Abstract
Cell therapy is a promising intervention for treating liver diseases and liver failure. Different animal models of human liver cell therapy have been developed in recent years. Rats and mice are the most commonly used liver failure models. In fact, rodent models of hepatic failure have shown significant improvement in liver function after cell infusion. With the advent of stem-cell technologies, it is now possible to re-programme adult somatic cells such as skin or hair-follicle cells from individual patients to stem-like cells and differentiate them into liver cells. Such regenerative stem cells are highly promising in the personalization of cell therapy. The present review article will summarize current approaches to liver stem cell therapy with rodent models. In addition, we discuss common cell tracking techniques and how tracking data help to direct liver cell therapy research in animal models of hepatic failure.
Keywords: Cell therapy, Hepatic failure, Regenerative medicine, Rodents
Introduction
The liver is one of the critical organs in the body and performs many important functions including homeostasis, synthesis and storage of glucose and proteins, detoxification and immune defence.1 Hepatocytes comprise the majority of liver cells. Functional disorders of these cells are related to many devastating diseases such as hepatitis, cirrhosis and hepatocellular carcinoma. Liver related diseases have a heavy burden on socioeconomics and affect 1.7% of population. Cirrhosis is an irreversible hepatocyte failure caused by long-term infection with hepatitis viruses, alcohol abuse,2 autoimmune inflammation and exposure to metabolic metals like iron and copper.3 Although the liver is a naturally regenerative organ, at the end stage failure of liver disease such as fibrosis and cirrhosis, therapeutic intervention is necessary.4,5 Liver transplantation is considered the most efficient therapeutic strategy in liver failure.1,6 However, because of the lack of appropriate donors,1,2 around 15% of list waiting patients die.6 Furthermore, risks associated with operation, immune suppression,1 immunological rejection and high-costs,5 and the need for high technology and an experienced team are obvious limitations of liver transplantation. According to these obstacles, cell therapy has been proposed as a novel alternative method for liver transplantation.
Cell therapy is defined as cell transplantation to damaged organs for repair. The transplanted cells will replace or enhance the function of damaged tissues or organs. The major candidate cells for cell therapy are primary autologous mesenchymal cells, genetically identical or syngeneic cells, immortalized hepatocytes7 and stem cells.2,8 Currently, cellular transplantation is developing for liver damage therapy9 and in comparison to liver transplantation has many advantages including overcoming the shortage of liver donors1,6,10 and immunological rejection,1,10 and a minimally invasive approach.7
Research in the area of cell replacement therapy in the liver, particularly with stem cells, is at the beginning stages. The development and evaluation of novel cell therapy for liver disease requires the use of an appropriate animal model. In this paper we focus on the principles and methods of liver stem cell therapy in rodents as models of human liver regeneration in hepatic failure.
Induction of liver damage
Experimental animal models of liver damage can be made using exogenous factor induction or surgical removal of the liver.
Drug induced damage
Acetaminophen (N-acetyl-p-aminophenol [APAP]) is the most common pharmaceutical poison that causes severe necrosis in hepatocytes.11-13 This necrosis in hepatocytes is followed by increasing mitochondrial permeability transition (MPT).12,13 The cytotoxic effects of APAP in the hepatocytes can be assessed by measuring the release of alanine aminotransferase (ALT), mitochondrial membrane potential using JC-1 or propidium iodide labeling.12 Koffman et al. have shown a dose dependent effect of APAP, administered intraperitoneally, on the destruction of liver in C57BL/6J.14 A single 300 mg/kg intraperitoneal injection of APAP is appropriate to cause severe acute parenchymatous liver injury in the rodents.15,16
Chemical induced damage
Carbon tetrachloride (CCl4) intoxication is a common method for induced liver injury in rodents that was originally described by Proctor and Chatamra.17 Injection of doses between 0.5-1 mL/kg CCl4 dissolved in olive18-20 or corn oil5,21 twice a week for 4-8 weeks18,22 have been used for liver injury in small animal models.12,20 CCl4 can be injected to rodents intraperitoneally12,23,24 or subcutaneously.5,18,20,21
Paracetamol,25 ally alcohol (AA)26 and dimethylnitroseamine (DMN) are other chemical agents for inducing liver damage in rodents.27 Mixture of D-galactoseamine (D-gal) and lipopolysaccharide (LPS) 0.5 mg/g and 1 ng/g, respectively, in 1 ml of saline via intraperitoneal have also been used for inducing fulminant hepatic failure (FHF) in mice.28
Surgery
Hepatectomy is another strategy to prepare rodent models with liver damage. In this method a one-third partial hepatectomy is performed under general anaesthesia, and is followed by a 2 mg/kg body weight 2-acetylaminofluoren administration to inhibit liver regeneration (Figure 1).29
Figure 1.
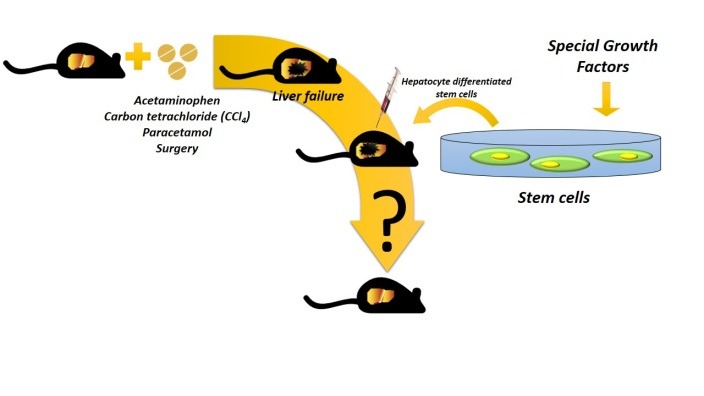
Schematic representation of liver stem cell therapy in rodents model. Stem cells can
be differentiated to hepatocytes in culture using specific condition and growth factors.
Exogenous inducing factors or surgical removal of the liver are used to produce experimental
animal model of acute liver failure. Finally, it is possible to transplant differentiated cells to liver
disease models through intrasplenic or intraportal infusion or tail vein injection.
Isolation and differentiation of stem cells
Stem cells are undifferentiated cells that are able to self-renew and differentiate into mature cell types of the specific tissue. Stem cells can be isolated from a variety of natural sources, such as blastocytes, somatic tissues, or whole blood. The first successful isolation of human embryonic stem cells was reported in 1998.30 Gradient separation, as with Percoll and isolation by enzymatic digests, as with trypsin and collagenase are the most basic techniques for stem cells. Isolation and differentiation procedures are still improved and expanding in their fields of application.
Stem cell in vitro differentiation is a novel therapeutic strategy in regenerative medicine. This strategy is based on the condition known to promote terminal differentiation of stem cells. This section provides an overview of the isolation and differentiation of different types of stem cells such as embryonic stem cells, adult stem cells, and induced pluripotent stem cells.
Embryonic stem cells
Embryonic stem cells (ESC) are pluripotent cells derived from the inner cell mass (ICM) of blastocysts, and can differentiate into all three germ layers.31 ESC are proliferated in an undifferentiated state by culturing them on feeder layers of mouse embryonic fibroblast cells (MEF) or in a medium containing leukaemia inhibitory factor (LIF). In order to differentiate ESC to cell linages, MEF or LIF is removed from the culture medium. ESC spontaneously differentiate and aggregate to form an embryoid body (EB) in hanging drop culture.32 EB contains all three germ layers which are capable of differentiating to several kinds of cells.29,32 For example, supplementation of ESC medium with sodium butyrate, recombinant mouse hepatocyte growth factor (rMuHGF) and dexamethasone resulted in hepatic differentiation in 19 days.33 Differentiated cells efficiently expressed liver biomarkers such as albumin (ALB), α- fetoprotein (AFP), α-1-antitrypsin (AAT) and transthyretin (TTR). Other researchers reported a more efficient differentiation protocol by adding the mid-stage factor oncostatin M (OSM).32 The synthesis of urea is also an important hepatocytes marker that is detectable from the ninth day of differentiation, reaching its maximum level on day 18.29
Adult stem cells
Mesenchymal stem cells (MSC) are the most available and important adult stem cells. MSC isolated from adult tissues such as bone marrow, adipose tissue, umbilical cord blood and placenta can be differentiated into functional hepatocytes.21 In recent studies the immunomodulatory effects of MSC have been documented.34 MSC that play a critical role in the bone marrow niche have the ability to self-renew or differentiate to other lineages.35 MSC are positive for CD90, CD105 and CD73 and negative for CD45, CD34, CD14, CD11b, CD79a and human leukocyte antigen (HLA)-DR.36 Fluorescence-activated cell sorting showed gene expression of CD90 and CD29 but not CD45 in MCS from rats.37 The natural environments of MSC are hypoxic and published data showed hypoxia preconditioning to have supporting effects on the cell survival and genetic instability of these cells, confirming a new insight towards overcoming poor engraftment after transplantation at the bed side.38
MSC can differentiate into hepatocytes by culturing on a specific medium containing growth factors such as FGF, HGF, EGF, insulin and dexamethasone.37 The first replacement of the medium is performed on the third day of differentiation protocol.18,22,39 Different concentrations of growth factors including human hepatocyte growth factor (rhHGF), human fibroblast growth factor-4 (rhFGF-4) and EGF have been used to differentiate MSC into hepatocytes.21,40 For assessing the differentiation of MSC to liver cells, hepatocytes marker genes such as ALB and α-fetoproteins analysed during in vitro differentiation.5,37ESC and MSC are two more common types of stem cells that are used to differentiate to hepatocytes.
Induced pluripotent stem cells
Induced pluripotent stem cells (iPSC) are actually patient specific pluripotent cells. iPSC were originally created by introduction of four genes, Oct4, Sox2, Kfl4, and cMyc, into a differentiated mouse cell.41 These reprogrammed cells were pluripotent. Technologies for the generation of iPSC are continuously evolving. For example, it has recently been shown that the presence of a single transcription factor, Oct4, was enough to induce pluripotency in adult cells.42 iPSC are now being used for disease modelling, drug development and organ synthesis. iPSC have been efficiently used to generate liver tissue for transplantation. A co-culture of iPSC with endothelial and MSC resulted in functional liver buds.43 For example, in one study, baicalin led to erythroid differentiation of CD133+ hematopoietic stem cells (HSC). Thus, baicalin application can be effective in the treatment of a wide range of disease.44 Then, HSC niches and other factors around HSC can promote malignancies,45-47 so it may be stem cells has notable clinical applications, especially in transplantation.44
The methods and ways of transplantation of functional mature cells
Generated functional mature cells from stem cells could be successfully transplanted into laboratory rat models. Transplanted cells could adhere, survive and regenerate the liver organ. The methods of transplantation of functional mature cells are discussed as follows.
Intrasplenic
A cell count of1×106-6×106 cells/ml in 0.5-1ml PBS could be injected through the lower pole of the spleen in laboratory rats or mice. To prevent bleeding and cell leakage after cell injection the lower pole of the spleen is ligated.18,22,29,40
Tail
A cell count of 5×105 suspended in 100µl PBS could be injected into the tail vein of rodents.1,19,28
Portal vein
Zhao and colleagues showed that intravenous injection of MSC is effective in the treatment of liver fibrosis in comparison with intrahepatic or intraperitoneal injection. As in intravenous injection liver lobules were normal, but in intrahepatic and intraperitoneal injection severe collagen deposition was observed5. A cell count of 1×106 suspended in 30µl of PBS or DMEM could be injected through the portal vein.20,21,26
Homing and efficacy of cell therapy
Histopathological methods such as Haematoxylin and Eosin (H&E),22,28 Masson Trichrome21,22 and Sirius Red22 staining were used for detection of tissue structure changes after cell therapy in liver failure.
The homing and engraftment of the transplanted cells to the target organ is key to the success of the cell therapy. Tracing of individual transplanted cells in the body of the recipient is particularly important in studies of cell therapy.
Tracing of transplanted cells
Several labelling techniques are currently used to trace transplanted cells in cell therapy. Carboxy fluorescein diacetate succinimidyl ester (CFSE)39 and 4,6-diamidino-2-phenylindole, dihydrochloride (DAPI),24 chloromethyl-benzamidodialkylcarbocyanine (CM- Dil)40 fluorescent dyes are commonly used to examine cell localization.
The red fluorochrome PKH26, which mainly binds to the cell membrane, is an efficient cell tracer to locate transplanted cells.20 The liver cell suspension could be easily labelled after around 4 minutes of incubation with PKH261. This fluorescent dye is not toxic, and labelled cells retain both biological and proliferative activity. The characteristics of PKH26 are compatible with rhodamine or phycoerythrin detection systems.1
Super paramagnetic particles of iron oxide (SPIO), such as Fe3O4- Poly- L- Lysin (PLL), could be used for tracking in cell therapy. In this method, the labelling efficiency of cells could be assessed in vitro by Prussian blue staining and atomic absorption spectrometry. The magnetically labelled cells are tracked in vivo by MR imaging.18,19
Alu sequence detection by polymerase chain reaction (PCR) and green fluorescent protein (GFP) fusions are other useful techniques for tracking and locating transplanted cells.9,19,21,28
Functional assay of transplanted cells
Characterization of cell viability and cell identity after administering the cells is very important in the long-term follow-up study of liver cell therapy. Mortality rate is a reliable indicator of transplant functionality and overall recipient health. Other cellular indicators include Y-chromosome and hepatic markers.
Detection of the Y-chromosome in the liver tissue of female recipients from male rodents is related to the efficacy of cell therapy. Both fluorescent in situ hybridization (FISH) and real time quantitative polymerase chain reaction (qPCR) may be used to identify and quantify Y chromosome material or a male-specific SRY.29 Investigations of immunohistochemical expression of hepatic markers, including ALB, AFP, CK18 and CK19, are popular quantitative functional assays of transplanted cells.26,28
Conclusion
Research is ongoing to identify an optimal strategy for long-term cell therapy treatment in liver failure. Rodents are suitable animal models of liver regeneration in hepatic failure. In fact, rodent models of hepatic failure have shown significant improvement in liver function after cell infusion. The major steps of liver stem cell therapy in rodents are induction of liver damage, stem cell in vitro differentiation, and transplantation of functional mature cells. In vivo identification and tracking of transplanted cells in animal models are important to determine their homing and fate. These parameters are actually related to and may even predict, the success or failure of liver stem cell therapy.
Acknowledgments
This research was based partially on a MSc student's thesis and was financially supported by a grant to M.D. (research project number 115/175) from the Liver and Gastrointestinal Disease Research Center of Tabriz University of Medical Sciences.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Piryaei A, Rezazadeh Valojerdi M, Baharvand H. Therapeutic Potential of Mouse Bone Marrow Mesenchymal Stem Cells in Carbon Tetrachloride (CCl4)-Induced Liver Fibrosis. J Iran Anat Sci. 2010;7(28-29):99–112. [Google Scholar]
- 2.Levicar N, Dimarakis I, Flores C, Tracey J, Gordon MY, Habib NA. Stem cells as a treatment for chronic liver disease and diabetes. Handb Exp Pharmacol. 2007;(180):243–62. doi: 10.1007/978-3-540-68976-8_11. [DOI] [PubMed] [Google Scholar]
- 3.Wu XB, Tao R. Hepatocyte differentiation of mesenchymal stem cells. Hepatobiliary Pancreat Dis Int. 2012;11(4):360–71. doi: 10.1016/s1499-3872(12)60193-3. [DOI] [PubMed] [Google Scholar]
- 4.Pournasr B, Farzaneh Z, Shahsanvani M, Baharvand H. Liver Development and In vitro Differentiation of Embryonic Stem Cells to Hepatocytes. Yakhteh Medical Journal. 2010;11(4):348–73. [Google Scholar]
- 5.Zhao Q, Ren H, Zhu D, Han Z. Stem/progenitor cells in liver injury repair and regeneration. Biol Cell. 2009;101(10):557–71. doi: 10.1042/BC20080105. [DOI] [PubMed] [Google Scholar]
- 6.Atta SA. Hepatocyte Transplantation. Euroasian J Hepato-Gastroenterol. 2013;3(1):59–63. [Google Scholar]
- 7.Jameson E. Cellular Transplantation for Liver Diseases. Gastroen Res. 2008;1(1):8–13. doi: 10.4021/gr2008.11.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou WL, Medine CN, Zhu L, Hay DC. Stem cell differentiation and human liver disease. World J Gastroenterol. 2012;18(17):2018–25. doi: 10.3748/wjg.v18.i17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seppen J, Filali EE, Elferink RO. Small animal models of hepatocyte transplantation. Methods Mol Biol. 2009;481:75–82. doi: 10.1007/978-1-59745-201-4_7. [DOI] [PubMed] [Google Scholar]
- 10.Nussler A, Konig S, Ott M, Sokal E, Christ B, Thasler W. et al. Present status and perspectives of cell-based therapies for liver diseases. J Hepatol. 2006;45(1):144–59. doi: 10.1016/j.jhep.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Botta D, Shi S, White CC, Dabrowski MJ, Keener CL, Srinouanprachanh SL. et al. Acetaminophen-induced liver injury is attenuated in male glutamate-cysteine ligase transgenic mice. J Biol Chem. 2006;281(39):28865–75. doi: 10.1074/jbc.m605143200. [DOI] [PubMed] [Google Scholar]
- 12.James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31(12):1499–506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 13.Reid AB, Kurten RC, McCullough SS, Brock RW, Hinson JA. Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J Pharmacol Exp Ther. 2005;312(2):509–16. doi: 10.1124/jpet.104.075945. [DOI] [PubMed] [Google Scholar]
- 14.Kofman AV, Morgan G, Kirschenbaum A, Osbeck J, Hussain M, Swenson S. et al. Dose- and time-dependent oval cell reaction in acetaminophen-induced murine liver injury. Hepatology. 2005;41(6):1252–61. doi: 10.1002/hep.20696. [DOI] [PubMed] [Google Scholar]
- 15.Shen K, Chang W, Gao X, Wang H, Niu W, Song L. et al. Depletion of activated hepatic stellate cell correlates with severe liver damage and abnormal liver regeneration in acetaminophen-induced liver injury. Acta Biochim Biophys Sin (Shanghai) 2011;43(4):307–15. doi: 10.1093/abbs/gmr005. [DOI] [PubMed] [Google Scholar]
- 16.Yin H, Cheng L, Holt M, Hail N Jr, Maclaren R, Ju C. Lactoferrin protects against acetaminophen-induced liver injury in mice. Hepatology. 2010;51(3):1007–16. doi: 10.1002/hep.23476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Proctor E, Chatamra K. High yield micronodular cirrhosis in the rat. Gastroenterology. 1982;83(6):1183–90. [PubMed] [Google Scholar]
- 18.Ju S, Teng GJ, Lu H, Zhang Y, Zhang A, Chen F. et al. In vivo MR tracking of mesenchymal stem cells in rat liver after intrasplenic transplantation. Radiology. 2007;245(1):206–15. doi: 10.1148/radiol.2443061290. [DOI] [PubMed] [Google Scholar]
- 19.Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H. et al. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40(6):1304–11. doi: 10.1002/hep.20452. [DOI] [PubMed] [Google Scholar]
- 20.Zhan Y, Wang Y, Wei L, Chen H, Cong X, Fei R. et al. Differentiation of hematopoietic stem cells into hepatocytes in liver fibrosis in rats. Transplant Proc. 2006;38(9):3082–5. doi: 10.1016/j.transproceed.2006.08.132. [DOI] [PubMed] [Google Scholar]
- 21.Chang YJ, Liu JW, Lin PC, Sun LY, Peng CW, Luo GH. et al. Mesenchymal stem cells facilitate recovery from chemically induced liver damage and decrease liver fibrosis. Life Sci. 2009;85(13-14):517–25. doi: 10.1016/j.lfs.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Rabani V, Shahsavani M, Gharavi M, Piryaei A, Azhdari Z, Baharvand H. Mesenchymal stem cell infusion therapy in a carbon tetrachloride-induced liver fibrosis model affects matrix metalloproteinase expression. Cell Biol Int. 2010;34(6):601–5. doi: 10.1042/cbi20090386. [DOI] [PubMed] [Google Scholar]
- 23.Khurana S, Mukhopadhyay A. Characterization of the potential subpopulation of bone marrow cells involved in the repair of injured liver tissue. Stem Cells. 2007;25(6):1439–47. doi: 10.1634/stemcells.2006-0656. [DOI] [PubMed] [Google Scholar]
- 24.Zhao W, Li JJ, Cao DY, Li X, Zhang LY, He Y. et al. Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis. World J Gastroenterol. 2012;18(10):1048–58. doi: 10.3748/wjg.v18.i10.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maheswari C, Maryammal R, Venkatanarayanan R. Hepatoprotective Activity of "Orthosiphon stamineus" on Liver Damage Caused by Paracetamol in Rats. Jordan J Biol Sci. 2008;1(3):105–8. [Google Scholar]
- 26.Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M. et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106(2):756–63. doi: 10.1182/blood-2005-02-0572. [DOI] [PubMed] [Google Scholar]
- 27.George J, Rao KR, Stern R, Chandrakasan G. Dimethylnitrosamine-induced liver injury in rats: the early deposition of collagen. Toxicology. 2001;156(2-3):129–38. doi: 10.1016/s0300-483x(00)00352-8. [DOI] [PubMed] [Google Scholar]
- 28.Yu J, Cao H, Yang J, Pan Q, Ma J, Li J. et al. In vivo hepatic differentiation of mesenchymal stem cells from human umbilical cord blood after transplantation into mice with liver injury. Biochem Biophys Res Commun. 2012;422(4):539–45. doi: 10.1016/j.bbrc.2012.04.156. [DOI] [PubMed] [Google Scholar]
- 29.Chinzei R, Tanaka Y, Shimizu-Saito K, Hara Y, Kakinuma S, Watanabe M. et al. Embryoid-body cells derived from a mouse embryonic stem cell line show differentiation into functional hepatocytes. Hepatology. 2002;36(1):22–9. doi: 10.1053/jhep.2002.34136. [DOI] [PubMed] [Google Scholar]
- 30.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS. et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 31.Poh YC, Chen J, Hong Y, Yi H, Zhang S, Chen J. et al. Generation of organized germ layers from a single mouse embryonic stem cell. Nat Commun. 2014;5:4000. doi: 10.1038/ncomms5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamazaki T, Iiboshi Y, Oka M, Papst PJ, Meacham AM, Zon LI. et al. Hepatic maturation in differentiating embryonic stem cells in vitro. FEBS Lett. 2001;497(1):15–9. doi: 10.1016/s0014-5793(01)02423-1. [DOI] [PubMed] [Google Scholar]
- 33.Yibo Y, Zhong B, Weiwei Q, Yang S, Yongcong W, Yixuan F. et al. In vitro differentiation of mouse embryonic stem cells into functional hepatocytes by sodium butyrate, hepatocyte growth factor and dexamethasone under chemically defined conditions. Afr J Biotechnol. 2011;10(46):9493–500. [Google Scholar]
- 34.Lotfinegad P, Shamsasenjan K, Movassaghpour A, Majidi J, Baradaran B. Immunomodulatory Nature and Site Specific Affinity of Mesenchymal Stem Cells: a Hope in Cell Therapy. Adv Pharm Bull. 2014;4(1):5–13. doi: 10.5681/apb.2014.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohammadian M, Shamsasenjan K, Lotfi Nezhad P, Talebi M, Jahedi M, Nickkhah H. et al. Mesenchymal Stem Cells: New Aspect in Cell-Based Regenerative Therapy. Adv Pharm Bull. 2013;3(2):433–7. doi: 10.5681/apb.2013.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sousa BR, Parreira RC, Fonseca EA, Amaya MJ, Tonelli FM, Lacerda SM. et al. Human adult stem cells from diverse origins: an overview from multiparametric immunophenotyping to clinical applications. Cytometry A. 2014;85(1):43–77. doi: 10.1002/cyto.a.22402. [DOI] [PubMed] [Google Scholar]
- 37.Meier RP, Müller YD, Morel P, Gonelle-Gispert C, Buhler LH. Transplantation of mesenchymal stem cells for the treatment of liver diseases, is there enough evidence? Stem Cell Res. 2013;11(3):1348–64. doi: 10.1016/j.scr.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Ejtehadifar M, Shamsasenjan K, Movassaghpour A, Akbarzadehlaleh P, Dehdilani N, Abbasi P. et al. The Effect of Hypoxia on Mesenchymal Stem Cell Biology. Adv Pharm Bull. 2015;5:In Press. doi: 10.15171/apb.2015.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia X, Chen W, Ma T, Xu G, Liu H, Liang C. et al. Mesenchymal stem cells administered after liver transplantation prevent acute graft-versus-host disease in rats. Liver Transpl. 2012;18(6):696–706. doi: 10.1002/lt.23414. [DOI] [PubMed] [Google Scholar]
- 40.Sun S, Chen G, Xu M, Qiao Y, Zheng S. Differentiation and migration of bone marrow mesenchymal stem cells transplanted through the spleen in rats with portal hypertension. PLoS One. 2013;8(12):e83523. doi: 10.1371/journal.pone.0083523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 42.Kim JB, Greber B, Arauzo-Bravo MJ, Meyer J, Park KI, Zaehres H. et al. Direct reprogramming of human neural stem cells by OCT4. Nature. 2009;461(7264):649–53. doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- 43.Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T. et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499(7459):481–4. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 44.Abbasi P, Shamsasenjan K, Movassaghpour A, Akbarzadeh P, Dehdilani N, Ejtehadifar M. The Effect of Baicalin, A PPAR Activator, on Erythroid Differentiation of CD133+ Hematopoietic Stem Cells in Umbilical Cord. Cell J. 2014;17(1):1–17. doi: 10.22074/cellj.2015.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114(16):3367–75. doi: 10.1182/blood-2009-06-225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu S, Otsuyama K, Ma Z, Abroun S, Shamsasenjan K, Amin J. et al. Induction of Multilineage Markers in Human Myeloma Cells and Their Down-Regulation by Interleukin 6. Int J Hematol. 2007;85(1):49–58. doi: 10.1532/ijh97.06132. [DOI] [PubMed] [Google Scholar]
- 47.Iqbal MS, Otsuyama K, Shamsasenjan K, Asaoku H, Mahmoud MS, Gondo T. et al. Constitutively lower expressions of CD54 on primary myeloma cells and their different localizations in bone marrow. Eur J Haematol. 2009;83(4):302–12. doi: 10.1111/j.1600-0609.2009.01284.x. [DOI] [PubMed] [Google Scholar]


