b+/SCD66b+ ratio versus low TCD66b+/SCD66b+ ratio; and (D) high TCD8+/TFoxp3+ ratio versus low TCD8+/TFoxp3+ ratio.
TABLE 5.
Multivariate Analyses of Factors Associated With Survival Outcomes
Prognostic Nomogram for Overall Survival and its Predictive Accuracy
Figure 3A revealed the prognostic nomogram integrating all significant independent factors identified in multivariate analysis. The C-index of our nomogram for predicting the prognosis was 0.83 with the 95% CI from 0.78 to 0.87. The calibration curve for likelihood of survival at 3 or 5 years demonstrated favorable consistency between the predictive value of the nomogram and actual observation (Figure 3B and C). Compared with the TNM staging system (0.70, 95% CI: 0.65–0.76), the C-index of our nomogram was statistically higher (P < 0.001), which validate the nomogram as a useful model to predict the long-term survival of gastric cancer patients.
FIGURE 3.
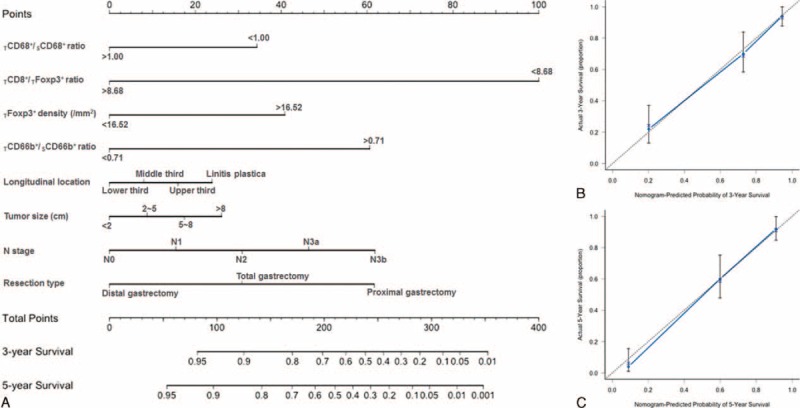
Gastric cancer survival nomogram (A). The calibration curve for predicting patient survival at 3 years (B) and 5 years (C).
DISCUSSION
Immune cells in the tumor microenvironment have been reported to impact on cancer development, progression, and cancer-related immune reactions, which has emerged as the hotspot of cancer research.22 In the present study, we performed an immunohistochemical evaluation of immune cells in gastric cancer. To our limited knowledge, this is the first report demonstrating prognostic values of various kinds of immune cells and their combined effects between different cells. In addition, this is also the first time that different immune cells were examined in intratumoral area, stromal area, and adjacent normal tissues simultaneously.
With respect to the association between TLCs and clinicopathologic factors, we found low-density TCD4+ and TCD8+ cells correlated positively with M1, lymph nodes metastasis, and more advanced stages. TFoxp3+ cell density was higher in tumors reported as M1 or more advanced stages. These results are in agreement with the hypothesis that CD4+ and CD8+ cells regulate the immune system positively, whereas Foxp3+ Tregs negatively. It is reported the CD4+/CD25+ Tregs populations in peripheral blood and tumor tissues of patients with gastrointestinal malignancies were significantly higher compared with healthy volunteers, which might means Tregs could aggress to peripheral blood with the progression of cancer.16,22 Therefore, Shen et al considered that tumor-related factors may induce the recruitment of CD4+ TICs and Foxp3+ Tregs.17
Univariate survival analysis in the present study confirmed high densities of TCD3+, TCD4+, and TCD8+ as well as SCD3+ and SCD4+ cells resulted in improved survival in gastric cancer. In contrast, neither SCD8+, NCD3+, NCD4+, nor NCD8+ cells were associated with survival. Furthermore, TCD8+/SCD8+ ratio and SCD4+/SCD8+ ratio could be expected to have antitumor reactivity. TCD3+/SCD3+ ratio, TCD4+/SCD4+ ratio, TCD4+/TCD8+ ratio, NCD4+/NCD8+ ratio, TCD3+/NCD3+ ratio, TCD4+/NCD4+ ratio, and TCD8+/NCD8+ ratio had no prognostic role in gastric cancer. However, none of the factors mentioned above were found to be associated with overall survival in multivariate survival analysis. Some research has reported that the density of TCD3+ TICs decreased during tumor progression,23 and survival outcomes were improved in patients with a higher density of TCD3+ cells.24 Patients in the high-density groups for TCD3+ and TCD8+ cells had a significantly longer survival time.25 However, other studies also reported CD8+ T cells producing interleukin-17 could promote tumor progression.26 We think there are likely several aspects that are responsible for our results and the discrepant research. First, CD4+ lymphocytes include a group of heterogeneous T lymphocytes, which can secret diverse cytokines.27 The presence of specific T-cells could be modulated by other components. Second, the activation status, rather than just the existence of CD8+ cells has great prognostic significance.28,29 It has been reported the activity and the number of cancer peptide-specific T cells need to be enhanced by vaccination with the appropriate cancer antigenic peptides.30 Third, our results showed the highest densities of various immune cells were distributed in different locations. Salama et al also reported that lymphocyte densities in both normal and tumor tissues had stronger prognostic significance in colorectal cancer.31 So the immune cells in different locations of the tumor microenvironment also could influence each other.
CD57 is a marker of natural killer (NK) cells. Our results of univariate analysis indicated the high density of SCD57+ cells was associated better overall survival, whereas TCD57+/SCD57+ ratio predicted the poor prognosis. Neither density of SCD57+ cells nor TCD57+/SCD57+ ratio was correlated to the overall survival in multivariate analysis. NK cells could attack tumor cells directly, representing an antitumor immunity.32 It has been reported that the recruitment of NK cells could exhibit strong antitumor activity and generate a better prognosis in gastric adenocarcinoma.33 However, an increased proportion of CD57+ cells in the circulation indicates a poor prognosis in advanced gastric cancer.34 One reasonable explanation for this is that the activity of NK cells is regulated by different cytokines and many cell subsets, even cancer itself. It has been reported that gastric cancer cells may decrease NK cytotoxicity through releasing the negative regulated cytokine, IL-10.35
Neutrophils comprising CD66b have been identified as a poor prognostic factor in many kinds of cancers, including gastric cancer.12,36–38 Our study is consistent with these studies, because we observed that densities of TCD66b+, NCD66b+, cells and TCD66b/SCD66b+ ratio were associated with poor outcome in univariate analysis although the density of SCD66b+ cells was showed to result in improved survival. Also, the TCD66b/SCD66b+ ratio was identified to be an unfavorable factor in multivariate analysis. Neutrophils are considered to have a protumorigenic role by promoting neoangiogenesis and reducing antitumor immune response.27 Nevertheless, Caruso et al reported that female patients, compared to male patients with higher density of intratumoral neutrophil have about a 39% reduction in their risk of mortality.18
Macrophage, which was recognized as CD68 positive, demonstrated poor survival outcomes in gastric cancer patients.39 It is suggested that tumor-infiltrating macrophages may cause increased CD44 expression through suppressing miR-328, resulting in tumor progression.40 And tumor-infiltrating macrophages could express thymidine phosphorylase, which is associated with tumor angiogenesis and poor survival in intestinal type gastric cancer.41 In the present study, both the univariate and multivariate analyses showed only TCD68+/SCD68+ ratio could favor overall survival. This may be explained by 3 possibilities. First, there is an inverse relationship between tumor-infiltrating macrophage cells and other subtypes. Wang et al reported the combination of high numbers of intratumoral CD68+ macrophage and Foxp3+ Tregs was associated with improved survival. Our results also showed the densities of CD4+ cells and CD68+ cells were significantly correlated in tumor tissues, and density of Foxp3+ Tregs was negatively correlated with CD68+ cells. Second, macrophages in different locations of the tumor microenvironment may have opposite functions and influences between each other, as illustrated by our study. Third, CD68+ macrophages display polarized versatile infiltration profiles comprising CD11c+ proinflammatory macrophages and CD206+ immunosuppressive macrophages in gastric cancer.42
Tregs are generally considered to be immunosuppressive and block of effective antitumor immunity, therefore are associated to poor outcome in several kinds of tumors.10,11,13,17,31 Various surface antigens, such as CD4, CD25, Foxp3, CTLA-4, and so on, are expressed on Tregs, among which Foxp3 is considered as the most specific marker for Tregs and it is possible to define Tregs more strictly as CD4+/CD25+ regulatory T cells.11,17,31,43 This is the reason why in the present study, we used Fxop3 + to identify Tregs. In univariate analysis, we demonstrated that densities of TFoxp3+ and TFoxp3+ cells, SCD8+/SFoxp3+ ratio and TFoxp3+/NFoxp3+ ratio were associated with worse survival and showed stronger prognostic significance. However, density of NFoxp3+ cells was associated with better prognosis, which opposed to previous results.31,44 A high TCD3+/TFoxp3+ ratio, TCD4+/TFoxp3+ ratio, TCD8+/TFoxp3+ ratio and SCD3+/SFoxp3+ ratio were associated with improved survival in gastric cancer, and we found that the TFoxp3+/SFoxp3+ ratio, SCD4+/SFoxp3+ ratio, NCD3+/NFoxp3+ ratio, NCD4+/NFoxp3+ ratio and NCD8+/NFoxp3+ ratio were not prognostic for survival. In multivariate analysis, only density of TFoxp3+ cells as negative prognostic factor and TCD8+/TFoxp3+ ratio as the positive factor were identified for survival. These results are in keeping with many reports,16,17,22,45 as well as ovarian cancer, colorectal cancer, breast cancer, and hepatocellular cancer.10,11,13,31 It has been proven in tumor models that the ratio of Tregs to effector T cells, instead of just the presence or absence of Tregs, played a more important role in determination of tumor development.46 It has been reported that Tregs can inhibit the function of effector T cells by direct touch or secretion of immune-suppressive cytokines.47,48 Our results have also shown that density of TFoxp3+ cells has a strong negative correlation with that of TCD8+ cells. This can partly explain the reason why the prognostic significance of TCD8+ cells was apparent in univariate analysis but not remarkable in multivariate analysis. Therefore, a combination of attenuation of Tregs and concomitant stimulation of tumor-specific effector T cells may be an effective immunotherapy strategy to improve the prognosis for patients with gastric cancer.11,17
Except for the immune parameters, the multivariate analysis revealed that some clinicopathological factors such as tumor size, longitudinal tumor location, and N stage, had independent prognostic significances. It has been reported that the T stage was a significant prognostic factor for gastric cancer. However, the T stage has not been identified as a significant prognostic factor in the present study, which may be attributed to 2 reasons. First, type II error probably existed in our results because the sample size may be relative small. Second, many variables were included into the multivariate analysis in our Cox model just like TICs, N stage, M stage, radical degree, and so on. Thus, there might be some interactions among these included factors. And the prognostic effect of T stage may be neutralized by other factors.
Our nomogram showed good performance in predicting survival, which was supported by the C-index and the calibration curve. Our nomogram also demonstrated more accuracy than the conventional TNM system for predicting prognosis in gastric cancer. These results could provide a possibility for doctors to predict the prognosis of gastric cancer accurately in clinic through evaluating the resected specimens with these identified novel independent predictors. However, we should notice that the prognostic accuracy of the suggested nomogram has been conducted in the same population where the nomogram was calculated as internal validation in the present study. Although internal validation could prevent against over-interpretation of current data, they cannot ensure external applicability. Whether our nomogram can be universally applied is still to be determined. Therefore, the nomogram needs to be validated externally in the future and it is a question that requires careful clinical judgment. On another hand, the immune reactions, which were proven to be associated with the overall survival in our multivariate analyses, were included in the nomogram comprising more prognostic variables than the traditional staging system. Thus, it can be inferred that part of the prognostic value of TNM system might derive from major underlying differences of quality and density of infiltrating immune cells.8 However, the exact mechanisms of how immune cells influence the overall survival and interact with each other are far from completely understood. Therefore, further studies are needed to focus on the relationship between the tumor microenvironment and immune cells.
In conclusion, high TCD68+/SCD68+ ratio and TCD8+/TFoxp3+ ratio were associated with improved overall survival, whereas high density of TFoxp3+ cells and TCD66b+/SCD66b+ ratio demonstrated poor overall survival, which are promising independent predictors for overall survival in gastric cancer.
Acknowledgments
Authors thank Ying Jie Xiu and Jian Ping Liu from Department of Pathology in West China Hospital for the assistance of pathological evaluation. This work was internal supported by Volunteer Team of Gastric Cancer Surgery (VOLTGA), West China Hospital, Sichuan University, PR China. The authors thank Dr. Trevor Hamilton (Department of Surgical Oncology, Princess Margaret Cancer Centre, University of Toronto, Canada), a native English-speaker, for his kind language modification of the manuscript.
Footnotes
Abbreviations: CI = confidence interval, C-index = concordance index, DAB = diamiobenzidine, HD = high density, HR = hazard ratio, IHC = Immunohistochemistry, IL-10 = interleukin-10, LD = low density, MAC = macrophages cells, NKC = natural killer cells.
Funding: Our study was supported by National Natural Science Foundation of China (Grant number: 81301867, 81372344) and National High-Technology Research and Development Program (863 Program) of China (Grant number: 2015AA020306)
KL, KY, BW, and HNC equally contributed to the study.
Author contributions: Conceptions and design: Kai Liu, Kun Yang, Bin Wu, Hai-Ning Chen, Zong-Guang Zhou, Xian-Ming Mo, Jian-Kun Hu; Development of methodology: Kai Liu, Kun Yang, Bin Wu, Hai-Ning Chen, Li-Li Jiang, Fu-Xiang Ye, Jian-Kun Hu; Acquisition of data: Kai Liu, Kun Yang, Bin Wu, Hai-Ning Chen, Xiao-Long Chen, Li-Li Jiang, Du He, Zheng-Hao Lu, Lian Xue, Wei-Han Zhang, Xin-Zu Chen; Analysis and interpretation of data: Kai Liu, Kun Yang, Bin Wu, Hai-Ning Chen, Li-Li Jiang, Fu-Xiang Ye, Du He, Qiu Li, Jian-Kun Hu; Writing, review and/or revision of the manuscript: All authors: Administrative, technical, or material support: Qiu Li, Zong-Guang Zhou, Xian-Ming Mo, Jian-Kun Hu; Study supervision: Jian-Kun Hu
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010; 11:439–449. [DOI] [PubMed] [Google Scholar]
- 3.Noh SH, Park SR, Yang HK, et al. CLASSIC trial investigators. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 2014; 15:1389–1396. [DOI] [PubMed] [Google Scholar]
- 4.Bang YJ, Van Cutsem E, Feyereislova A, et al. ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376:687–697. [DOI] [PubMed] [Google Scholar]
- 5.Wen L, Chen XZ, Yang K, et al. Prognostic value of cancer stem cell marker CD133 expression in gastric cancer: a systematic review. PLoS One 2013; 8:e59154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Y, Li N, Zhuang W, et al. P53 codon 72 polymorphism and gastric cancer: a meta-analysis of the literature. Int J Cancer 2007; 121:1481–1486. [DOI] [PubMed] [Google Scholar]
- 7.McLean MH, El-Omar EM. Genetics of gastric cancer. Nat Rev Gastroenterol Hepatol 2014; 11:664–674. [DOI] [PubMed] [Google Scholar]
- 8.Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 2011; 29:610–618. [DOI] [PubMed] [Google Scholar]
- 9.Pagès F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353:2654–2666. [DOI] [PubMed] [Google Scholar]
- 10.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8 + tumor-infiltrating lymphocytes and a high CD8 + /regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005; 102:18538–18543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 2007; 25:2586–2593. [DOI] [PubMed] [Google Scholar]
- 12.Jensen HK, Donskov F, Marcussen N, et al. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol 2009; 27:4709–4717. [DOI] [PubMed] [Google Scholar]
- 13.Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 2006; 24:5373–5380. [DOI] [PubMed] [Google Scholar]
- 14.Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8 + lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011; 29:1949–1955. [DOI] [PubMed] [Google Scholar]
- 15.Wang B, Xu D, Yu X, et al. Association of intra-tumoral infiltrating macrophages and regulatory T cells is an independent prognostic factor in gastric cancer after radical resection. Ann Surg Oncol 2011; 18:2585–2593. [DOI] [PubMed] [Google Scholar]
- 16.Ichihara F, Kono K, Takahashi A, et al. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res 2003; 9:4404–4408. [PubMed] [Google Scholar]
- 17.Shen Z, Zhou S, Wang Y, et al. Higher intratumoral infiltrated Foxp3+Treg numbers and Foxp3 + /CD8 + ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol 2010; 136:1585–1595. [DOI] [PubMed] [Google Scholar]
- 18.Caruso RA, Bellocco R, Pagano M, et al. Prognostic value of intratumoral neutrophils in advanced gastric carcinoma in a high-risk area in northern Italy. Mod Pathol 2002; 15:831–837. [DOI] [PubMed] [Google Scholar]
- 19.Amedei A, Della Bella C, Silvestri E, et al. T cells in gastric cancer: friends or foes. Clin Dev Immunol 2012; 2012:690571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Japanese gastric cancer association. Japanese classification of gastric carcinoma: 3rd English version. Gastric Cancer 2011; 14:101–112. [DOI] [PubMed] [Google Scholar]
- 21.Ajani JA, Bentrem DJ, Besh S, et al. National Comprehensive Cancer Network. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2013; 11:531–546. [DOI] [PubMed] [Google Scholar]
- 22.Sasada T, Kimura M, Yoshida Y, et al. CD4+CD25+regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer 2003; 98:1089–1099. [DOI] [PubMed] [Google Scholar]
- 23.Arigami T, Uenosono Y, Ishigami S, et al. Decreased density of CD3+tumor-infiltrating lymphocytes during gastric cancer progression. J Gastroenterol Hepatol 2014; 29:1435–1441. [DOI] [PubMed] [Google Scholar]
- 24.Kim JW, Nam KH, Ahn SH, et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer 2014; [Epub ahead of print]. doi:10.1007/s10120-014-0440-5. [DOI] [PubMed] [Google Scholar]
- 25.Lee HE, Chae SW, Lee YJ, et al. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer 2008; 99:1704–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhuang Y, Peng LS, Zhao YL, et al. CD8(+) T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology 2012; 143:951–962.e8. [DOI] [PubMed] [Google Scholar]
- 27.Chang WJ, Du Y, Zhao X, et al. Inflammation-related factors predicting prognosis of gastric cancer. World J Gastroenterol 2014; 20:4586–4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schumacher K, Haensch W, Röefzaad C, et al. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res 2001; 61:3932–3936. [PubMed] [Google Scholar]
- 29.van Beek J, zur Hausen A, Snel SN, et al. Morphological evidence of an activated cytotoxic T-cell infiltrate in EBV-positive gastric carcinoma preventing lymph node metastases. Am J Surg Pathol 2006; 30:59–65. [DOI] [PubMed] [Google Scholar]
- 30.Amedei A, Niccolai E, Della Bella C, et al. Characterization of tumor antigen peptide-specific T cells isolated from the neoplastic tissue of patients with gastric adenocarcinoma. Cancer Immunol Immunother 2009; 58:1819–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salama P, Phillips M, Grieu F, et al. Tumor-infiltrating FOXP3 + T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol 2009; 27:186–192. [DOI] [PubMed] [Google Scholar]
- 32.Mimura K, Kamiya T, Shiraishi K, et al. Therapeutic potential of highly cytotoxic natural killer cells for gastric cancer. Int J Cancer 2014; 135:1390–1398. [DOI] [PubMed] [Google Scholar]
- 33.Hyakudomi M, Matsubara T, Hyakudomi R, et al. Increased expression of fractalkine is correlated with a better prognosis and an increased number of both CD8+T cells and natural killer cells in gastric adenocarcinoma. Ann Surg Oncol 2008; 15:1775–1782. [DOI] [PubMed] [Google Scholar]
- 34.Akagi J, Baba H. Prognostic value of CD57(+) T lymphocytes in the peripheral blood of patients with advanced gastric cancer. Int J Clin Oncol 2008; 13:528–535. [DOI] [PubMed] [Google Scholar]
- 35.Szkaradkiewicz A, Karpiński TM, Drews M, et al. Natural killer cell cytotoxicity and immunosuppressive cytokines (IL-10, TGF-beta1) in patients with gastric cancer. J Biomed Biotechnol 2010; 2010:901564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt H, Suciu S, Punt CJ, et al. American Joint Committee on Cancer Stage IV Melanoma; EORTC 18951. Pretreatment levels of peripheral neutrophils and leukocytes as independent predictors of overall survival in patients with American Joint Committee on Cancer Stage IV Melanoma: results of the EORTC 18951 Biochemotherapy Trial. J Clin Oncol 2007; 25:1562–1569. [DOI] [PubMed] [Google Scholar]
- 37.Yamashita J, Ogawa M, Shirakusa T. Free-form neutrophil elastase is an independent marker predicting recurrence in primary breast cancer. J Leukoc Biol 1995; 57:375–378. [DOI] [PubMed] [Google Scholar]
- 38.Sakitani K, Hirata Y, Watabe H, et al. Gastric cancer risk according to the distribution of intestinal metaplasia and neutrophil infiltration. J Gastroenterol Hepatol 2011; 26:1570–1575. [DOI] [PubMed] [Google Scholar]
- 39.Ishigami S, Natsugoe S, Tokuda K, et al. Tumor-associated macrophage (TAM) infiltration in gastric cancer. Anticancer Res 2003; 23:4079–4083. [PubMed] [Google Scholar]
- 40.Ishimoto T, Sugihara H, Watanabe M, et al. Macrophage-derived reactive oxygen species suppress miR-328 targeting CD44 in cancer cells and promote redox adaptation. Carcinogenesis 2014; 35:1003–1011. [DOI] [PubMed] [Google Scholar]
- 41.Kawahara A, Hattori S, Akiba J, et al. Infiltration of thymidine phosphorylase-positive macrophages is closely associated with tumor angiogenesis and survival in intestinal type gastric cancer. Oncol Rep 2010; 24:405–415. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, Wang X, Shen Z, et al. Infiltration of diametrically polarized macrophages predicts overall survival of patients with gastric cancer after surgical resection. Gastric Cancer 2014; [Epub ahead of print]. doi:10.1007/s10120-014-0422-7. [DOI] [PubMed] [Google Scholar]
- 43.Hori S, Sakaguchi S. Foxp3: a critical regulator of the development and function of regulatory T cells. Microbes Infect 2004; 6:745–751. [DOI] [PubMed] [Google Scholar]
- 44.Haas M, Dimmler A, Hohenberger W, et al. Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC Gastroenterol 2009; 9:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kono K, Kawaida H, Takahashi A, et al. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother 2006; 55:1064–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bui JD, Uppaluri R, Hsieh CS, et al. Comparative analysis of regulatory and effector T cells in progressively growing versus rejecting tumors of similar origins. Cancer Res 2006; 66:7301–7309. [DOI] [PubMed] [Google Scholar]
- 47.Dieckmann D, Bruett CH, Ploettner H, et al. Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected]. J Exp Med 2002; 196:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Longhi MS, Hussain MJ, Mitry RR, et al. Functional study of CD4+CD25+regulatory T cells in health and autoimmune hepatitis. J Immunol 2006; 176:4484–4491. [DOI] [PubMed] [Google Scholar]


