Abstract
Purpose: Solid dispersions have been efficient in improving the dissolution rate and bioavailability of hydrophobic drugs. The aim of the present study was enhancement of the dissolution profile of Spironolactone using solid dispersion.
Methods: Spironolactone solid dispersions (1:1, 1:2 and 2:1 drug: carrier weight ratio) were prepared by polyethylene glycol (PEG) 6000 as a carrier by hot melt method. The influence of several amounts of Tween 20, 60, and 80 were also studied. The dissolution profile was evaluated by USP Apparatus II.
Results: The results showed that solid dispersions were efficacious to enhance the dissolution rate of Spironolactone in water; and the procedure indicated that there was an increase in dissolution rate for solid dispersions containing the surfactant Tweens. The solid dispersions were evaluated using Fourier-transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD) and Differential Scanning Calorimetry (DSC) studies; and the results showed no complex formation or change in crystal shape of drug.
Conclusion: It is concluded that solid dispersion technique can be successfully used for improvement of dissolution of Spironolactone.
Keywords: Solid dispersion, Spironolactone, PEG 6000, Tweens, Dissolution
Introduction
Solid dispersion (SD) refers to a group of solid products consisting of at least two different components, generally a hydrophilic matrix and a hydrophobic drug.1 The matrix can be either crystalline or amorphous. The drug can be dispersed molecularly, in amorphous particles or in crystalline particles.2 In other words, SD involved a dispersion of one or more active ingredients in an inner carrier in solid state.3
Some of these carriers are PEGs, Gelucires, Poloxamers, PVP, Sugar or Urea. PEG polymers are used widely because of their low melting point, low toxicity, wide drug compatibility and hydrophilicity.4 A water-soluble carrier results in a faster release of the drug from the matrix.5 Many methods like spray drying, co-precipitation, co-evaporation and freeze dying are used for solid dispersion manufacturing. Apart from these techniques, fusion method is a method of choice because it is environmentally friendly, cost effective, represents no stability nor toxicity problems and can be easily scaled up for commercial purpose.6
Spironolactone is a synthetic steroid that acts as a competitive antagonist to aldosterone. Potassium-sparing diuretics are most useful in states of mineralocorticoid excess or hyperaldosteronism (also called aldosteronism), due either to primary hypersecretion (Crohn's syndrome, ectopic adrenocorticotropic hormone production) or secondary hyperaldosteronism (evoked by heart failure, hepatic cirrhosis, nephrotic syndrome, or other conditions associated with diminished effective intravascular volume).7 Spironolactone has a low water solubility (0.028 mg/ml, at 25°C) and slow dissolution rate.8 Solubility and dissolution rate are key determinant of oral bioavailability of a drug and dissolution may be rate determining step for appearance of medicinal effect, therefore efforts to increase dissolution of drug with limited water solubility is often needed. Various methods used for the improvement of the dissolution rate of poorly water-soluble drugs. SD is one of these techniques. Surface-active agents (surfactants) are substances that at low concentrations adsorb onto the surfaces or interfaces of a system and alter the surface or interfacial free energy and the surface and the interfacial tension. Surface-active agents have a characteristic structure, possessing both polar (hydrophilic) and non-polar (hydrophobic) regions in the same molecule. The surface active carriers are said to be amphipathic in nature.9 Surfactants are used to enhance solubility.
The aim of this study was to prepare different solid dispersions of Spironolactone by fusion method with PEG and several types of Tweens, and evaluate the effect of them on solubility profile of the drug.
Materials and Methods
Materials
Spironolactone was obtained from Behdasht-Kar Co. (Behdasht-Kar Co., Iran). PEG 6000, Tween 20, 60, and 80 were purchased from Merck (Merck, Germany). All other chemicals and solvents used were of pharmaceutical grade.
Preparation of solid dispersion
Fusion (hot melt) method was used for the preparation of Spironolactone SD. Several formulations were prepared by adding required amount of PEG 6000 to accurately weighed drug with the drug: carrier weight ratio of 1:1, 1:2 and 2:1; and several amounts of Tween 20, 60, and 80 (Table 1). The formulations were heated on a water bath (Behdad, Iran) at the temperature of 70°C to melt the carrier and stirred to make homogenous mixing. The combination was cooled rapidly, and grinded to obtain the SDs as powder that is easy to handle.10 Finally the preparations were passed through 50-mesh sieve and stored in desiccators for next evaluations.
Table 1. Drug –carrier and Tween (20, 60, 80) weight ratio in prepared formulations.F0 is pure spironolactone,F1 to F12 are solid dispersions,F13 to F16 are physical mixtures.
| Formulation code | Drug: Carrier ratio | Tween 80 | Tween 60 | Tween 20 |
| F0 | 1: 0 | - | - | - |
| F1 | 1:1 | - | - | - |
| F2 | 1:2 | - | - | - |
| F3 | 2:1 | - | - | - |
| F4 | 1:1 | 1% | - | - |
| F5 | 1:1 | 0.5% | - | - |
| F6 | 1:1 | 1.5% | - | - |
| F7 | 1:1 | - | 1% | - |
| F8 | 1:1 | - | 0.5% | - |
| F9 | 1:1 | - | 1.5% | - |
| F10 | 1:1 | - | - | 1% |
| F11 | 1:1 | - | - | 0.5% |
| F12 | 1:1 | - | 1.5% | |
| F13* | 1:1 | 1% | - | - |
| F14* | 1:1 | - | 1% | - |
| F15* | 1:1 | - | - | 1% |
| F16* | 1:1 | - | - | - |
* Physical mixture
Preparation of Physical mixture
The physical mixtures were prepared by weighing Spironolactone and PEG accurately in the ratio of 1:1 w/w of drug to carrier and mixing them with determined amount of Tween. The formulations were obtained by mixing the components using spatula in a mortar for 3 minutes. They were passed through 50-mesh sieve and kept in desiccators for other studies.4
Release studies of Solid Dispersions
In vitro dissolution of samples equivalent to 25 mg of pure drug was carried out in a USP apparatus No. II (Erweka, Germany) in 900ml of distilled water as medium at 37±0.5ºC for 8 hours and at a rotational speed of 100 rpm, the system was allowed to equilibrate for 60 minutes. Dissolution samples (5ml) were withdrawn at predetermined intervals of 5, 10, 15, 20, 30, 45, 60 then every 30 minutes up to 8 hours , and were filtered through Whatmann number 41 filter papers. The samples withdrawn were replaced by distilled water to maintain the constant volume of dissolution medium. The samples were analyzed at 239.8 nm in UV spectrophotometer (Varian, Italy). Each preparation was tested three times and the mean values were figured out. Percentage of drug release was calculated using the equation obtained from the standard curve prepared in the media.11,12
The percent dissolution efficiency (%DE) was computed to compare the relative performance of various type and concentrations of surfactant in the investigated formulations. The %DE of a solid dispersion is defined as the area under the dissolution curve up to a certain time, t, expressed as a percentage of the area of the rectangle described by 100% dissolution at the same time. The %DE can be calculated from %DE.
Where Y is the percent drug dissolved at time t.
To understand the extent of dissolution-rate increase from its solid dispersion, the dissolution data were used to calculate the mean dissolution time (MDT). The MDT can be calculated as follow:
Where, i is the dissolution sample number, n is the number of dissolution sampling times, Tmid is the midpoint between times Ti and Ti-1, and DM is the amount of dissolved drug between times Ti and Ti-1.13
Fourier-Transform Infrared Spectroscopy
IR spectroscopy is used to assess the interaction between carrier or complexing agent and guest molecule in solid state. Because of their high structural resolution this technique is valuable for characterization of an amorphous system.14 The samples were previously ground and mixed thoroughly with potassium bromide, an infrared transparent matrix, at 1:5 (Sample: KBr) ratio, respectively. The KBr discs were prepared by compressing the powder at a pressure of 5 tons for 5 min in a hydraulic press. Scans were obtained at a resolution of 4 cm-1, from 4000 to 500 cm-1.14 FT-IR spectra were obtained by using an FT-IR spectrometer (Perkin Elmer , Spectrum One , USA ).
X-ray diffraction
Powder X-ray diffraction can be used to qualitatively detect material with long range order. Sharper diffraction peaks indicate more crystalline material.15
X-ray powder diffraction patterns were obtained at room temperature using a D8 ADVANCE X-ray diffractometer (Bruker, Germany) with Cu as anode material and graphite monochromator, operated at a voltage of 40 kV and 20 mA current. The samples were analyzed in the 2θ angle range of 4°–40° and the process parameters were set as: scan step size of 0.02° (2θ), and scan step time of 0.5 degree/min.15
Differential scanning calorimetry (DSC)
In DSC, samples are heated with a constant heating rate and the amount of energy necessary for that is detected. With DSC the temperatures at which thermal events occur can be detected. Thermal events can be a glass to rubber transition, (re)crystallization, melting or degradation. Furthermore, the melting- and (re)crystallization energy can be quantified. The melting energy can be used to detect the amount of crystalline material.16
The DSC thermograms of pure drug, physical mixtures and solid dispersions were recorded using Differential scanning calorimeter (Perkin Elmer Pyris 6). About 5 mg of each sample was weighed and hermitically sealed in aluminum pans prior to analysis, and the thermal behavior of each sample was investigated at a scanning rate of 10°C/min, from 0 to 300°C. Dry nitrogen at a flow rate of 50 ml/min was used to purge the DSC cell.
Statistical Analysis
ANOVA followed Tukey test was used to determine significant differences between groups and “P<0.05” was considered significant.
Results and Discussion
Dissolution profiles
Comparison of release behaviour of The various formulations are shown in Figure 1-4. The Spironolactone solid dispersions were prepared employing hot melt method. Drug –carrier and Tween (20, 60, 80) weight ratio applied in prepared formulations is shown in Table 1. The dissolution study showed that the release percentage of pure Spironolactone after one hour was only 55.06% (Figure 1). During dissolution studies, it was noted that drug carrier systems sink immediately, whereas pure drug keeps floating on the surface for a longer time interval. There was an appeal difference between the release rate of pure Spironolactone and solid dispersions (F1, F2, and F3) (P<0.001). Comparative dissolution profile showed that an increase in the percentage of PEG 6000 from (formulation F1 to F3) resulted in an increase in the release rate of Spironolactone (P<0.01). Chaulang et al. showed that the solid dispersion (SD) technique has improved the dissolution rate of Furosemide to a great extent. The release percentage of drug was increased when the concentrations of carrier increased.8 In vitro dissolution in Islam et al. studies indicated that drug release from SDs was faster from their pure drug. Drug release from SDs was found to depend on the nature and amount of carrier.15 Bolourchian et al. evaluated the effect of several PEG (6000, 12000, and 20000) as solid dispersion carriers on the dissolution behavior of simvastatin. The results showed that the kind of carrier had effect on drug dissolution. This study revealed that release rate was increased by increase in ratio of carrier to drug.17
Figure 1.
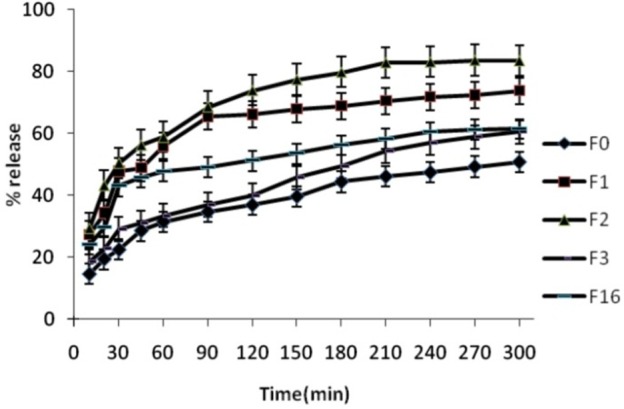
Release percentage of prepared formulations, dissolution condition: distilled water; RPM: 100; 37°C ± 0.5°C (n=3).
Figure 2.
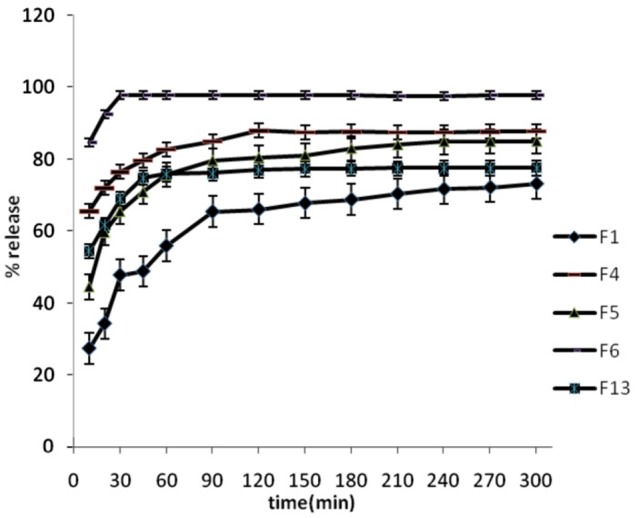
Release percentage of prepared formulations, dissolution condition: distilled water; RPM: 100; 37°C ± 0.5°C (n=3).
Figure 3.
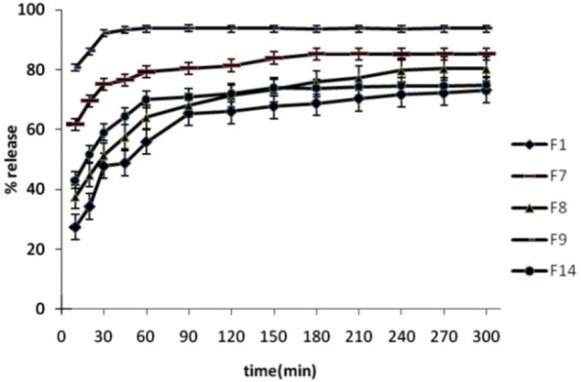
Release percentage of prepared formulations, dissolution condition: distilled water; RPM: 100; 37°C±0.5°C (n=3).
Figure 4.
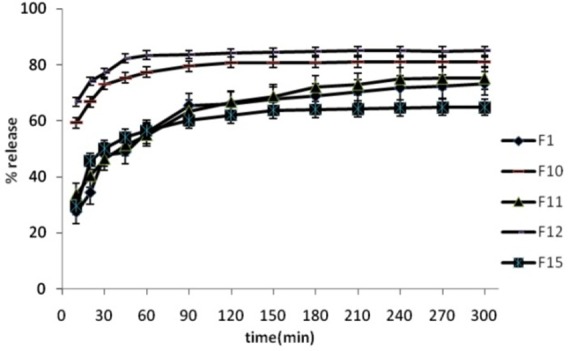
Release percentage of prepared formulations, dissolution condition: distilled water; RPM: 100; 37°C±0.5°C (n=3).
Solid dispersions containing Spironolactone with both PEG 6000 and Tween 60 or 80 provided higher release rate than the release rate of pure Spironolactone and SD preparations containing PEG (P<0.01). The release profile showed that release rate was increased by increasing in amount of Tween 60 or 80 (P<0.05). The drug release in SD formulation containing 0.5% Tween 20 (F11) was similar to SD preparation containing drug and PEG 6000 (F1) (P>0.05). The drug release increased by increasing in amount of Tween 20 to 1 and 1.5% (P<0.05). Comparing the dissolution profile of SDs with other preparations at similar ratios of Tween revealed that, the release rates of SDs containing Tween 80 was higher than release rates in formulations containing Tween 60 and 20 (P<0.05 for all investigations).
DE% and MDT of investigated solid dispersions are shown in Table 2. The DE% of F0 (drug powder) was significantly lower than solid dispersions. The onset of release was faster than drug powder in all of formulations. The most DE% was observed in F6 containing 1.5% of tween 80.
Table 2. Dissolution parameters of spironolactone solid dispersions.
| Formulation code | %DE | MDT(min) |
| F0 | 43.69 | 131.17 |
| F1 | 67.11 | 94.69 |
| F2 | 75.47 | 74.42 |
| F3 | 50.77 | 146.57 |
| F4 | 84.39 | 46.38 |
| F5 | 79.80 | 52.18 |
| F6 | 95.50 | 22.62 |
| F7 | 81.41 | 51.50 |
| F8 | 73.07 | 77.46 |
| F9 | 91.37 | 25.45 |
| F10 | 78.04 | 37.81 |
| F11 | 68.30 | 81.99 |
| F12 | 82.18 | 35.82 |
| F13* | 75.11 | 49.97 |
| F14* | 71.05 | 51.00 |
| F15* | 61.00 | 50.98 |
| F16* | 55.15 | 89.55 |
The MDT of F0 was 131.17 min. This time was decreased in investigated solid dispersions significantly. This proved the increase in release rate by solid dispersion preparation.
In one study, solid dispersions of Meloxicam containing PEG 6000 and SLS showed a significant increase in dissolution rate with an increase in PEG 6000 and the surfactant sodium lauryl sulphate.12 In another research the effect of sucrose laurate as surfactant was evaluated in gemfibrosil solid dispersions containing PEG 6000 as carrier. Drug released increased significantly in solid dispersions containing this surfactant.18
Possible mechanisms of increased dissolution rates of solid dispersions have been proposed by Craig, and include: reduction of drug crystallite size, a solubilisation effect of the carrier, absence of aggregation of drug crystallites, improved wettability and dispersibility of a drug from the dispersion, dissolution of the drug in the hydrophilic carrier, conversion of the drug to the amorphous state and finally the combination of the above mentioned methods. Dry mixing of Spironolactone with PEG and Tween brings drug in close contact with the hydrophilic carrier. The increased dissolution rate in this study can thus be contributed by several factors such as a solubilisation effect of the carrier, conversion of drug to amorphous state, improved wettability and dispersibility of the drug.19
The dissolution of the physical mixtures was higher compared to pure Spironolactone. SDs of Spironolactone in hydrophilic carrier considerably enhanced dissolution compared to the physical mixtures (Figure 1). Dehghan et al. study showed the solid dispersions of Meloxicam with PEG 6000 improved dissolution when compared with physical mixtures and pure drug.12
FTIR analysis
Figure 5 represents the FTIR spectra of the samples. PEG 6000 spectrum showed characteristic peaks at 1107(–C-O stretching), 2890(–C-H stretching), 3445(–O-H stretching). Pure Spironolactone spectrum showed sharp peaks at wave numbers 1617 (-C=C stretching of α, β-unsaturated ring), 1673(-C=O stretching of α, β-unsaturated ring), 1768(-C=O stretching of the thioacetyl group), 1791 (-C=O stretching of the lactone ring), 2950cm–1 (-C=H stretching). In the spectra of all physical mixture and SD formulations, major characteristic peaks of both drug individually and polymer were retained. This confirms that there were no physical or chemical interactions amongst the components of the formulation and compatibility of the drug with the carrier.20
Figure 5.
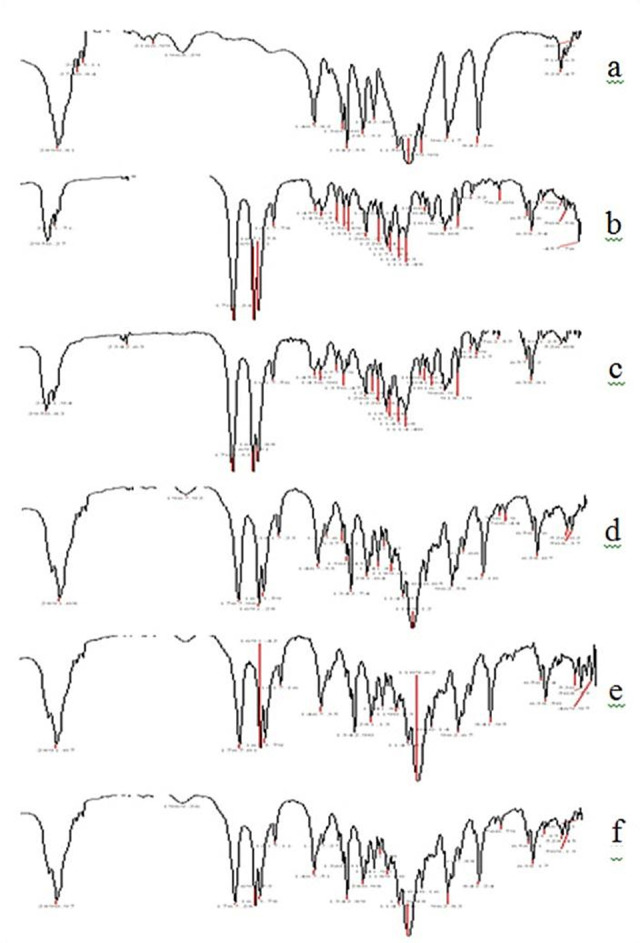
FTIR spectra of PEG and Spironolactone preparations
a: PEG, b: Spironolactone, c: Physical mixture (1:1 Drug-carrier), d: Solid dispersion (1:1 Drug carrier), e: Solid dispersion (1:1 Drug-carrier and 1.5% Tween 80), f: Solid dispersion (1:1 Drug-carrier and 1.5% Tween 60)
Powder X-Ray diffraction
Powder X-ray diffraction patterns of pure spironolactone, polymer, physical mixtures and solid dispersions of spironolactone are displayed in Figure 6. The X-ray diffraction spectrum of Spironolactone showed numerous distinct lines (9.21, 11.5, 12.5, 16.3,17, 17.5, 18.8, 20.21) indicating that it is present in highly crystalline state. XRD spectrum of formulation 1, 6 and 9 showed peaks at 9.1, 11.5, 12.5, 16.3, 17.1, 17.5, 18.8, 19.2, 20.21, and 23.1. The position of characteristic peaks was not changed in physical mixtures which suggest absence of change in the polymorph.4,16 These peaks were observed in solid dispersions too, but intensity of them was decreased which proved changing from crystalline to amorphous form.
Figure 6.
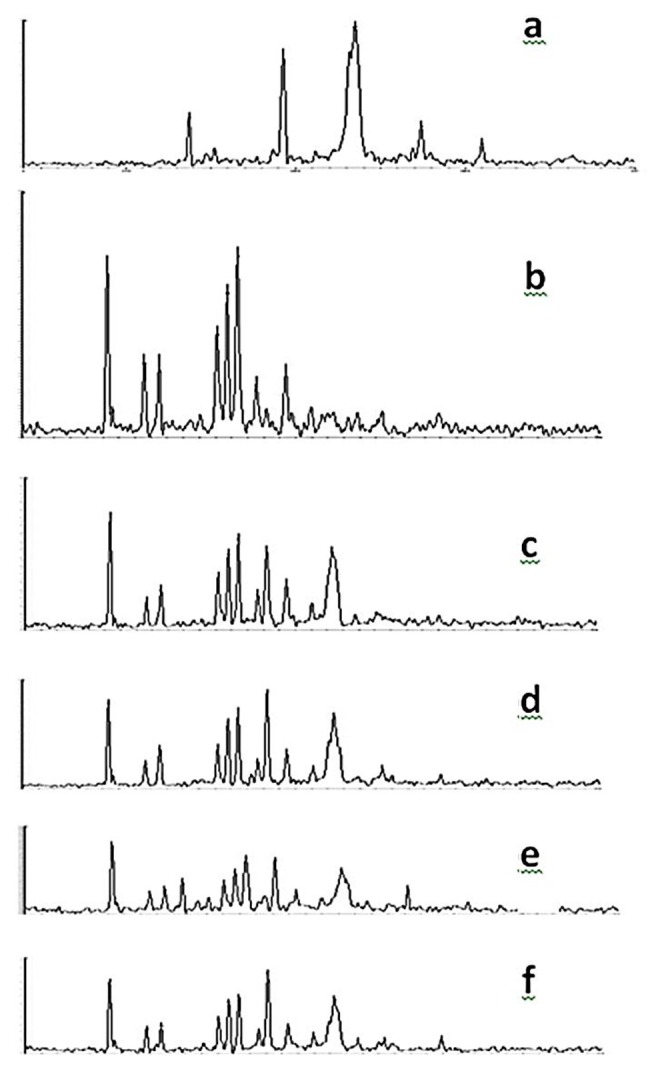
X-ray diffraction (XRD) patterns of PEG and Spironolactone preparations
a: PEG, b: Spironolactone, c: Physical mixture (1:1 Drug-carrier), d: Solid dispersion (1:1 Drug-carrier), e: Solid dispersion (1:1 Drug-carrier and 1.5% Tween 80), f: Solid dispersion (1:1 Drug-carrier and 1.5% Tween 60)
DSC analysis
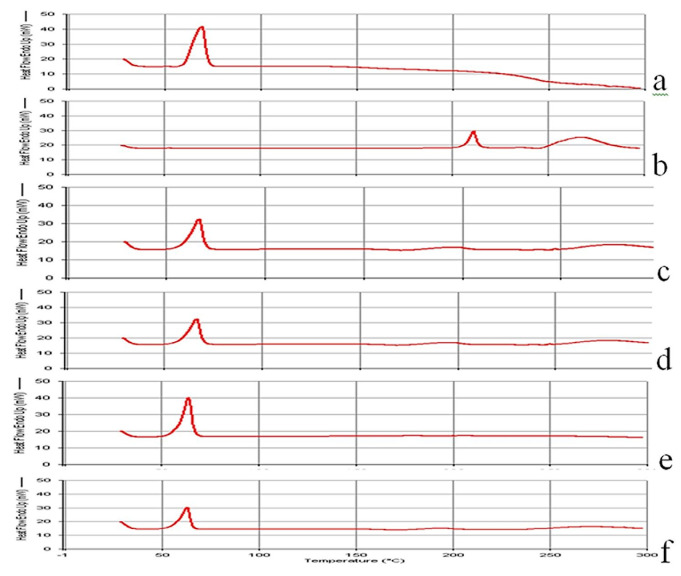
The DSC thermograms of Spironolactone and PEG as well as their solid dispersions and physical mixtures are shown in Figure 7. The DSC thermogram of pure Spironolactone showed sharp endothermic peak at 208.7°C, with a melting enthalpy of 47.2 J/g, which is associated with melting point of the drug and indicates the crystalline nature of the drug. There is also a broad peak at 267.7°C with an enthalpy of 182.0 J/g, which may be suggestive of Spironolactone decomposition at this high temperature. From the thermograms of SDs and physical mixtures the endothermic peak of spironolactone was shifted to a lower temperature, around 198 to 202°C, and the calorimetric enthalpy was markedly reduced from 47.2 J/g (for pure drug) to 0.65±0.08 J/g. This signifies that most of the drug has dissolved in the liquid vehicle, and there is reduced number of drug crystal particles. A broadened peak with markedly reduced intensity was observed at approximately 250°C. The broad peak at about 250°C in SD formulations might indicate that Spironolactone decomposition was minimized as the decomposition enthalpy was reduced from 182.0 J/g at 267.7°C to 38.35±5.41 J/g and some of crystalline form was changed to amorphous form. DSC thermograms of solid dispersion and physical mixture formulations after storage at 25°C and 76% relative humidity for 4 weeks revealed that there are no significant changes in thermal properties of the formulations compared to fresh formulations.3,4,8
Figure 7.
DSC thermograms of PEG and Spironolactone preparations
a: PEG, b: Spironolactone, c: Physical mixture (1:1 Drug-carrier), d: Solid dispersion (1:1 Drug-carrier), e: Solid dispersion (1:1 Drug-carrier and 1.5% Tween 80), f: Solid dispersion (1:1 Drug-carrier and 1.5% Tween 60)
Conclusion
In this experiment an attempt has been taken to evaluate Spironolactone release from the different polymer-drug concentrations loaded formulation of Solid dispersions. Observations of In-vitro dissolution of the solid dispersion of Spironolactone using distilled water as the media to study the drug release efficiency from the solid dispersion. These distorted solid dispersions may cause a wide variation in drug release pattern. Spironolactone is a potassium-sparing diuretic, acts as a competitive antagonist to aldosterone and is poorly water soluble drug. The present study was aimed to enhance the dissolution property of Spironolactone. So this study was endeavored to observe release pattern of drug from the solid dispersion of Spironolactone by using different concentrations of PEG as a carrier and Tween as a surfactant. The variables affecting drug dissolution was matrix property, hydrophilic excipients loading from the solid dispersion and also depend on the physicochemical property of the drug molecule. The major problem of poorly water soluble drugs especially for new molecules is bioavailability. Thus the main target is to increase the solubility of poorly water soluble drugs. So the present study reveals that solid dispersion may be an ideal means of drug delivery system for poorly water soluble drugs. Further study in this field is required to establish these drug delivery systems so that in future it can be used effectively in commercial basis.
Acknowledgments
This work was supported by a grant from the Research Council of the Mazandaran University of Medical Sciences.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Ford JL. The current status of solid dispersions. Pharm Acta Helv. 1986;61(3):69–88. [PubMed] [Google Scholar]
- 2.Nokhodchi A, Talari R, Valizadeh H, Jalali MB. An investigation on the solid dispersions of chlordiazepoxide. Int J Biomed Sci. 2007;3(3):211–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Chaulang G, Patil K, Ghodke D, Khan S, Yeole P. Preparation and characterization of solid dispersion tablet of Furosemide with crospovidone. Res J Pharm Tech. 2008;1(4):386–89. [Google Scholar]
- 4.Kshirsagar SJ, Ubhe A, Malshe J, Sengaokar V. A Modified Solvent Method for Preparation of Solid Dispersions. Iran J Pharm Sci. 2011;8(1):287–98. [Google Scholar]
- 5.Arunachalam A, Karthikeyan M, Konam K, Prasad P, Sethuraman S, Ashutoshkumar S. Solid Dispersions: A Review. Curr Pharm Res. 2010;1:82–90. [Google Scholar]
- 6.Saffoon N, Jhanker YM, Huda NH. Dissolution profile of Ibuprofen solid dispersion prepared with cellulosic polymers and sugar by fusion method. Stamford J Pharm Sci. 2011;4(1):31–7. doi: 10.3329/sjps.v4i1.8864. [DOI] [Google Scholar]
- 7. Katzung BG. Introduction to autonomic pharmacology. In: Katzung BG, Masters SB, Trevor AJ, editors. Basic and Clinical Pharmacology. 11th ed. New York: McGraw-Hill Companies Inc; 2009.
- 8.Elkordy AA, Tan XN, Essa EA. Spironolactone release from liquisolid formulations prepared with Capryol 90, Solutol(R) HS-15 and Kollicoat(R) SR 30 D as non-volatile liquid vehicles. Eur J Pharm Biopharm. 2013;83(2):203–23. doi: 10.1016/j.ejpb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Patidar K, Kshirsagar MD, Saini V, Joshi PB, Soni M. Solid dispersion technology: a boon for poor water soluble drugs. Indian J Novel Drug Deliv. 2011;3(2):83–90. [Google Scholar]
- 10.Dhirendra K, Lewis S, Udupa N, Atin K. Solid dispersions: a review. Pak J Pharm Sci. 2009;22(2):234–46. [PubMed] [Google Scholar]
- 11.Dewan I, Islam SM, Shahriar M. Dissolution study of Spironolactone by using solid dispersion technique. Stamford J Pharm Sci. 2011;4(2):42–7. doi: 10.3329/sjps.v4i2.7776. [DOI] [Google Scholar]
- 12.Dehghan MH, Jafar M. Improving dissolution of Meloxicam using solid dispersions. Iran J Pharm Res. 2006;4(5):231–38. [Google Scholar]
- 13.Sruti J, Patra CN, Swain S, Panigrahi KC, Patro AP, Beg S. et al. Improvement in the dissolution rate and tableting properties of cefuroxime axetil by melt-granulated dispersion and surface adsorption. Acta Pharm Sinica B. 2013;3(2):113–22. doi: 10.1016/j.apsb.2013.01.001. [DOI] [Google Scholar]
- 14.Saindane DS, Kulkarni AS, Khade TS, Patil MD. Enhancing drug solubility and oral bioavailability using solid dispersions: A review. Int J Biopharm. 2011;2(1):22–30. [Google Scholar]
- 15.Kumar P, Mohan C, Kanamsrinivasan Uma Shankar M, Gulati M. Physiochemical Characterization and Release Rate Studies of SolidDispersions of Ketoconazole with Pluronic F127 and PVP K-30. Iran J Pharm Res. 2011;10(4):685–94. [PMC free article] [PubMed] [Google Scholar]
- 16.Rani KS, Poornima G, Krishnaveni A, Brahmaiah B, Nama S. A review on solid dispersions. Asian J Pharm Res. 2013;3(2):93–8. [Google Scholar]
- 17.Bolourchian N, Mahboobian MM, Dadashzadeh S. The effect of PEG molecular weights on dissolution behavior of simvastatin in solid dispersions. Iran J Pharm Res. 2013;12(Suppl):11–20. [PMC free article] [PubMed] [Google Scholar]
- 18.Szuts A, Lang P, Ambrus R, Kiss L, Deli MA, Szabo-Revesz P. Applicability of sucrose laurate as surfactant in solid dispersions prepared by melt technology. Int J Pharm. 2011;410(1-2):107–10. doi: 10.1016/j.ijpharm.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 19.Craig DQ. The mechanisms of drug release from solid dispersions in water-soluble polymers. Int J Pharm. 2002;231(2):131–44. doi: 10.1016/s0378-5173(01)00891-2. [DOI] [PubMed] [Google Scholar]
- 20.Shah S, Joshi S, Lin S, Madan PL. Preparation and characterization of Spironolactone solid dispersions using hydrophilic carrier. Asian J Pharm Sci. 2012;7(1):40–9. [Google Scholar]