Abstract
Purpose: The aim of this study was to find a relationship between drug solubility and its transdermal permeation and find the best vehicle composition to improve transdermal permeation of Tadalafil.
Methods: Pure or binary mixtures of commonly used solvents in pharmaceutical sciences including ethanol, glycerin, N-methyl pyrrolidone (NMP), polyethylene glycol (PEG) 400 and propylene glycol (PG) were evaluated for drug solubility and transdermal delivery through the exercised rat skin employing Franz diffusion cells.
Results: Tadalafil showed higher solubility in NMP compared to the other solvents. The amount of Tadalafil permeation from the pure vehicles was ranked as follow: Ethanol >glycerin >NMP>PEG 400 >PG. Furthermore, the solubility and transdermal delivery from binary mixtures of NMP and PG were higher than that obtained from pure PG, and accordingly, both increased with increasing NMP concentration in the binary solvent mixtures. The Flux values were determined as following order for Ethanol>NMP>glycerin>PG>PEG 400.
Conclusion: Generally, increase in Tadalafil solubility resulted in a decrease in its skin penetration rate and amount. However, NMP exhibited substantial drug skin penetration rate and amount accompanying with appropriate drug solvency. In conclusion, the results of this study introduced NMP as a solvent suitable for application in the formulation of topically applied drug delivery systems.
Keywords: Solubility, Transdermal drug delivery, Tadalafil, N-methyl pyrrolidone
Introduction
Transdermal and dermal delivery systems represent an attractive alternative route of administration to oral delivery systems, which are available for systemic and local effect of drugs. Dermal and transdermal delivery systems have proven to be effective in reducing dose frequency, long duration of activity, achieving target delivery, easily administrating and avoiding hepatic first pass metabolism.1-4 The stratum corneum, or horny layer, is the outermost layer of the skin and has been identified as the main barrier of the most drug permeation. Various strategies have been employed in attempts to improve the efficacy of topical delivery of drugs, including iontophoresis, electroporation, occlusion and ultrasound.5-9 Furthermore, an alternative approach is to use chemicals, known as permeation enhancers, which are materials that can partition into, and interact with, skin constituents to induce a temporary and reversible decrease in the skin barrier properties.10,11 The therapeutic effects of transdermal formulations depend on the drug action and also on other structural factors of the vehicle. Therefore, when a drug is formulated to be administered by transdermal route, it is essential to investigate the excipients and vehicles properties.3,12-16 There are a few solvents which have been used frequently in the formulation of TDDSs as carrier and penetration enhancer, as well, such as ethanol, glycerin, propylene glycol (PG) and polyethylene glycols (PEGs).17-20 Pyrrolidones, such as N-Methyl-2-pyrrolidone (NMP), have been proposed as penetration enhancers. As with many other penetration enhancers, pyrrolidones are able to promote both the penetration of hydrophilic drugs and the penetration of lipophilic drug substances. NMP is also a cosolvent and very strong solubilizing agent that has important applications in different fields of industry.21-24 NMP is a biodegradable FDA approved solvent listed in GRAS (generally recognized as safe), therefore, environmental contamination considerations are fewer in its applications.22,25
Tadalafil is a potent, reversible and competitive inhibitor of phosphodiesterase 5 which inactivates cyclic guanosine monophosphate used in the treatment of erectile dysfunction by facilitating relaxation of smooth muscle resulting to penile erection. Tadalafil clearance is mainly via hepatic metabolism by CYP3A to a catechol that undergoes methylation and is extensively conjugated to form a methyl catechol glucuronide which is a major circulating metabolite.26-28
The administration of Tadalafil through the skin could provide many benefits compared with the oral administration, where the first pass metabolism as well as gastro intestinal side effects will be avoided. Moreover, side effects like headache, stomach upset, back pain, muscle pain, stuffy nose, flushing, or dizziness were reported as well. Therefore, it is valuable local therapy application in male genital system.29-31 However, it is difficult to reach therapeutic levels because of the difficulty for the drug to penetrate through the skin barrier. It was shown that increase in drug solubility in the carrier causes decrease in skin penetration ability of the topically designed dosage form. On the other hand, drug solubility guarantees content uniformity of dosage form which is important for topical delivery that intrinsically suffers from erratic and unpredictable drug absorption.32-34 Therefore, investigation on the selection of proper solvent for formulation of TDDSs which simultaneously provides adequate drug solubility and appropriate skin penetration would be invaluable. Therefore, the aim of the present study was to investigate the effects of different vehicles on the solubility and transdermal delivery of Tadalafil.
Materials and Methods
Materials
Tadalafil was kindly donated from OSVE Pharmaceutical Company (Tehran, Iran). Acetonitrile, glycerin, propylene glycol, polyethylene glycol 400 (PEG 400) and N-methyl pyrrolidone (NMP) were purchased from Merck Company (Darmstadt, Germany).
Solubility study
Solubility of Tadalafil in ethanol, acetonitrile, glycerin, propylene glycol, PEG 400 and N-methyl pyrrolidone and different combinations of mixture of NMP-PG was checked. Excess amounts of Tadalafil were added to sealed vials containing solvents or mixtures. All dispersions were shaken for 24 h at room temperature (25 °C). The dispersions were then filtered using hydrophilic Durapore filters (0.45 μm, Milipore, Ireland). To determine the amount of drug dissolved, HPLC method was employed. Aliquots were examined and the solubility of drug was identified in each sample. Experiments were carried out in triplicates.
In vitro skin permeation studies
The abdominal skin of Wistar male rats, weighing 140-180 g, was shaved using an electric razor after scarifying animals by excess chloroform anesthesia (24 h before the treatment). The experiments were performed in accordance with ethical committee and the guide lines of the Care and Use of Laboratory Animals of Tabriz University of Medical Sciences, Tabriz-Iran (National Institutes of Health Publication No 85-23, revised 1985). The abdominal skin was surgically excised and excess subcutaneous fat was carefully removed. To remove extraneous debris and leachable enzymes, the dermal side of the skin was kept in contact with a normal saline solution up to 12 h before using for permeation studies. The skins were mounted on the Franz diffusion cells (Erweka HDT6, Germany) (with an available diffusion area of 3.14 cm2) with the stratum corneum facing the donor compartment and the dermis facing the receptor. Each set of experiments was performed with three diffusion cells. Equal amounts of Tadalafil was dissolved in solvents. Twenty four milliliter of hydroethanolic solution (50:50, v:v) was used as the receptor medium and 2 mL of the each solution was placed on the skin surface in the donor compartment. The temperature of the receptor medium was maintained at 37±2 °C by circulating of warm water between two layers of the diffusion cells and contents of receptor medium were stirred magnetically at a constant rate of 750 rpm. Samples (0.1 mL) were withdrawn from the receptor compartment at different time intervals (30, 60, 120, 240, 360, 480 and 720 min) and replaced with the same volume of hydroethanolic solution at 37±2 °C to maintain a constant volume. The amount of Tadalafil in the receptor phase was assayed with an HPLC method. The cumulative amount of the permeated Tadalafil per unit area of skin was plotted versus time. The permeability test was performed in triplicate.
Analytical procedure
The HPLC apparatus (Knauer, Germany) equipped with UV detector and an ODS C18 (250×4.6 mm, 5 μm) column was used to assay the amount of Tadalafil. The mobile phase consisting of phosphate buffered saline (PBS): acetonitrile (30:70) mixture eluted the column at the flow rate of 0.7 mL/min and the effluent was monitored at 290 nm using a UV detector. 20 μl of sample was injected into the HPLC column and the retention time of Tadalafil at this HPLC condition was 5.1 min. Calibration curve with standard concentrations ranging from 0.125 to 5 μg/mL of Tadalafil in mobile phase was constructed to measure the drug concentration in the samples.
Data treatment
The cumulative amount of the permeated drug was plotted against time. Slope of the linear portion of the cumulative drug permeated vs. time plot and the intercept on the time axis are considered as the steady-state Flux (J) value.
Statistical analysis
The data was demonstrated as mean ± SD. Analysis of variance (ANOVA) was performed for multiple comparisons using SPSS software (version 15). A level of significance of P< 0.05 was set to determine any significant difference between the formulations.
Results and Discussion
Solubility of Tadalafil in different vehicles
Table 1 shows the solubility of Tadalafil in different vehicles used for transdermal drug delivery. Results demonstrated that Tadalafil showed the highest solubility in NMP compared to the other used pure or binary vehicles. Results also indicated that the solubility of Tadalafil was increased significantly via increasing the ratio of NMP in the binary solvent mixtures with PG. Although the studied solvents are routinely used in the formulation of TDDSs, few disadvantages are reported such as skin dryness using ethanol35 as well as sticky and greasy characteristics for glycerin36 which cause patient incompliance and even stop the treatment procedure. PG have shown some merits among these solvents for application in TDDSs such as lack of above mentioned drawbacks and skin permeation enhancement ability.3,37 Therefore, the binary mixtures of PG and NMP in different ratios were prepared to provide the benefits of skin permeation enhancement and high drug loading and solubility providing content uniformity of dosage form, simultaneously.
Table 1. Permeated Tadalafil from the rat skin during 12 h (µg/cm2), Flux values (µg/cm2/h) and solubility (mg/mL) of Tadalafil in different vehicles. Data are presented as mean ± standard deviation (n=3).
| Vehicles | Glycerin | Ethanol | PG | PEG 400 | NMP |
PG: NMP (75: 25) |
PG: NMP (50: 50) |
PG: NMP (25: 75) |
| Permeated drug after 12h | 5.37 ± 0.44 | 6.01 ± 0.10 | 1.88 ± 0.12 | 2.41 ± 0.16 | 4.55 ± 0.31 | 3.21 ± 0.05 | 2.30 ± 0.19 | 1.57 ± 0.55 |
| Flux | 0.26 ± 0.02 | 0.41 ± 0.03 | 0.14 ± 0.01 | 0.10 ± 0.02 | 0.34 ± 0.02 | 0.23 ± 0.01 | 0.11 ± 0.02 | 0.06 ± 0.02 |
| Solubility | 0.17 ± 0.05 | 1.98 ± 0.30 | 1.99 ± 0.08 | 14.7 ± 2.86 | 344.99 ± 13.10 | 13.71 ± 0.56 | 26.77 ± 3.80 | 220.87 ± 10.12 |
Skin permeation study of Tadalafil from different vehicles
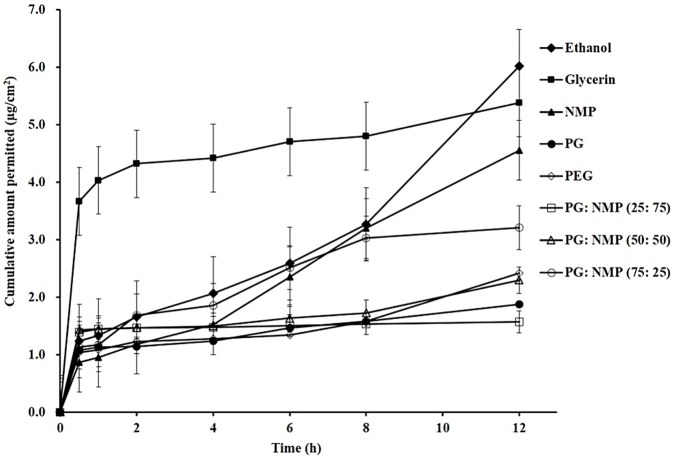
Figure 1 illustrates the skin permeation of Tadalafil from different vehicles. As it is shown, Tadalafil which dissolved in glycerin, ethanol or NMP showed higher skin permeation over 12 h than that of dissolved in PG or PEG 400. Accordingly, calculated Flux values of Tadalafil from different formulations also indicated that formulations containing ethanol, NMP and glycerin possess the higher Flux values compared with those possess PG and PEG 400. Furthermore, incorporation of PG and NMP as Tadalafil vehicle increased the Flux value and amount of permeated drug over 12 h. On the other hand, as shown in Figure 1, Glycerin not only increased the total amount of permeated drug over 12 h, but also enhanced the rate of permeated drug, where highest amount of Tadalafil was permeated during 1 h compared to the other vehicles.38 This could be advantageous in the reducing the time needed for the onset of action especially for drugs such as Tadalafil.
Figure 1.
Tadalafil skin permeation from different vehicles. [N-Methylpyrrolidone: NMP, propylene glycol (PG) and poly ethylene glycol (PEG) 400].
Although it was shown that improving the drug solubility by addition of NMP to PG decreased drug penetration rate and amount through skin, but NMP with almost more than 20 times higher capacity for drug solubility illustrated better drug transdermal potential and homogenous distribution for NMP than PG and PEG400.
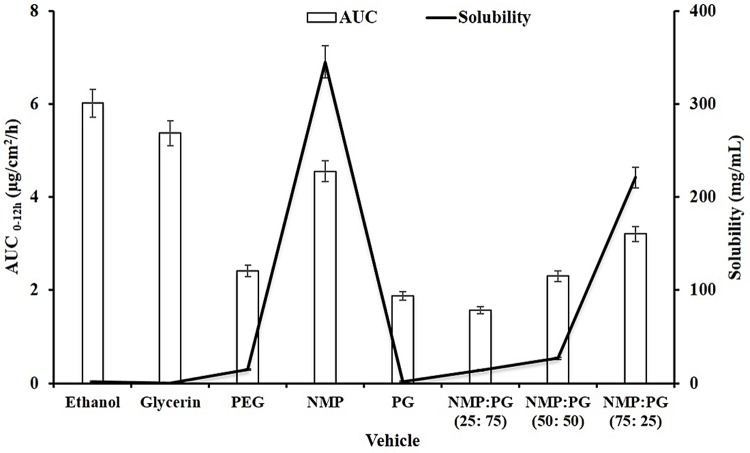
Figure 2 compares the solubility and total permeated Tadalafil during 12 h (AUC 0-12h) by using different vehicles. Although glycerin and ethanol showed higher permeability, Tadalafil exhibited lowest solubility in these solvents. On the other hand, Tadalafil exhibited the highest solubility and AUC 0-12h value in the case of NMP compared with other solvents. Figure 2 also indicates that by using the binary solvent mixtures, composed of PG and NMP, and increasing the ratio of NMP, both of the solubility and AUC 0-12h values were increased simultaneously.
Figure 2.
Solubility and Permeated Tadalafil from the rat skin (AUC 0-12h) using different vehicles
Although total permeated Tadalafil during 12 h from formulations containing ethanol and glycerin were higher than that contains NMP, higher solubility of Tadalafil in NMP allows using minimum amount of NMP with ideal homogeneity in the formulation. This resulted in lower probable harmful effects of NMP on the skin. Results of using binary solvent mixtures also indicated that by increasing the ratio of NMP, solubility and skin permeation values of Tadalafil were increased concurrently.
Pyrrolidone is a component of natural moisturizing factor, therefore its derivatives and structurally related compounds are studied as penetration enhancers.21,23,25 These enhancers are known to improve transport of drugs of varied lipophilic (betamethasone-17-benzoate, hydrocortisone and progesterone)/hydrophilic (mannitol, 5-fluorouracil and sulphaguanidine) properties. Mechanism of action of the pyrrolidones is partition into human stratum corneum. NMP is a polar and clear solvent, liquid at room temperature, miscible with most common solvents and was used to extract aromatic moieties from oils, olefins and animal feeds.22,24,39,40 The mechanism of solubilization of drugs by NMP is ambiguous, and there are various theories for the same, including its action as a cosolvent, complexing agent and surfactant. The NMP molecule has nonpolar carbons, which can weaken the hydrogen-bonded structure of water, thus enabling it to act as a cosolvent. In addition, the presence of a large planar nonpolar region can lead to hydrophobic interactions between NMP and drugs. NMP exerts its direct influence on the aqueous regions of skin between the polar lipid head groups of the bilayer. It penetrates into this region of skin in such amounts that they alter the solubilizing ability of this site, thereby promoting drug partition into skin, which subsequently results in increased flux of the penetrant.41 Comparison of the Tadalafil penetration patterns of different solvents demonstrated that NMP caused a sustained penetration for Tadalafil (Figure 2). It was reported that, inside the tissue, NMP alters the solvent surrounding the membrane and creates reservoirs in skin membranes, giving potential for sustained release of a drug from the stratum corneum during prolonged time periods.23,25,37,40
In Lee et al.’s investigation, NMP was found to be an effective enhancer for transdermal lidocaine from a hydrophobic formulation and also mixtures of NMP with isopropyl myristate resulted in synergistic improvement in lidocaine transdermal delivery.14 Our findings showed that binary mixture of NMP and PG did not enhance drug permeation synergically, but our goal was to improve enhancement efficacy of PG with NMP which was successfully carried out. Further investigations indicated that combination of NMP with lauryl-2pyrrolidone and isopropyl myristate significantly increased the permeation of phenol red and 5-flouorouracil. Furthermore, NMP in combination with Isopropyl myristate increased metronidazole penetration through human skin.22,23,42
NMP has been also used to accelerate the permeation of mefanamic acid across rabbit skin in vivo.43 Other study showed that NMP in combination with propylene glycol enhanced the transport of naloxone through human skin.44
Although NMP in the present study and previous reports exhibited substantial results in transdermal delivery of various classes of drugs, however, the clinical use of pyrrolidones as skin penetration enhancers is limited because of adverse reactions to these compounds which are associated with local adverse reactions, including irritant dermatitis and erythema.22,41 Therefore, the acute and chronic toxic effect of NMP on skin is open to be studied.
Conclusion
Skin permeation enhancement technology is a rapidly developing field which would significantly increases the number of drugs suitable for transdermal drug delivery. The results of this study showed that the type and concentration of solvent are very important variables for solubility, rate and extent of transdermal delivery of Tadalafil. The results indicated that using NMP as Tadalafil solvent resulted in the substantial improvement in skin penetration of Tadalafil comparable with routinely used solvents such as ethanol and glycerin. NMP showed the fast penetration rate of Tadalafil, as well. Further investigation in this area will be needed to determine the safety of chronic administration of NMP in TDDS.
Acknowledgments
This paper was extracted from Pharm.D. thesis (No. 3652) that was submitted to the Faculty of Pharmacy of Tabriz University of Medical Sciences and financially supported by grant (No. 91/23) from the Drug Applied Research Center of the same university.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Azagury A, Khoury L, Enden G, Kost J. Ultrasound mediated transdermal drug delivery. Adv Drug Deliv Rev. 2014;72:127–43. doi: 10.1016/j.addr.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Badran MM, Taha EI, Tayel MM, Al-Suwayeh SA. Ultra-fine self nanoemulsifying drug delivery system for transdermal delivery of meloxicam: Dependency on the type of surfactants. J Mol Liq. 2014;190:16–22. doi: 10.1016/j.molliq.2013.10.015. [DOI] [Google Scholar]
- 3.Chen Y, Quan P, Liu X, Wang M, Fang L. Novel chemical permeation enhancers for transdermal drug delivery. AJPS. 2014;9(2):51–64. doi: 10.1016/j.ajps.2014.01.001. [DOI] [Google Scholar]
- 4.Delgado-Charro MB, Guy RH. Effective use of transdermal drug delivery in children. Adv Drug Deliv Rev. 2014;73:63–82. doi: 10.1016/j.addr.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 5.El Maghraby GM, Ahmed AA, Osman MA. Penetration enhancers in proniosomes as a new strategy for enhanced transdermal drug delivery. Saudi Pharm J. 2015;23(1):67–74. doi: 10.1016/j.jsps.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giannos SA. Skin microporation: Strategies to enhance and expand transdermal drug delivery. J Drug Deliv Sci Technol. 2014;24(3):293–9. doi: 10.1016/S1773-2247(14)50048-2. [DOI] [Google Scholar]
- 7.Loftsson T, Masson M. Cyclodextrins in topical drug formulations: Theory and practice. Int J Pharm. 2001;225(1-2):15–30. doi: 10.1016/S0378-5173(01)00761-X. [DOI] [PubMed] [Google Scholar]
- 8.Rubio L, Alonso C, Rodríguez G, Cócera M, López-Iglesias C, Coderch L. et al. Bicellar systems as new delivery strategy for topical application of flufenamic acid. Int J Pharm. 2013;444(1-2):60–9. doi: 10.1016/j.ijpharm.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 9.Ghanbarzadeh S, Arami S. Formulation and evaluation of piroxicam transferosomal gel: An approach for penetration enhancement. J Drug Deliv Sci Technol. 2013;23(6):587–90. doi: 10.1016/S1773-2247(13)50089-X. [DOI] [Google Scholar]
- 10.Javadzadeh Y, Hamishehkar H. Enhancing percutaneous delivery of methotrexate using different types of surfactants. Colloids Surf B Biointerfaces. 2011;82(2):422–6. doi: 10.1016/j.colsurfb.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Ghanbarzadeh S, Arami S. Enhanced transdermal delivery of diclofenac sodium via conventional liposomes, ethosomes, and transfersomes. BioMed Res Int. 2013;2013:616810. doi: 10.1155/2013/616810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung E, Kang YP, Yoon IS, Kim JS, Kwon SW, Chung SJ. et al. Effect of permeation enhancers on transdermal delivery of fluoxetine: In vitro and in vivo evaluation. Int J Pharm. 2013;456(2):362–9. doi: 10.1016/j.ijpharm.2013.08.080. [DOI] [PubMed] [Google Scholar]
- 13.Kalhapure RS, Akamanchi KG. Oleodendrimers: A novel class of multicephalous heterolipids as chemical penetration enhancers for transdermal drug delivery. Int J Pharm. 2013;454(1):158–66. doi: 10.1016/j.ijpharm.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 14.Lee PJ, Ahmad N, Langer R, Mitragotri S, Prasad Shastri V. Evaluation of chemical enhancers in the transdermal delivery of lidocaine. Int J Pharm. 2006;308(1-2):33–9. doi: 10.1016/j.ijpharm.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Taghizadeh SM, Moghimi-Ardakani A, Mohamadnia F. A statistical experimental design approach to evaluate the influence of various penetration enhancers on transdermal drug delivery of buprenorphine. J Adv Res. 2015;6(2):155–62. doi: 10.1016/j.jare.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tank CJ, Kapse GK, Sarvaiya JI. Transdermal drug delivery of fluvastatin sodium: Effect of permeation enhancers and pressure sensitive adhesive. J Pharm Res. 2013;6(5):573–8. doi: 10.1016/j.jopr.2013.03.029. [DOI] [Google Scholar]
- 17.Karande P, Jain A, Mitragotri S. Insights into synergistic interactions in binary mixtures of chemical permeation enhancers for transdermal drug delivery. J Control Release. 2006;115(1):85–93. doi: 10.1016/j.jconrel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Megrab NA, Williams AC, Barry BW. Oestradiol permeation through human skin and silastic membrane: Effects of propylene glycol and supersaturation. J Control Release. 1995;36(3):277–94. doi: 10.1016/0168-3659(95)00062-D. [DOI] [Google Scholar]
- 19.Moser K, Kriwet K, Kalia YN, Guy RH. Enhanced skin permeation of a lipophilic drug using supersaturated formulations. J Control Release. 2001;73(2-3):245–53. doi: 10.1016/S0168-3659(01)00290-5. [DOI] [PubMed] [Google Scholar]
- 20.Verma DD, Fahr A. Synergistic penetration enhancement effect of ethanol and phospholipids on the topical delivery of cyclosporin a. J Control Release. 2004;97(1):55–66. doi: 10.1016/j.jconrel.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 21.Kim CK, Hong MS, Kim YB, Han SK. Effect of penetration enhancers (pyrrolidone derivatives) on multilamellar liposomes of stratum corneum lipid: A study by uv spectroscopy and differential scanning calorimetry. Int J Pharm. 1993;95(1-3):43–50. doi: 10.1016/0378-5173(93)90388-V. [DOI] [Google Scholar]
- 22.Sasaki H, Kojima M, Mori Y, Nakamura J, Shibasaki J. Enhancing effect of pyrrolidone derivatives on transdermal drug delivery. I. Int J Pharm. 1988;44(1-3):15–24. doi: 10.1016/0378-5173(88)90095-6. [DOI] [Google Scholar]
- 23.Sasaki H, Kojima M, Mori Y, Nakamura J, Shibasaki J. Enhancing effect of pyrrolidone derivatives on transdermal drug delivery ii. Effect of application concentration and pre-treatment of enhancer. Int J Pharm. 1990;60(3):177–83. doi: 10.1016/0378-5173(90)90070-K. [DOI] [Google Scholar]
- 24.Saw CL, Heng PW, Chin WW, Soo KC, Olivo M. Enhanced photodynamic activity of hypericin by penetration enhancer n-methyl pyrrolidone formulations in the chick chorioallantoic membrane model. Cancer Lett. 2006;238(1):104–10. doi: 10.1016/j.canlet.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 25.Lambert WJ, Kudla RJ, Holland JM, Curry JT. A biodegradable transdermal penetration enhancer based on n-(2-hydroxyethyl)-2-pyrrolidone i. Synthesis and characterization. Int J Pharm. 1993;95(1-3):181–92. doi: 10.1016/0378-5173(93)90405-5. [DOI] [Google Scholar]
- 26.Ko IG, Shin MS, Kim BK, Kim SE, Sung YH, Kim TS. et al. Tadalafil improves short-term memory by suppressing ischemia-induced apoptosis of hippocampal neuronal cells in gerbils. Pharmacol Biochem Behav. 2009;91(4):629–35. doi: 10.1016/j.pbb.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Kruuse C, Gupta S, Nilsson E, Kruse L, Edvinsson L. Differential vasoactive effects of sildenafil and tadalafil on cerebral arteries. Eur J Pharmacol. 2012;674(2-3):345–51. doi: 10.1016/j.ejphar.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 28.Lau LC, Adaikan PG. Mechanisms of direct relaxant effect of sildenafil, tadalafil and vardenafil on corpus cavernosum. Eur J Pharmacol. 2006;541(3):184–90. doi: 10.1016/j.ejphar.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Cavallini G, Biagiotti G. The role of tadalafil in treatment of infertility. AJPS. 2012;1(4):258–61. doi: 10.1016/S2305-0500(13)60088-3. [DOI] [Google Scholar]
- 30.Vilela VR, de Oliveira AL, Comar JF, Peralta RM, Bracht A. Tadalafil inhibits the camp stimulated glucose output in the rat liver. Chem Biol Interact. 2014;220:1–11. doi: 10.1016/j.cbi.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 31.Montorsi F, Verheyden B, Jünermann K, Moncada I, Valiquette L, Denne J. et al. Long-term safety experience with tadalafil. Eur Urol Suppl. 2003;2(1):96. doi: 10.1016/S1569-9056(03)80379-2. [DOI] [PubMed] [Google Scholar]
- 32.Batheja P, Sheihet L, Kohn J, Singer AJ, Michniak-Kohn B. Topical drug delivery by a polymeric nanosphere gel: Formulation optimization and in vitro and in vivo skin distribution studies. J Control Release. 2011;149(2):159–67. doi: 10.1016/j.jconrel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Ashara KC, Paun JS, Soniwala MM, Chavada JR, Mori NM. Micro-emulsion based emulgel: A novel topical drug delivery system. Asian Pac J Trop Dis. 2014;4(Supplement 1):S27–32. doi: 10.1016/S2222-1808(14)60411-4. [DOI] [Google Scholar]
- 34.Frederiksen K, Guy RH, Petersson K. Formulation considerations in the design of topical, polymeric film-forming systems for sustained drug delivery to the skin. Eur J Pharm Biopharm. 2015;91:9–15. doi: 10.1016/j.ejpb.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed-Lecheheb D, Cunat L, Hartemann P, Hautemanière A. Prospective observational study to assess hand skin condition after application of alcohol-based hand rub solutions. Am J Infect Control. 2012;40(2):160–4. doi: 10.1016/j.ajic.2011.04.323. [DOI] [PubMed] [Google Scholar]
- 36.Lodén M. The skin barrier and use of moisturizers in atopic dermatitis. Clin Dermatol. 2003;21(2):145–57. doi: 10.1016/S0738-081X(02)00373-5. [DOI] [PubMed] [Google Scholar]
- 37.Alexander A, Dwivedi S, Ajazuddin, Giri TK, Saraf S, Saraf S. et al. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J Control Release. 2012;164(1):26–40. doi: 10.1016/j.jconrel.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 38.El Maghraby GM, Alanazi FK, Alsarra IA. Transdermal delivery of tadalafil. I. Effect of vehicles on skin permeation. Drug Dev Ind Pharm. 2009;35(3):329–36. doi: 10.1080/03639040802360494. [DOI] [PubMed] [Google Scholar]
- 39.Fujii M, Koizumi A, Kinoshita Y, Suzuki R, Nio J, Kondoh M. et al. Effect of n-methyl-2-pyrrolidone on the skin permeation of estradiol and levonorgestrel from adhesive strips prepared using eudragit EPO. J Drug Deliv Sci Technol. 2006;16(2):121–5. doi: 10.1016/S1773-2247(06)50018-8. [DOI] [Google Scholar]
- 40.Koizumi A, Fujii M, Kondoh M, Watanabe Y. Effect of n-methyl-2-pyrrolidone on skin permeation of estradiol. Eur J Pharm Biopharm. 2004;57(3):473–8. doi: 10.1016/j.ejpb.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Jouyban A, Fakhree MA, Shayanfar A. Review of pharmaceutical applications of n-methyl-2-pyrrolidone. J Pharm Pharm Sci. 2010;13(4):524–35. doi: 10.18433/j3p306. [DOI] [PubMed] [Google Scholar]
- 42.Wu YH, Freeman BD. Structure, water sorption, and transport properties of crosslinked n-vinyl-2-pyrrolidone/n,n′-methylenebisacrylamide films. J Membr Sci. 2009;344(1-2):182–9. doi: 10.1016/j.memsci.2009.07.050. [DOI] [Google Scholar]
- 43.Shun-Ichi N, Shogo N, Mikio A, Takehiro N, Hideharu T. Observations on and pharmacokinetic discussion of percutaneous absorption of mefenamic acid. Int J Pharm. 1985;24(2-3):127–47. doi: 10.1016/0378-5173(85)90015-8. [DOI] [Google Scholar]
- 44.Aungst BJ, Rogers NJ, Shefter E. Enhancement of naloxone penetration through human skin in vitro using fatty acids, fatty alcohols, surfactants, sulfoxides and amides. Int J Pharm. 1986;33(1-3):225–34. doi: 10.1016/0378-5173(86)90057-8. [DOI] [Google Scholar]