Abstract
Purpose: Doxorubicin is administrated as a single agent in first-line therapy of breast cancer to induce apoptosis in tumor cells. Bax, Bcl-xL, Caspase-8 and 9 proteins are involved in induction of apoptosis. The present study describes Bax, Bcl-xL gene expression and Caspase-8 and 9 protein levels in MCF-7 cells incubated with doxorubicin at different doses an incubation times.
Methods: The cytotoxic effects of doxorubicin were studied using MTT assay. MCF-7 cells were treated with three concentrations of doxorubicin (0.1, 0.5, 1 μM) and incubated for 24, 48 and 72 hours then expression levels of Bax and Bcl-xL genes were elucidated by Real-time RT-PCR technique and protein levels of caspase-8 and caspase-9 proteins were measured using ELISA method. Morphological modifications of the cells were also monitored via light microscopic images.
Results: Doxorubicin decreased the anti-apoptotic Bcl-xL and increased pro-apoptotic Bax mRNA levels. Doxorubicin induced a significant increase in Bax /Bcl-xL ratio in all doses and incubation times (p<0.05). Highest (more than 10 fold) increase in Bax /Bcl-xL ratio was revealed after 48 h incubation of the cells with in all doses of doxorubicin. Doxorubicin also increased caspase-9 level in a time and dose-dependent manner, while caspase-8 level didn't follow time and dose dependency pattern.
Conclusion: Our results confirm that doxorubicin induces mitochondrial-dependent apoptosis by down-regulation of Bcl-xL and up- regulation of Bax and caspase-9 expressions.
Keywords: MCF-7, Apoptosis, Doxorubicin, Breast cancer, Bax /Bcl-xL, Caspase-9
Introduction
Apoptosis is the process of programmed cell death that occurs in response to environmental stimuli. Regulation of apoptosis is important for normal growth and homeostasis, during development, embryogenesis, and modification of normal tissues, also cancer treatment. Disorders of abnormal induction of apoptosis can cause severe outcomes.1 Impaired regulation of apoptotic mechanisms provides an opportunity to growth cancerous cells and chemo-resistance. Apoptosis plays an important role in the treatment of cancer as it is the general goal of many treatment strategies.2,3
In mammalian cells, there are at least two main pathways that lead to apoptosis including extrinsic death-receptor dependent apoptosis and intrinsic mitochondrial dependent apoptosis.4,5 In both extrinsic and intrinsic apoptotic pathways induction of cell death is associated with the activation of caspases.6,7 Caspases are two types, initiator caspases (e.g. caspase-8 and -9) and effector caspases (e.g. caspase-3, -6, and -7). Caspase-8 is mainly activated in extrinsic apoptotic pathway, but caspase-9 activation is related to the mitochondrial or intrinsic pathway.8 In anticancer chemotherapy, activation of caspases can be started through stimulating of the extrinsic pathway or by activation of intrinsic pathway at the mitochondria.9,10
One of the key regulators in the molecular mechanisms of apoptosis is the family of Bcl-2.11 These proteins are attached to cell membrane or free in cytosol. They are operating in intracellular membranes of mitochondria that organize intrinsic apoptosis.12,13 This family includes proapoptotic members including Bax, Bak and antiapoptotic members including Bcl-2, Bcl-xL.11,14-16 The localization of certain Bcl-2 family proteins changes when stimulation of apoptosis occurs. For example, Bax moves from cytosol to the membrane of mitochondria followed induction of apoptotic signals by an apoptotic stimulant.17 The Bcl-2 gene is important in regulation of apoptosis that encodes variety of proteins that play key roles in regulation of cell apoptosis. Bax is another pro-apoptotic gene that meaningfully is homologous with Bcl-2.14 However, Bax operates as an enhancer of apoptosis in contrast to Bcl-2 with antiapoptotic properties. Complex network of interactions between Bcl-2 family members both in the cytosol and on mitochondria determines the fate of the cell for death or survive.13,18 Enhanced expression of Bax in breast cancer cells increases sensitivity to apoptotic stimuli and decreases tumour enlargement.19 Bcl-xL is another anti-apoptotic protein that inhibits apoptosis.20 Progress of breast cancer has been associated with of Bcl-xL expression.21,22 Furthermore, it has been associated in advanced grade and development of metastatic cancer.22 Since Bcl-xL prevents apoptosis, this protein is considered as a key molecule to induce chemoresistance.23
A variety of stimuli and the molecular mechanisms of apoptosis are explained intensively.24 Several chemotrapoetic agents have been identified to persuade apoptosis in cancer cells.25 Doxorubicin is one of anthracycline drugs that used for treatment of variety of tumors especially solid tumors.26 The mechanism for cytotoxic effects of doxorubicin is free radical production, DNA intercalating and blocking of topoisomerase II followed by preventing DNA replication and eventually DNA breakage.27 Since doxorubicin is extensively utilized as a first line treatment of variety of different cancers including breast cancer, investigating the mechanisms of apoptosis induced by this chemotherapeutic agent is important.27
Breast cancer is second prevalent and deadly cancer in women worldwide.28 Breast cancer treatment consists of radiotherapy, hormonal therapy and chemotherapy that stimulate apoptotic pathways followed by cancer cell death.29 Different strategies have been developed for analysis and study of this cancer.30-33 MCF-7 cell line is an excellent in vitro model for studying the mechanisms of chemoresistance as it is susceptible to apoptosis and applied for various investigations on apoptosis or survival of cancer cells.34-36 Although the extensive clinical application of doxorubicin for cancer patients has been studied, its anti-proliferative and death-inducing signalling cascades are yet unclear. In this study, we investigated the molecular basis of induction of apoptosis by doxorubicin on MCF-7 cells. Cytotoxic effects of doxorubicin have been evaluated with MTT assay. Alteration in the expression of pro-apoptotic Bax and anti-apoptotic Bcl-xL genes and Bax/Bcl-xL ratio were investigated via Real-time RT-PCR method. Furthermore, the levels of caspase-8 and caspase-9 proteins have been evaluated with ELISA method.
Material and Methods
Cell culture
The human MCF-7 breast cancer cells were obtained from National Cell Bank of Iran (Pasteur Institute, Iran). The MCF-7 cells were cultivated in RPMI 1640 medium (Sigma, St Louis, MO, USA) enriched with 10% fetal bovine serum (FBS; Invitrogen), 100 mg/ml streptomycin and 100 units/ml penicillin G (Sigma, St Louis, MO, USA). Cells were incubated in a humidified atmosphere with 5% CO2 at 37°C.
Cell viability assessment using MTT assay
Cell proliferation and viability of doxorubicin treated MCF-7 cells determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl- tetrazolium bromide (MTT) which evaluates the percentage of viable cells. The MCF-7 cells with 70% confluency were detached from the dish with 0.05% trypsin/EDTA solution. Cells were seeded into 96- well plate with concentration of 4×104 cells/cm2.
Cells with about 50% confluency were exposed with increasing concentrations of the doxorubicin (Ebewe, Unterach, Austria) 0.1-10 μM to induce cytotoxicity. Four wells were remained untreated as control. MTT assay was carried out 24, 48 and 72 hours after treatments. To prepare MTT (Sigma-Aldrich, St. Louis, MO) reagent, 2mg of MTT powder was dissolved in 1 ml PBS. The culture medium was exchanged with 150 μl fresh media plus 50 μl MTT reagent (2mg/ml in PBS); the cell-free wells were considered as blank controls. Cells were incubated in 37°C with 5% CO2 and humidified atmosphere for 4 hours. Then the MTT solution was removed and 200μl of DMSO were added to each well. The plate was maintained for 15 min at37 °C and then the optical density (OD) of the wells was determined at 570 nm through a spectrophotometric microplate reader (Biotek, EL x 800. USA).
RNA isolation and Assessment of mRNA expression via Real-time RT-PCR
Cultivated MCF-7 cells were treated with three concentrations of doxorubicin (0.1, 0.5, 1 μM) and incubated for 24, 48 and 72 hours. RNA extraction of treated cells was performed by RNX™-Plus Kit (CinnaGen, Iran) according to the protocol.
Quality of RNA was evaluated by agarose gel electrophoresis and concentration of extracted RNA was estimated by optical density measurement (A260/A280 ratio) with NanoDrop 1000 Spectrophotometer (Wilmington, DE, USA).
For synthesis of cDNA, reaction mixture was composed to 5μg extracted RNA, 1μg random hexonucleotides primersand 1μM deoxyribonucleotides (dNTP), 10 units RNase inhibitor and Moloney Murine Leukemia Virus SuperScript II® Reverse Transcriptase (M-MLV RT, Invitrogen). Then, the reaction tubes were maintained at 42°C for 60 min.
Real-time PCR technique was carried out using the SYBR Green-based PCR Master Mix. The iQ5 Optical System (Bio-Rad Laboratories, Inc., CA-USA) was used for performing all amplification reactions in a total volume of 25 µL. Each well in this experiment contained 1 µl of cDNA, 70-100 nM of each Primer (MWG Biotech, Ebersberg, Germany) and 12.5 µl of 2X Power SYBR green PCR Master Mix (Applied Biosystems, Warrington, UK). Applied Primer sequences and annealing temperature of them for Quantitative PCR showed in Table 1.
Table 1. Applied Primer sequences for Quantitative PCR of Bax and Bcl-xL genes.
| Gene name | Primer sequence | Tm | Amplicon length (bp) |
| 18S rRNA | F: 5’-CGATGCGGCGGCGTTATTC-3’ R:5’-TCTGTCAATCCTGTCCGTGTCC-3’ |
59.4 | 198 |
| Bcl-x L | F: 5’- GTTCCCTTTCCTTCCATCC -3’(19) R: 5’- TAGCCAGTCCAGAGGTGAG -3’(19) |
58 | 123 |
|
Bax
|
F: 5’- GATGCGTCCACCAAGAAG -3’(18) R: 5’- AGTTGAAGTTGCCGTCAG-3’(18) |
56 | 163 |
For PCR thermal cycling program, the samples were included 10 min at 95 °C, 35 cycles of 30 s at 95 °C for denaturation step, 30 s at annealing temperature, and 30 s at 72°C for the extension respectively. Final 10 min incubation at 72°C was carried out to completion of amplicons. mRNA expressions were calculated in compare with 18S rRNA (housekeeping gene) expression. Melting-curve analysis was carried out after amplification to verification the validity of the amplicons. For reporting of gene expression level Pfaffl method was applied.
Analysis of Caspase-8 and Caspase-9 production
To measure Caspase-8 and Caspase-9 protein levels, MCF-7 cells were treated with various concentrations of doxorubicin (0.1, 0.5, 1 μM) and incubated for 24, 48 and 72 hours. Then Caspase-8 and Caspase-9 levels were measured using enzyme-linked immunosorbent assay (Platinum ELISA; eBioscience©) according to the instructions of the manufacturer.
Morphology of doxorubicin treated cells
To study the alteration in morphology of the cells, doxorubicin were applied with concentrations of 0.1, 0.5, 1 μM and the cells were incubated for 24, 48 and 72 h. Light Microscopy was applied to study the morphology of alive and dead cells.
Statistical analysis
All statistical analyses were performed using SPSS 16.0 software. Data obtained from three or more individual experiments were expressed as mean ± SD. Data were analyzed by One-Way ANOVA and Tukey post Hoc tests. p-values less than 0.05 were considered as statistically significant.
Results
Cell viability and IC50 values in doxorubicin treated MCF-7 cells
MCF-7 cells were treated with various concentrations of the doxorubicin ranging from 0.1-10 μM for 24, 48 and 72 hours, and cell viability was measured by MTT assay. MTT assay showed that increased concentrations of the doxorubicin decreased the viability of cells in a time and concentration dependent manner. IC50 values were 0.75 μM for the cells incubated for 24 h and 0.25 μM for the cells incubated for both 48 and 72 h (Figure 1).
Figure 1.
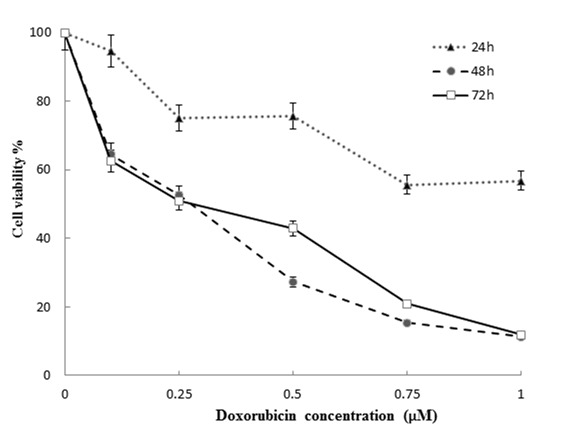
Column charts of MTT assay results of MCF-7 cell treated with different concentrations of doxorubicin.
Expression of Bax, Bcl-xL genes and Bax /Bcl-xLratio in doxorubicin treated MCF-7 cells
Expression of Bax Gene significantly increased by increasing concentrations of doxorubicin (0.1, 0.5, 1 μM) after 24, 48 and 72 hours incubations (p<0.05) (Figur 2). Highest increase (4.5 fold) in expression of Bax revealed with 1μM doxorubicin after 72 h incubation. Bcl-xL gene expression was decreased in 48 and 72 h (Figur 2. b). Highest decrease in Bcl-xL mRNA level was shown after 48 h incubation. Change in Bcl-xL gene expression was not dose dependent. Our results also revealed a significant increase in Bax /Bcl-xL ratio in all doxorubicin concentrations (p<0.05). Highest increase in Bax/Bcl-xL ratio (more than 10 fold) was revealed after 48 h incubation (Table 2).
Table 2. Bax/Bcl-xL ratio was calculated and show that it was significantly increased in all of doxorubicin treated MCF-7 cells (p<0.05).
| doxorubicin concentration | Bax/Bcl-xLratio | |
| 24 h | 0.1 μM | 1.774 |
| 0.5 μM | 1.354 | |
| 1 μM | 1.125 | |
| 48 h | 0.1 μM | 13.445 |
| 0.5 μM | 14.976 | |
| 1 μM | 11.950 | |
| 72 h | 0.1 μM | 4.702 |
| 0.5 μM | 4.884 | |
| 1 μM | 5.484 |
Figure 2.
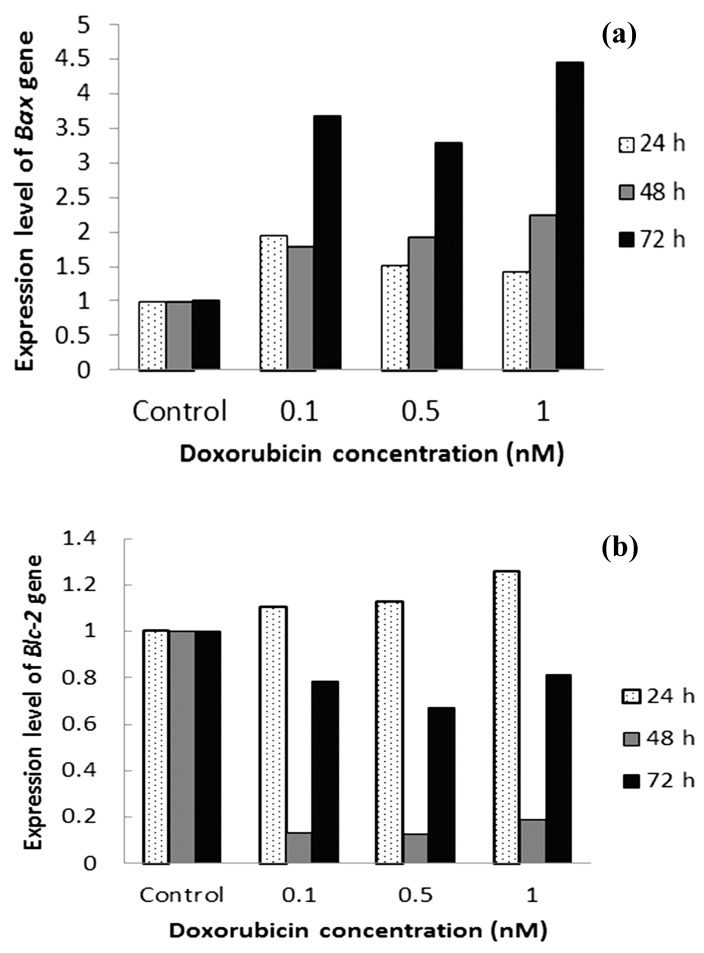
Expression of Bcl-xL and Bax genes in apoptosis pathway were investigated to demonstrate the induction of apoptosis by doxorubicin. Cultivated MCF-7 cells were treated with three concentrations of doxorubicin (0.1, 0.5, 1 μM) and incubated for 24, 48 and 72 hours.
Caspase-8 and 9 proteins level in doxorubicin treated MCF-7 cells
Caspase-8 levels in the cells exposed to doxorubicin (0.1, 0.5 and 1 μM) didn't show any significant increase after 24 h incubation (p>0.05). Caspase-8 levels increased with 0.1 and 1 μM concentrations of doxorubicin after 48 and 72 h incubation (p<0.05) (Figure. 3a).
Caspase-9 level showed no significant increase when we incubated the cells with different concentrations of doxorubicin after 24 h incubation (p>0.05). Doxorubicin (0.1 μM, 0.5 μM) also caused a significant increase in caspase-9 after 48 h and 72 h incubations (p<0.05) (Figure. 3b).
Figure 3.
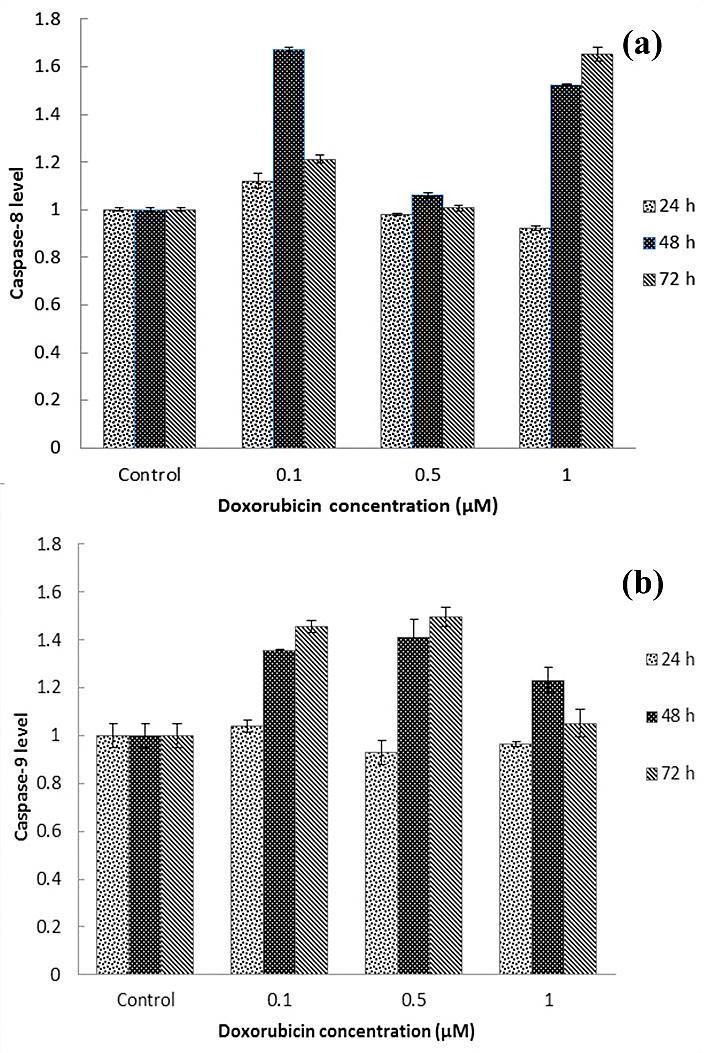
For evaluation of Caspase-8 and 9 was used from ELISA method. MCF-7 cell was treated with various concentrations of doxorubicin (0.1, 0.5, 1 μM) for 24, 48 and 72 hours. a: level of Caspase-8 and b: level of Caspase-9 in MCF-7 cell was treated with various concentrations of doxorubicin (0.1, 0.5, 1 μM) for 24, 48 72 hours compare with untreated MCF-7 cell.
Alteration in morphology of MCF-7 cells by doxorubicin
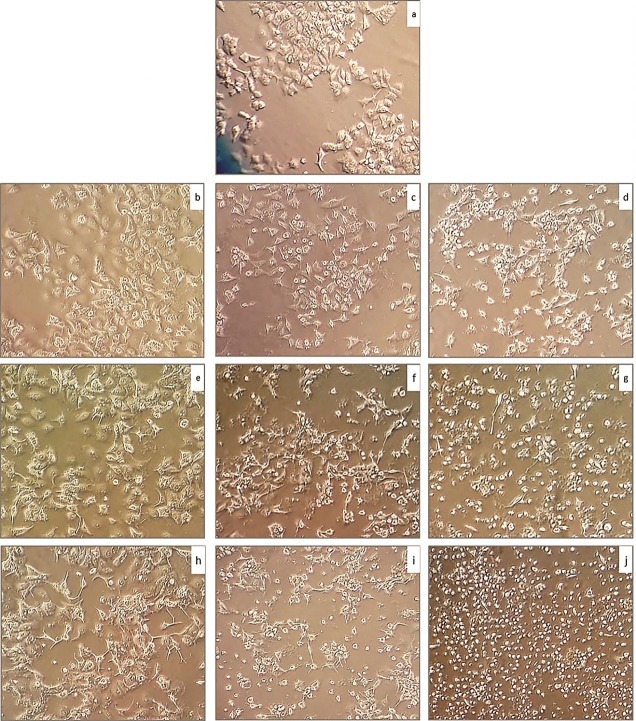
Doxorubicin displayed morphological alterations in MCF-7 cells. Adherent cells after treated with doxorubicin became rounded, condensed and detached from the dish. Number of detached and rounded cells was increased by increase in the dose of doxorubicin and incubation time. Doxorubicin (0.1 μM) after 24 h incubation showed least and 1 μM doxorubicin with 72 h incubation showed the most rounded and detached cells in comparison with other doxorubicin concentrations and incubation times (Figure. 4 a-j).
Figure 4.
Morphology and cell death of doxorubicin treated MCF-7 cells was investigated via light Microscopic image. a: untreated MCF-7 cell line, b: MCF-7 cells treated with 0.1 μM for 24 h, c: cells treated with 0.5 μM for 24 h, d: cells treated with 1 μM for 24 h, e: treated with 0.1 μM for 48 h, f: cells treated with 0.5 μM for 48 h, g: cells treated with 1 μM for 48 h, h:cells treated with 0.1 μM for 72 h, i: cells treated with 0.5 μM for 72 h, j: cells treated with 1 μM for 72 h.
Discussion
Cytotoxic agents induce apoptosis by initiating death signalling pathways in susceptible target cells.37 Activating of apoptosis by chemotherapeutic agents is involved in simultaneous or consequent activation of death receptor systems, disturbance in mitochondrial function and proteolytic processing of caspases.38 Thus, the cell death pathway may occur in several locations; however the accurate molecular mechanisms for each particular drug and special target cell have not been determined completely. In this study, the alteration in Bcl-xL(anti-apoptotic), Bax (pro-apoptotic) genes' expression, Bax /Bcl-xL ratio and caspase 8 and 9 proteins' level following the stimulation of apoptosis by doxorubicin in MCF-7 cells were reported.
For evaluation of cytotoxicity induced by doxorubicin MTT assay was applied. The results demonstrated that the viability of MCF-7 cells was clearly decreased in a time and dose-dependent manner. IC50 values were 0.75, 0.25 and 0.25 μM for 24, 48 h and 72 h incubations respectively. Based on Lukyanova study, the IC50 of doxorubicin on MCF-7 was about 0.5 μM which is consistent with our findings.39 Moreover, the reported IC50 of doxorubicin on MCF-7 were approximately 0.1 μM and 1.19 μM using MTT assay based on Fornari et al. and Taherian et al. studies, respectively. The mentioned differences can be explained regarding different brands of doxorubicin or technical strategies.40,41
Members of the Bcl-2 family are main regulators of cell death or cell survival. Chemotherapeutic drugs apply their effects in part by controlling the expression of numerous members of the Bcl-2 family in MCF-7 cells. The Bcl-2 family proteins play a significant role in apoptosis, either as apoptotic activators (Bax) or as apoptotic inhibitors (Bcl-xL).42,43
Bax and Bak are the effectors of the Bcl-2 family as upon activation, they change conformation, insert into the outer mitochondrial membrane, oligomerize, and induce mitochondrial outer membrane permeabilization (MOMP).11,18 On the other hand, Bcl-xL is a strong inhibitor of apoptosis that heterodimerize with Bax and neutralizes the effects of the latter. When Bcl-xL is present in excess, cells are protected against apoptosis. In contrast, when Bax is in excess and the homodimers of Bax dominate, cells are susceptible to programmed death.
High expression of Bax gene in the cells incubated with doxorubicin demonstrated that doxorubicin has Bax enhancer effects followed by induction in intrinsic apoptosis pathway on MCF-7 breast cancer cells. April measured Bax in rats and declared that Bax declined markedly in the cells after treatment with doxorubicin.44
Our data showed that doxorubicin was reduced expression of Bcl-xL protein, suggesting the importance of Bcl-2 family proteins for breast cancer cell survival. Clinical studies have showed that increased level of Bcl-xL is related to a poor response to chemotherapy in breast cancer.22,45
In this study, expression of Bcl-xL gene in doxorubicin treated MCF-7 cells was not time and dose dependent. The most decrease in expression of this gene was in the treated cells with 0.1, 0.5, 1 μM doxorubicin for 48 h which showed more than 5 fold decrease compared with non-treated MCF-7 cells (p<0.05).
Several apoptosis regulators have been associated with various human malignancies. For example, studies on human tumors have demonstrated an overall positive correlation between increased expression of Bcl-xL, decreased expression of Bax and uncontrolled tumour cell growth (due to suppressed apoptosis). A wide range of experimental and clinical reports have demonstrated biological effects for doxorubicin. Similar results were shown with doxorubicin, which causes a decrease in Bcl-2 expression and increase in Bax expression.46 Other chemotherapeutic agents such as paclitaxel and thiotepa up-regulate several proapoptotic Bcl-2 proteins and down-regulate antiapoptotic Bcl-2 proteins.47 Additionally, the present study evaluated the effects of doxorubicin on Bcl-xL and Bax expression ratio in induction of apoptosis in vitro. The Bax/Bcl-xL ratios determine the fate of a cell rather than the absolute concentration of either.48 The treatment of MCF-7 cells with doxorubicin originate a significant percentage in the Bax/Bcl-xL ratios (p<0.05). Thus, the imbalance between Bcl-xL and Bax expressions during doxorubicin treatment is believed to play important role in doxorubicin-induced apoptosis as observed in this study and in other investigation.49
Mariana and Gonzalez suggest that Bax/Bcl-xL expression ratio may be a sensitive monitor of cancer progression and an early predictor for cancer patients.50 In this study, we investigated at least three distinct parameters: absolute expression levels of Bax and Bcl-xL genes and the ratio of these pro and antiapoptotic proteins. Our results support the existence of a specific apoptotic mechanism in MCF-7 cells that increasing in Bax/Bcl-xL ratio causes the loss of cell viability and enhanced apoptosis.
Doxorubicin-induced apoptosis is regulated by the Bcl-2 family of proteins upstream of caspase activation. The Bax gene expression is known to cause the activation of caspases followed by apoptosis.51-53 Caspases are members of a family of cysteine proteases that are divided into initiator caspases, such as caspase-8 and -9 and executioner caspases such as caspase-3 or -7.54 Initiator caspase-8 is known to be activated through extrinsic pathway, whereas caspase-9 is activated in the event of mitochondrial cytochrome c leakage.54,55
In our study, treatment of the cells with doxorubicin caused a time dependent increase in caspase-9 levels, while caspase-8 level didn't follow time and dose dependency pattern. Liang et al.56 showed that MCF-7 cells can undergo apoptosis by the sequential activation of caspases-9, -7, and -6. An increased level of caspase-9 is similar to Bax gene expression, therefore, both of these proteins increased with increase in doxorubicin dose and incubation time. Up regulation of caspase-9 takes place in doxorubicin treated MCF-7 cells. Bcl-xL interacts with Apaf1 to prevent apoptosis by inhibiting Apaf1 dependent activation of caspase-9.57 These results suggest that doxorubicin induced apoptosis via mitochondrial dependent intrinsic pathway.
In this study, decreased level of Bcl-xL caused increase in caspase-9 levels consequently in apoptotic cells. These results are compatible with our other findings for the Bax /Bcl-xLratio, since BCL-2 family controls the intrinsic apoptotic pathway in doxorubicin treated MCF-7 breast cancer cells.
MCF7 cells are deficient in capase-3 expression because of a deletion mutation in their caspase-3 gene, suggesting the existence of caspase-3-independent apoptotic pathways.58,59 Participation of activated caspase-3 is crucial for activation of caspase-8 which explains our results on caspase 8 protein level in the cells with lack of caspase-3 protein level.60 Therefore, increased level of caspase-8 couldn't trigger extrinsic apoptosis pathway in MCF-7 cells. This is compatible with other studies on a disabled Fas pathway in this cell line.61
The presence of caspase-3 can increase cell sensitivity to apoptosis-inducing agents. MCF-7 cells, which are relatively insensitive to many chemotherapeutic agents, acquire greater sensitivity to doxorubicin and etoposide-induced apoptosis when caspase-3 is reconstituted.62 Future research may show whether maximizing cross-talk between the intrinsic and extrinsic pathways of apoptosis and up-regulation of caspase-3 are potential targets for breast cancer therapy.
In conclusion, our data suggest that increase in the Bax/Bcl-xL ratio and caspase-9 level can play a key role in doxorubicin-induced apoptosis in MCF-7 cells, however further studies are warranted to validate these findings.
Acknowledgments
Authors would like to thank Research Center for Pharmaceutical Nanotechnology (RCPN) and Faculty of Advanced Biomedical Sciences for supporting this project (which was a part of PhD thesis No: 76).
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88(3):347–54. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 2.Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30(1):87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108(2):153–64. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281(5381):1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 5.Harris MH, Thompson CB. The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ. 2000;7(12):1182–91. doi: 10.1038/sj.cdd.4400781. [DOI] [PubMed] [Google Scholar]
- 6.Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25(34):4798–811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 7.Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22(53):8543–67. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- 8.Cryns V, Yuan J. Proteases to die for. Genes Dev. 1998;12(11):1551–70. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- 9.Olsson M, Zhivotovsky B. Caspases and cancer. Cell Death Differ. 2011;18(9):1441–9. doi: 10.1038/cdd.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fulda S, Debatin KM. Targeting apoptosis pathways in cancer therapy. Curr Cancer Drug Targets. 2004;4(7):569–76. doi: 10.2174/1568009043332763. [DOI] [PubMed] [Google Scholar]
- 11.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37(3):299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krajewski S, Tanaka S, Takayama S, Schibler MJ, Fenton W, Reed JC. Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993;53(19):4701–14. [PubMed] [Google Scholar]
- 13.Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351(1-2):41–58. doi: 10.1007/s11010-010-0709-x. [DOI] [PubMed] [Google Scholar]
- 14.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74(4):609–19. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 15.Kelly PN, Strasser A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 2011;18(9):1414–24. doi: 10.1038/cdd.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed JC. Bcl-2 family proteins. Oncogene. 1998;17(25):3225–36. doi: 10.1038/sj.onc.1202591. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Heim J, Meyhack B. Redistribution of Bax from cytosol to membranes is induced by apoptotic stimuli and is an early step in the apoptotic pathway. Biochem Biophys Res Commun. 1998;251(2):454–9. doi: 10.1006/bbrc.1998.9485. [DOI] [PubMed] [Google Scholar]
- 18.Lindsay J, Esposti MD, Gilmore AP. Bcl-2 proteins and mitochondria--specificity in membrane targeting for death. Biochim Biophys Acta. 2011;1813(4):532–9. doi: 10.1016/j.bbamcr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Bargou RC, Wagener C, Bommert K, Mapara MY, Daniel PT, Arnold W. et al. Overexpression of the death-promoting gene bax-alpha which is downregulated in breast cancer restores sensitivity to different apoptotic stimuli and reduces tumor growth in SCID mice. J Clin Invest. 1996;97(11):2651–9. doi: 10.1172/jci118715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simonian PL, Grillot DaM, Nunez G. Bcl-2 and Bcl-XL can differentially block chemotherapy-induced cell death. Blood. 1997;90(3):1208–16. [PubMed] [Google Scholar]
- 21.Zapata JM, Krajewska M, Krajewski S, Huang RP, Takayama S, Wang HG. et al. Expression of multiple apoptosis-regulatory genes in human breast cancer cell lines and primary tumors. Breast Cancer Res Treat. 1998;47(2):129–40. doi: 10.1023/a:1005940832123. [DOI] [PubMed] [Google Scholar]
- 22.Olopade OI, Adeyanju MO, Safa AR, Hagos F, Mick R, Thompson CB. et al. Overexpression of BCL-x protein in primary breast cancer is associated with high tumor grade and nodal metastases. Cancer J Sci Am. 1997;3(4):230–7. [PubMed] [Google Scholar]
- 23.Kojima H, Endo K, Moriyama H, Tanaka Y, Alnemri ES, Slapak CA. et al. Abrogation of mitochondrial cytochrome c release and caspase-3 activation in acquired multidrug resistance. J Biol Chem. 1998;273(27):16647–50. doi: 10.1074/jbc.273.27.16647. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Nieto S, Zhivotovsky B. Role of alterations in the apoptotic machinery in sensitivity of cancer cells to treatment. Curr Pharm Des. 2006;12(34):4411–25. doi: 10.2174/138161206779010495. [DOI] [PubMed] [Google Scholar]
- 25.Adjei AA, Rowinsky EK. Novel anticancer agents in clinical development. Cancer Biol Ther. 2003;2(4 Suppl 1):S5–15. doi: 10.4161/cbt.218. [DOI] [PubMed] [Google Scholar]
- 26.Hortobagyi GN. Anthracyclines in the treatment of cancer. An overview. Drugs. 1997;54 Suppl 4:1–7. doi: 10.2165/00003495-199700544-00003. [DOI] [PubMed] [Google Scholar]
- 27.Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57(7):727–41. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 28.Moulder S, Hortobagyi GN. Advances in the treatment of breast cancer. Clin Pharmacol Ther. 2008;83(1):26–36. doi: 10.1038/sj.clpt.6100449. [DOI] [PubMed] [Google Scholar]
- 29.Makin G, Dive C. Apoptosis and cancer chemotherapy. Trends Cell Biol. 2001;11(11):S22–6. doi: 10.1016/S0962-8924(01)02124-9. [DOI] [PubMed] [Google Scholar]
- 30.Ghanbari P, Mohseni M, Tabasinezhad M, Yousefi B, Saei AA, Sharifi S. et al. Inhibition of survivin restores the sensitivity of breast cancer cells to docetaxel and vinblastine. Appl Biochem Biotechnol. 2014;174(2):667–81. doi: 10.1007/s12010-014-1125-6. [DOI] [PubMed] [Google Scholar]
- 31.Sabzichi M, Hamishehkar H, Ramezani F, Sharifi S, Tabasinezhad M, Pirouzpanah M. et al. Luteolin-loaded phytosomes sensitize human breast carcinoma MDA-MB 231 cells to doxorubicin by suppressing Nrf2 mediated signalling. Asian Pac J Cancer Prev. 2014;15(13):5311–6. doi: 10.7314/apjcp.2014.15.13.5311. [DOI] [PubMed] [Google Scholar]
- 32.Samadi N, Ghanbari P, Mohseni M, Tabasinezhad M, Sharifi S, Nazemieh H. et al. Combination therapy increases the efficacy of docetaxel, vinblastine and tamoxifen in cancer cells. J Cancer Res Ther. 2014;10(3):715–21. doi: 10.4103/0973-1482.139152. [DOI] [PubMed] [Google Scholar]
- 33.Sharifi S, Barar J, Hejazi MS, Samadi N. Roles of the Bcl-2/Bax ratio, caspase-8 and 9 in resistance of breast cancer cells to paclitaxel. Asian Pac J Cancer Prev. 2014;15(20):8617–22. doi: 10.7314/apjcp.2014.15.20.8617. [DOI] [PubMed] [Google Scholar]
- 34.Levenson AS, Jordan VC. MCF-7: the first hormone-responsive breast cancer cell line. Cancer Res. 1997;57(15):3071–8. [PubMed] [Google Scholar]
- 35.Hamedeyazdan S, Fathiazad F, Sharifi S, Nazemiyeh H. Antiproliferative activity of Marrubium persicum extract in the MCF-7 human breast cancer cell line. Asian Pac J Cancer Prev. 2012;13(11):5843–8. doi: 10.7314/apjcp.2012.13.11.5843. [DOI] [PubMed] [Google Scholar]
- 36.Simstein R, Burow M, Parker A, Weldon C, Beckman B. Apoptosis, chemoresistance, and breast cancer: insights from the MCF-7 cell model system. Exp Biol Med (Maywood) 2003;228(9):995–1003. doi: 10.1177/153537020322800903. [DOI] [PubMed] [Google Scholar]
- 37.Fisher DE. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994;78(4):539–42. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 38.Debatin KM. Cytotoxic drugs, programmed cell death, and the immune system: defining new roles in an old play. J Natl Cancer Inst. 1997;89(11):750–1. doi: 10.1093/jnci/89.11.750. [DOI] [PubMed] [Google Scholar]
- 39.Lukyanova NY, Rusetskya NV, Tregubova NA, Chekhun VF. Molecular profile and cell cycle in MCF-7 cells resistant to cisplatin and doxorubicin. Exp Oncol. 2009;31(2):87–91. [PubMed] [Google Scholar]
- 40.Taherian A, Mazoochi T. Different Expression of Extracellular Signal-Regulated Kinases (ERK) 1/2 and Phospho-Erk Proteins in MBA-MB-231 and MCF-7 Cells after Chemotherapy with Doxorubicin or Docetaxel. Iran J Basic Med Sci. 2012;15(1):669–77. [PMC free article] [PubMed] [Google Scholar]
- 41.Fornari FA, Randolph JK, Yalowich JC, Ritke MK, Gewirtz DA. Interference by doxorubicin with DNA unwinding in MCF-7 breast tumor cells. Mol Pharmacol. 1994;45(4):649–56. [PubMed] [Google Scholar]
- 42.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281(5381):1309–12. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 43.Rao L, White E. Bcl-2 and the ICE family of apoptotic regulators: making a connection. Curr Opin Genet Dev. 1997;7(1):52–8. doi: 10.1016/s0959-437x(97)80109-8. [DOI] [PubMed] [Google Scholar]
- 44.Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res. 2002;62(16):4592–8. [PubMed] [Google Scholar]
- 45.Campos L, Rouault JP, Sabido O, Oriol P, Roubi N, Vasselon C. et al. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993;81(11):3091–6. [PubMed] [Google Scholar]
- 46.Leung LK, Wang TT. Differential effects of chemotherapeutic agents on the Bcl-2/Bax apoptosis pathway in human breast cancer cell line MCF-7. Breast Cancer Res Treat. 1999;55(1):73–83. doi: 10.1023/a:1006190802590. [DOI] [PubMed] [Google Scholar]
- 47.Tudor G, Aguilera A, Halverson DO, Laing ND, Sausville EA. Susceptibility to drug-induced apoptosis correlates with differential modulation of Bad, Bcl-2 and Bcl-xL protein levels. Cell Death Differ. 2000;7(6):574–86. doi: 10.1038/sj.cdd.4400688. [DOI] [PubMed] [Google Scholar]
- 48.Gross A. BCL-2 proteins: regulators of the mitochondrial apoptotic program. IUBMB life. 2001;52(3-5):231–6. doi: 10.1080/15216540152846046. [DOI] [PubMed] [Google Scholar]
- 49.Huynh H. Induction of apoptosis in rat ventral prostate by finasteride is associated with alteration in MAP kinase pathways and Bcl-2 related family of proteins. Int J Oncol. 2002;20(6):1297–303. doi: 10.3892/ijo.20.6.1297. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez MS, De Brasi CD, Bianchini M, Gargallo P, Moiraghi B, Bengio R. et al. BAX/BCL-XL gene expression ratio inversely correlates with disease progression in chronic myeloid leukemia. Blood Cells Mol Dis. 2010;45(3):192–6. doi: 10.1016/j.bcmd.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Rosse T, Olivier R, Monney L, Rager M, Conus S, Fellay I. et al. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391(6666):496–9. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 52.Jurgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci U S A. 1998;95(9):4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kagawa S, Pearson SA, Ji L, Xu K, Mcdonnell TJ, Swisher SG. et al. A binary adenoviral vector system for expressing high levels of the proapoptotic gene bax. Gene Ther. 2000;7(1):75–9. doi: 10.1038/sj.gt.3301048. [DOI] [PubMed] [Google Scholar]
- 54.Kumar S. Regulation of caspase activation in apoptosis: implications in pathogenesis and treatment of disease. Clin Exp Pharmacol Physiol. 1999;26(4):295–303. doi: 10.1046/j.1440-1681.1999.03031.x. [DOI] [PubMed] [Google Scholar]
- 55.Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B. et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45(6):487–98. doi: 10.1111/j.1365-2184.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang Y, Yan C, Schor NF. Apoptosis in the absence of caspase 3. Oncogene. 2001;20(45):6570–8. doi: 10.1038/sj.onc.1204815. [DOI] [PubMed] [Google Scholar]
- 57.Hu Y, Benedict MA, Wu D, Inohara N, Nunez G. Bcl-XL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activation. Proc Natl Acad Sci U S A. 1998;95(8):4386–91. doi: 10.1073/pnas.95.8.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kurokawa H, Nishio K, Fukumoto H, Tomonari A, Suzuki T, Saijo N. Alteration of caspase-3 (CPP32/Yama/apopain) in wild-type MCF-7, breast cancer cells. Oncol Rep. 1999;6(1):33–7. doi: 10.3892/or.6.1.33. [DOI] [PubMed] [Google Scholar]
- 59.Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273(16):9357–60. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- 60.Ferreira KS, Kreutz C, Macnelly S, Neubert K, Haber A, Bogyo M. et al. Caspase-3 feeds back on caspase-8, Bid and XIAP in type I Fas signaling in primary mouse hepatocytes. Apoptosis. 2012;17(5):503–15. doi: 10.1007/s10495-011-0691-0. [DOI] [PubMed] [Google Scholar]
- 61.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ. et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17(6):1675–87. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang XH, Sladek TL, Liu X, Butler BR, Froelich CJ, Thor AD. Reconstitution of caspase 3 sensitizes MCF-7 breast cancer cells to doxorubicin- and etoposide-induced apoptosis. Cancer Res. 2001;61(1):348–54. [PubMed] [Google Scholar]