Abstract
Purpose: Insulin resistance plays a key role in the onset and development of type 2 diabetes mellitus (T2DM) and its complications. In this study, we evaluated the effect of swim training on insulin resistance in diabetic rats.
Methods: Forty male Wistar rats were randomly divided into four groups (n=10): sedentary control (Con), sedentary diabetic (Dia), swim trained control (Exe) and swim trained diabetic (Dia+Exe) rats. Diabetes was induced by high fat diet (HFD) and a low dose of streptozotocin (35 mg/kg, i.p). In trained groups, one week after the induction of diabetes, animals were subjected to swimming (60 min/5 days a week) for 10 weeks. At the end of training, fasting blood sugar (FBS), oral glucose tolerance test (OGTT), fasting/basal insulin, glycosylated hemoglobin (HbA1c) levels, insulin resistance index, homeostasis model assessment method (HOMA-IR), triglycerides (TG,) total cholesterol (TCh), and high density lipoprotein (HDL) levels in blood were measured.
Results: Swimming significantly improved OGTT (P<0.01) and HOMA-IR (P<0.01). Swim training also significantly decreased FBS (p<0.01), fasting/basal insulin (P<0.01), HbA1C (p<0.01), TG (P<0.05), and TCh (P<0.05) levels. It also significantly increased HDL (p<0.05) level.
Conclusion: Our findings indicate that swim training improved glycemic control and insulin sensitivity in type 2 diabetes caused by high fat diet in male rats.
Keywords: High fat diet, HOMA-IR, Insulin resistance, Swim training, Rat
Introduction
Unhealthy lifestyles (increased sedentary lifestyles and high-fat, energy-dense diets) are strong predictors of obesity, insulin resistance and type 2 diabetes.1 T2DM is the most widespread type of diabetes that includes about 80% of all diabetic cases.2 According to the World Health Organization, T2DM has been estimated in developing countries to have growth rate of 170 percent and increase from 84 to 228 million until 2030.2,3 The main feature of type 2 diabetes mellitus is the insufficient production of insulin due to beta-cell dysfunction and insulin resistance.3 Insulin resistance is defined as a condition in which insulin’s action on peripheral tissues such as adipose tissue, skeletal muscle, and liver is reduced and this statement has a fundamental role in the improvement and beginning of type 2 diabetes.4,5 Insulin resistance initiates earlier than the beginning of T2DM, in which tolerance of glucose impairs due to beta cell deterioration and comparative insulin deficiency.5,6 Many factors such as genetics and environmental influences and obesity are linked to the improvement of insulin resistance in individuals with damaged glucose tolerance and T2DM.7,8 Epidemiological researches have exhibited that the incidence of insulin resistance and T2DM in people with the similar genetic texture exhibition increases whenever they receive a high fat diet9,10 Recently, studies demonstrated that fat-rich diet shave pro-oxidant and pro-inflammatory compounds that have been linked to impaired insulin sensitivity.11-13 Because of the high universal occurrence of diabetes, wide investigations are still being implemented to develop new anti-diabetic medications and methods to manage it. Therefore, a number of diabetic animal models have been employed and developed and are the most thoroughly described over the years.14,15 These models can be classified into two wide kinds: 1) inherently and genetically induced spontaneous diabetes models; and 2) experimentally induced non spontaneous diabetes models.
High fat diet-fed STZ model of T2DM is associated with insulin resistance and is very similar to T2DM in humans.15,16 Because of the important role of skeletal muscle in insulin resistance in the situation of the ‘‘insulin resistance syndrome’’ and in the progress of T2DM, interventions that improve insulin action on skeletal muscle are important therapeutic strategies in these insulin resistant situations.17,18 Useful non pharmacological interventions comprise exercise training, particularly activities including running, biking, or swimming, energy limitation and special dietary modifications,19 which rise insulin action or sensitivity, result in a reduced request for insulin and thus reduce beta cell exhaustion. Regular exercise is useful for the prevention and treatment of insulin-resistant disorders such as T2DM. It has been shown that in T2DM cases, regular exercise increases insulin release to either hyperglycemia or arginine stimulation proposing that exercise may have direct effects on pancreatic function.19,20 Epidemiological researches have shown that moderate-to-vigorous physical activity suppresses the development of T2DM.21 Among , types of exercise, running and cycle ergometer exercises are at 50–70% of maximal oxygen uptake (VO2 max), assumed as low to moderate intensity have beneficial effects in preventing or alleviating insulin resistanc.22 Also aerobic and resistance exercise have important effects on management of T2DM.22 Recent studies have shown that aerobic trainings such as swimming and treadmill, and resistance trainings such as weight lifting and combined forms have similar beneficial effects on the glycemic control and on the improvement of insulin resistance and T2DM, although aerobic training has a larger effect on body composition and insulin resistance.23 The present study was designed to investigate the effect of ten weeks swim training on insulin resistance in type2 diabetic male rats.
Materials and Methods
Animals
Forty male Wistar rats (200-250 g) were obtained from laboratory animal house of Tabriz University of Medical Sciences. Animals were kept in an animal room at 22-24°C with free access to rat chow and blow water. All the employed experimental processes, as well as rat care and handling were in agreement with guidelines provided by the Animal Care Committee of the Tabriz University of Medical Sciences. Animals were randomly divided into four groups (n = 10): Sedentary control group (Con), sedentary diabetic group (Dia), exercise group (Exe) and diabetic- exercise group (Dia+Exe).
Induction of type2 diabetes
In order to induction of diabetes, rats were fed with HFD regimens consisting 22% fat, 48% carbohydrates and 20% protein for a period of 4 weeks. The composition and preparation of HFD were as described previously.24,25 After the 4 weeks of dietary manipulation, the animals were injected intraperitoneally (i.p) with a low dose of STZ (35 mg/kg). After 72h of injection, animals with the non-fasting basal plasma glucose (PGL) of ≥ 300 mg/dl were considered diabetic and selected for further studies.
Swim training protocol
Three days after induction of diabetes, swim training protocol began and increased gradually to a maximum of 60 min daily, 5days a week for 10 weeks. The training was initiated from 5 minutes on the first day to reach to 60mindaily during 12 days.26 Swimming exercise carried out in a rectangular tank (100 ×60 ×80cm) with water maintained at 34–36°C. After swimming, animals were dried and kept in a warm place, and then returned to their cages.
Fasting blood glucose Oral glucose tolerance test, Serum Insulin, Insulin resistance, and HbA1c estimate
At the end of training period, fasting/basal plasma glucose and serum insulin levels were measured before and a glucose tolerance test was performed after oral glucose administration (1 g/kg). Animals were fasted for a period of 12 hours before their blood glucose level measurement. Blood samples were collected from the tip of tail and plasma glucose levels were measured using a digital glucometer (Gluco Sure, Star, Taiwan). Quantitative estimation of serum insulin was performed by rat insulin ELISA kit (Bioseps, Co. ltd, China). Insulin resistance estimation carried out using homeostasis model assessment method, HOMA-IR, and was calculated using the following formula:
Plasma glucose (mg/dl) × fasting plasma insulin (IU mg /L in the fasting state divided by 405.27
HbA1c was also measured by the quantitative estimation using immunoturbidimetry kit (Pars Azmoon HbA1c kit, Iran). The manufacturer’s technical recommendations were followed.
Detection of serum total cholesterol, triglyceride and HDL
Serum samples were analyzed for estimation of total cholesterol, triglycerides and HDL levels using an auto blood analyzer (Bayer Corp. USA). Triglycerides, total cholesterol and HDL kits were obtained from Pars Azmoon CO, Iran.
Statistical analysis
Software SPSS 16 was used for data analysis. After ensuring normal distribution of data, they were statistically analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s test. The significant level was set at p < 0.05. Results are expressed as means ± SEM.
Results
Plasma glucose, serum insulin and glycosylated hemoglobin levels
Figure 1 shows the plasma glucose levels of control and experimental groups before and after oral administration of glucose. Fasting blood glucose in diabetic and diabetic-exercise groups were significantly (P<0.01) higher than that of control group as shown in time zero of Figure 1. The plasma glucose level in the control and exercise groups increased to a peak level thirty minutes after glucose administration and reduced to normal values at 120 min. In diabetic rats, the peak rise of plasma glucose concentration was detected after 30 min and remained high over the 120 min. Swim training in diabetic rats resulted in a significant (P<0.01) decrease in plasma glucose level at 120 min compared with diabetic rats.
Figure 1.
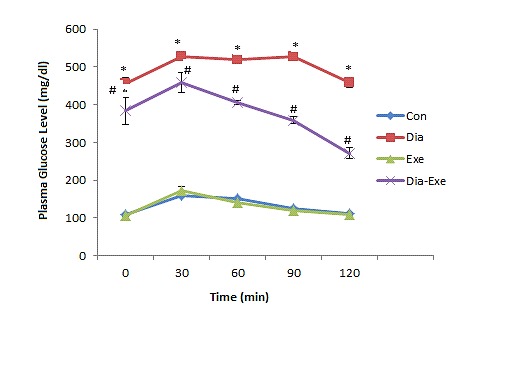
Plasma glucose levels after oral glucose tolerance test (OGTT) in sedentary control (Con), sedentary diabetic (Dia), swim trained control (Exe), and swim trained diabetic rats (Dia+Exe). Data are shown as Mean ± SEM. *P< 0.01 vs Control group; #P< 0.01, vs Diabetic group.
Serum insulin levels were measured in fasted animals after ten weeks swim training. One way ANOVA results showed that serum insulin levels were significantly (P<0.01) higher in diabetic rats compared to control group (Figure 2). Also, swim training significantly (P<0.01) reduced serum insulin level of the diabetic rats compared to diabetic group.
Figure 2.
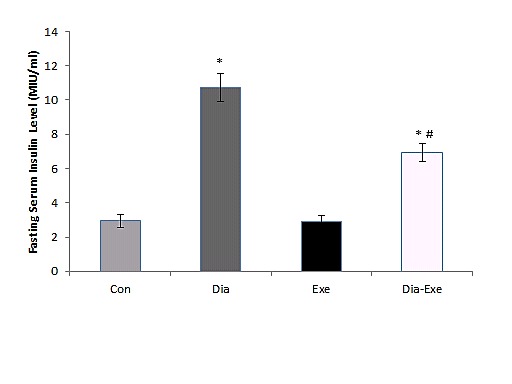
Fasting/basal serum insulin levels in sedentary control (Con), sedentary diabetic (Dia), swim trained control (Exe) and swim trained diabetic rats (Dia+Exe). Data are shown as Mean ± SEM. *P< 0.01 vs Control group; #P< 0.01, vs Diabetic group.
Blood HbA1C levels were also measured in all groups; it was significantly (P<0.01) higher in both diabetic and swim trained diabetic rats compared to the control rats (Figure 3). Swim training also significantly (P<0.01) decreased HbA1C level in the exercised diabetic rats compared to the diabetic group.
Figure 3.
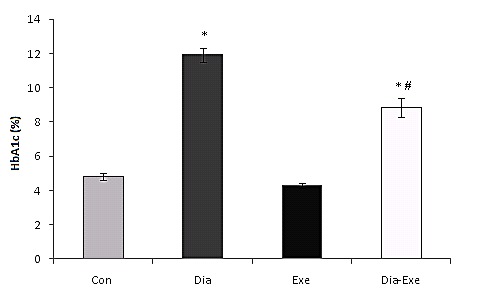
HbA1c (glycosylated hemoglobin) level in sedentary control (Con), sedentary diabetic (Dia), swim trained control (Exe), and swim trained diabetic rats (Dia+Exe). Data are shown as Mean ± SEM. *P< 0.01 vs Control group; #P< 0.01, vs Diabetic group.
Insulin resistance index(HOMA-IR)
In HOMA-IR, both diabetic and swim trained diabetic groups showed significantly (P<0.01) higher resistance in comparison with the control group (Figure 4). Swim training significantly (P<0.01) reduced insulin resistance in the diabetic+exercise group compared to the diabetic group (Figure 4).
Figure 4.
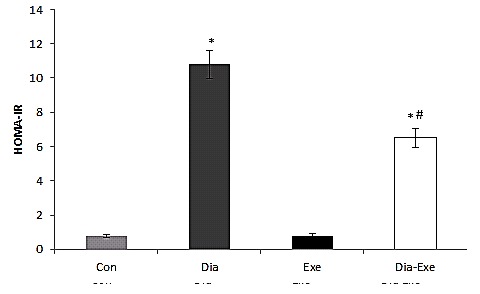
Insulin resistance index, HOMA-IR (homeostasismodel assessment) in sedentary control (Con), sedentary diabetic (Dia), swim trained control (Exe), and swim trained diabetic rats (Dia+Exe). Data are shown as Mean ± SEM. *P< 0.01 vs Control group; #P< 0.01, vs Diabetic group.
Serum lipid profile
No significant differences were seen in cholesterol and triglycerides and HDL levels between exercise and control groups. The HDL-cholesterol concentration was also significantly (P<0.05) lowered by the induction of diabetes. However, it was significantly (p<0.05) higher in the diabetic+exercise group compared to the diabetic group. Total cholesterol and triglyceride levels in the serum of diabetic groups were significantly (P<0.05) higher than in the control rats (Table 1). Swim training significantly (P<0.05) reversed the increase in the total cholesterol and triglyceride levels in the serum of the diabetic rats.
Table 1. The serum levels of total cholesterol (TCh), triglyceride (TG), and high density lipoprotein (HDL) in sedentary control (Con), sedentary diabetic (Dia), exercised control (Exe) and swim trained diabetic rats (Dia+Exe).
| Groups | Con | Dia | Exe | Dia-Exe |
| TCh (mg/dl) | 77.00±3.21 | 87.42±2.59* | 73.57±1.92 | 77.42±1.98# |
| TG (mg/dl) | 50.57±6.18 | 89.85±4.31* | 48.57±4.81 | 61.57±2.52# |
| HDL (mg/dl) | 46.85±3.96 | 28.71±4.01* | 52.14±2.28 | 44.85±1.84# |
Data are shown as Mean ± SEM, *P< 0.05vs Control group; #P< 0.05, vs Diabetic group.
Discussion
In the current study, in order to induction of T2DM associated with insulin resistance, rats received a high fat diet for 4 weeks and subsequently a low dose of STZ (35mg/kg, i.p). This model is similar to the T2DM in humans.24,25 In this study, plasma glucose levels increased both in fasting and postprandial OGTT, blood levels of HbA1C and serum levels of fasting/basal insulin escalated in diabetic rats, as they have shown by other studies.28-30 Our results also showed that HOMA-IR, serum total cholesterol, and triglycerides increased but serum HDL level decreased in diabetic rats. Coincidence of increased serum fasting/basal insulin and plasma fasting/basal glucose indicates that insulin resistance and T2DM in these animals have been created.28-30 The insulin resistance induced by high fat diet is mostly associated with an increased lipid accessibility and oxidation of the glucose-fatty acid cycle.31,32 High-fat diet produces a state of insulin resistance related to decreased insulin stimulated glycolysis and glycogen synthesis.32 Also, enhanced lipid oxidation impairs insulin evoked glycogen synthesis (GS) in skeletal muscle.31 The exact molecular and cellular relationship between high fat diet, obesity and insulin resistance and therefore T2DM has not been fully understood. Although, there are few theories that describe the different situations, and various types of insulin resistance related with high fat diet.1,13,31,32 One of the theories emphasizes the pathological roles of lipid disturbance following a high-fat intake that increases accumulation of fatty acids or fatty acid derivatives in muscle and liver, and secondary insulin resistance.33Moreover, high-fat diet is related to diminished GLUT-4 expression31 or impaired insulin function on glucose transport in skeletal muscle.22,33 Recently, studies demonstrated that fat-rich diets have pro-oxidant and pro-inflammatory compounds that have been linked to impaired insulin sensitivity.13 In the current study10 weeks of swim training had some beneficial effects on improvement of insulin sensitivity. Swim training altered glucose tolerance test, reversed the increase of plasma glucose, attenuated the HbA1Cand serum fasting/basal insulin levels in swim trained diabetic rats. In addition, swim training reduced HOMA-IR, serum total cholesterol and triglycerides and increased serum HDL level in swim trained diabetic rats. Such that, at the end of the training period, postprandial and fasted glucose were lower in swim trained diabetic rats than sedentary diabetic rats. Our findings of improved insulin function in exercised diabetic rats are in agreement with other studies.34-36 Other studies have also shown that aerobic and resistance exercise improve situations in T2DM.22,23 For instance, Medeiros et al showed that a 12- week swim training in obese rats, induced by a high-fat diet, reduced epididymal fat, fasting serum insulin, and plasma glucose levels.34 Another study indicated that 8 weeks circuit training (CT) program, combined aerobic and resistance exercise, decreased HbA1C, FBS, and plasma adiponectin levels and improved insulin resistance in T1DM.36 Also, findings of And El-Kader demonstrated that in obese type 2 diabetic patients, submitted to a 40 min aerobic session on a treadmill or resistance stretch, HOMA-IR and HbA1C levels were lower than untrained diabetic patients.35 The main benefit of swimming exercise, as a non–weight-bearing exercise, is elimination of the limitations of exercise induced by obesity and related problems. In addition, in the obese persons that suffer from joint pain, a pool as low- influenced environment can help effective performance of exercise in comparison to other types of exercise such as walking or running.37 Many studies demonstrated that resistance exercise leads to a hypertrophic response and a muscle-fiber type change in exercising muscles,38 which could increase the whole-body glucose use. It appears, therefore, that a number of potential mechanisms exist to delay or prevent the development of diabetes in this animal model.
Based on intraperitoneal glucose tolerance test, 6 weeks of treadmill and swim training in obese diabetic animals improved peripheral insulin sensitivity.39 Increased peripheral glucose uptake with exercise may be due to an up-regulation of GLUT 4 protein expression, GLUT 4 translocation, and the content of cell surface GLUT4.22,38 A subsequent increase in GLUT4 proteins may improve glycemic control.38 Mechanisms that are fundamentally involved in improvement of glucose tolerance in T2DM in exercise trained animals comprise enhancement in the glucose clearance rate (related to an increased muscular blood flow) and increased ability to extract glucose by each muscle fiber. This reveals that exercise training can involve in the improvement of glucose tolerance and insulin resistance.38,39 Other factors are also likely involved in improving insulin sensitivity. For example, the regulation of lipid turnover and utilization are also among mechanisms by which exercise training may improve insulin resistance.22,38 In addition, exercise training, by increasing the expression of proteins related to mitochondrial biogenesis such as peroxisome proliferator-activated receptor c coativator (PGC1), peroxisome proliferator-activated receptor-a (PPAR-a) and nuclear respiratory factor 1.38 enhance the oxidative capacity of skeletal muscle.22,40 Another important mechanism at cellular level,41 which exercise training can improve insulin resistance and diabetes, is enhancement of insulin signaling on insulin receptors, insulin receptor substrate or phosphatidyl- inositol- 3- kinase (PI3K).38,42
Conclusion; Our findings indicate that swim training is a suitable intervention for confronting insulin resistance and T2DM caused by HFD. The current study shows that swim training increased insulin sensitivity of diabetic rats. It may therefore be a beneficial tool for the reduction of insulin resistancein patients with T2DM.
Acknowledgments
This study was financially supported by Liver and Gastrointestinal Diseases Research Center of Tabriz University of Medical Sciences (project No: 5/4/610). This article is derived from PhD dissertation of Rafigheh Ghiasi, entitled “Evaluating the effect of swimming exercise onpanceratic inflamatory miRs signaling (miR-34a and miR146a) in type 2 diabetic male rats”.
Ethical Issues
The study protocol was designed in accordance with NIH guidelines and Ethics Committee for the Use of Animals in Research at Tabriz University of Medical Sciences.
Conflict of Interest
The authors have declared that there is no conflict of interest.
References
- 1.Parillo M, Riccardi G. Diet composition and the risk of type 2 diabetes: epidemiological and clinical evidence. Br J Nutr. 2004;92(1):7–19. doi: 10.1079/bjn20041117. [DOI] [PubMed] [Google Scholar]
- 2.Bisht R, Bhattacharya S, Jaliwala YA. Evaluating the use of Desmodium gangeticum as Alpha Glucosidase and DPP-IV Inhibitor for Type-II Diabetes. Am J Phytomed Clin Ther. 2014;2(4):530–9. [Google Scholar]
- 3.Singh K, Bal BS, Chopra S, Sigh S, Malhotra N. Ameliorative effect of lycopene on lipid peroxidation and certain antioxidant enzymes in diabetic patients. Diabetes Metab. 2012;3(6):61–75. doi: 10.4172/2155-6156.1000202. [DOI] [Google Scholar]
- 4.Virally M, Blickle JF, Girard J, Halimi S, Simon D, Guillausseau PJ. Type 2 diabetes mellitus: epidemiology, pathophysiology, unmet needs and therapeutical perspectives. Diabetes Metab. 2007;33(4):231–44. doi: 10.1016/j.diabet.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Ozougwu JC, Obimba KC, Belonwu CD, Unakalamba C. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J Physiol Pathophysiol. 2013;4(4):46–57. [Google Scholar]
- 6.Spellman CW. Pathophysiology of type 2 diabetes: targeting islet cell dysfunction. J Am Osteopath Assoc. 2010;110(3 Suppl 2):S2–7. [PubMed] [Google Scholar]
- 7.Zimmet P. Type 2 (non-insulin-dependent) diabetes--an epidemiological overview. Diabetologia. 1982;22(6):399–411. doi: 10.1007/bf00282581. [DOI] [PubMed] [Google Scholar]
- 8.Cuff DJ, Meneilly GS, Martin A, Ignaszewski A, Tildesley HD, Frohlich JJ. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care. 2003;26(11):2977–82. doi: 10.2337/diacare.26.11.2977. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes, Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33 Suppl 1:S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speakman JR. Obesity: the integrated roles of environment and genetics. J Nutr. 2004;134(8 Suppl):2090S–105S. doi: 10.1093/jn/134.8.2090S. [DOI] [PubMed] [Google Scholar]
- 11.Shafrir E, Ziv E, Mosthaf L. Nutritionally induced insulin resistance and receptor defect leading to beta-cell failure in animal models. Ann N Y Acad Sci. 1999;892:223–46. doi: 10.1111/j.1749-6632.1999.tb07798.x. [DOI] [PubMed] [Google Scholar]
- 12.Haag M, Dippenaar NG. Dietary fats, fatty acids and insulin resistance: short review of a multifaceted connection. Med Sci Monit. 2005;11(12):RA359–67. [PubMed] [Google Scholar]
- 13.Sandu O, Song K, Cai W, Zheng F, Uribarri J, Vlassara H. Insulin resistance and type 2 diabetes in high-fat-fed mice are linked to high glycotoxin intake. Diabetes. 2005;54(8):2314–9. doi: 10.2337/diabetes.54.8.2314. [DOI] [PubMed] [Google Scholar]
- 14.Masiello P. Animal models of type 2 diabetes with reduced pancreatic beta-cell mass. Int J Biochem Cell Biol. 2006;38(5-6):873–93. doi: 10.1016/j.biocel.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Islam MS, Loots Du T. Experimental rodent models of type 2 diabetes: a review. Methods Find Exp Clin Pharmacol. 2009;31(4):249–61. doi: 10.1358/mf.2009.31.4.1362513. [DOI] [PubMed] [Google Scholar]
- 16.Henriksen EJ. Invited review: Effects of acute exercise and exercise training on insulin resistance. J Appl Physiol (1985) 2002;93(2):788–96. doi: 10.1152/japplphysiol.01219.2001. [DOI] [PubMed] [Google Scholar]
- 17.Henriksen EJ. Exercise training and the antioxidant alpha-lipoic acid in the treatment of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2006;40(1):3–12. doi: 10.1016/j.freeradbiomed.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286(10):1218–27. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 19.Christ CY, Hunt D, Hancock J, Garcia-Macedo R, Mandarino LJ, Ivy JL. Exercise training improves muscle insulin resistance but not insulin receptor signaling in obese Zucker rats. J Appl Physiol (1985) 2002;92(2):736–44. doi: 10.1152/japplphysiol.00784.2001. [DOI] [PubMed] [Google Scholar]
- 20.Dela F, Von Linstow ME, Mikines KJ, Galbo H. Physical training may enhance beta-cell function in type 2 diabetes. Am J Physiol Endocrinol Metab. 2004;287(5):E1024–31. doi: 10.1152/ajpendo.00056.2004. [DOI] [PubMed] [Google Scholar]
- 21.Houmard JA, Tanner CJ, Slentz CA, Duscha BD, Mccartney JS, Kraus WE. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol (1985) 2004;96(1):101–6. doi: 10.1152/japplphysiol.00707.2003. [DOI] [PubMed] [Google Scholar]
- 22.Turcotte LP, Fisher JS. Skeletal muscle insulin resistance: roles of fatty acid metabolism and exercise. Phys Ther. 2008;88(11):1279–96. doi: 10.2522/ptj.20080018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terada S, Yokozeki T, Kawanaka K, Ogawa K, Higuchi M, Ezaki O. et al. Effects of high-intensity swimming training on GLUT-4 and glucose transport activity in rat skeletal muscle. J Appl Physiol (1985) 2001;90(6):2019–24. doi: 10.1152/jappl.2001.90.6.2019. [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Lv XY, Li J, Xu ZG, Chen L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res. 2008;2008:704045. doi: 10.1155/2008/704045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52(4):313–20. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 26.De Lemos E, Reis F, Baptista S, Pinto R, Sepodes B, Vala H. et al. Exercise training decreases proinflammatory profile in Zucker diabetic (type 2) fatty rats. Nutrition. 2009;25(3):330–9. doi: 10.1016/j.nut.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/bf00280883. [DOI] [PubMed] [Google Scholar]
- 28.Sun H, Deng X, Xiao Xiao, Chen L, Li H. Role of exercise training on insulin resistance and TNF-α in high-fat diet rats. Front Med China. 2009;3(4):403–7. doi: 10.1007/s11684-009-0071-0. [DOI] [Google Scholar]
- 29.Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53 Suppl 3:S215–9. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 30.Riserus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009;48(1):44–51. doi: 10.1016/j.plipres.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim CH, Youn JH, Park JY, Hong SK, Park KS, Park SW. et al. Effects of high-fat diet and exercise training on intracellular glucose metabolism in rats. Am J Physiol Endocrinol Metab. 2000;278(6):E977–84. doi: 10.1152/ajpendo.2000.278.6.E977. [DOI] [PubMed] [Google Scholar]
- 32.Ravussin E, Smith SR. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann N Y Acad Sci. 2002;967:363–78. doi: 10.1111/j.1749-6632.2002.tb04292.x. [DOI] [PubMed] [Google Scholar]
- 33.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and β-cell dysfunction. Eur J Clin Invest. 2002;32 Suppl 3:14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 34.Medeiros C, Frederico MJ, Da Luz G, Pauli JR, Silva AS, Pinho RA. et al. Exercise training reduces insulin resistance and upregulates the mTOR/p70S6k pathway in cardiac muscle of diet-induced obesity rats. J Cell Physiol. 2011;226(3):666–74. doi: 10.1002/jcp.22387. [DOI] [PubMed] [Google Scholar]
- 35.Abd El-Kader SM. Aerobic versus resistance exercise training in modulation of insulin resistance, adipocytokines and inflammatory cytokine levels in obese type 2 diabetic patients. Journal of Advanced Research. 2011;2(2):179–83. doi: 10.1016/j.jare.2010.09.003. [DOI] [Google Scholar]
- 36.Maiorana A, O'driscoll G, Goodman C, Taylor R, Green D. Combined aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes. Diabetes Res Clin Pract. 2002;56(2):115–23. doi: 10.1016/s0168-8227(01)00368-0. [DOI] [PubMed] [Google Scholar]
- 37.Marwick TH, Hordern MD, Miller T, Chyun DA, Bertoni AG, Blumenthal RS. et al. Exercise training for type 2 diabetes mellitus: impact on cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119(25):3244–62. doi: 10.1161/CIRCULATIONAHA.109.192521. [DOI] [PubMed] [Google Scholar]
- 38.O'gorman DJ, Krook A. Exercise and the treatment of diabetes and obesity. Med Clin North Am. 2011;95(5):953–69. doi: 10.1016/j.mcna.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Kiraly MA, Bates HE, Yue JT, Goche-Montes D, Fediuc S, Park E. et al. Attenuation of type 2 diabetes mellitus in the male Zucker diabetic fatty rat: the effects of stress and non-volitional exercise. Metabolism. 2007;56(6):732–44. doi: 10.1016/j.metabol.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 40.Fazelian S, Hoseini M, Namazi N, Heshmati J, Sepidar Kish M, Mirfatahi M. et al. Effects of L- Arginine Supplementation on Antioxidant Status and Body Composition in Obese Patients with Pre-diabetes: A Randomized Controlled Clinical Trial. Adv Pharm Bull. 2014;4(Suppl 1):449–54. doi: 10.5681/apb.2014.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jonnalagadda VG, Ram Raju AV, Pittala S, Shaik A, Selkar NA. The Prelude on Novel Receptor and Ligand Targets Involved in the Treatment of Diabetes Mellitus. Adv Pharm Bull. 2014;4(3):209–17. doi: 10.5681/apb.2014.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hawley JA, Lessard SJ. Exercise training-induced improvements in insulin action. Acta Physiol (Oxf) 2008;192(1):127–35. doi: 10.1111/j.1748-1716.2007.01783.x. [DOI] [PubMed] [Google Scholar]


