Abstract
Through extensive microbial-mammalian co-metabolism, the intestinal microbiota have evolved to exert a marked influence on health and disease via gut-brain-microbiota interactions. In this addendum, we summarize the findings of our recent study on the fecal microbiota and metabolomes of children with pervasive developmental disorder–not otherwise specified (PDD-NOS) or autism (AD) compared with healthy children (HC). Children with PDD-NOS or AD have altered fecal microbiota and metabolomes (including neurotransmitter molecules). We hypothesize that the degree of microbial alteration correlates with the severity of the disease since fecal microbiota and metabolomes alterations were higher in children with PDD-NOS and, especially, AD compared to HC. Our study indicates that the levels of free amino acids (FAA) and volatile organic compounds (VOC) differ in AD subjects compared to children with PDD-NOS, who are more similar to HC. Finally, we propose a new perspective on the implications for the interaction between intestinal microbiota and AD.
Keywords: ASD, dysbiosis, intestinal microbiota, metabolome, perspective
Introduction
The human microbiota plays a key role in health and disease.1,2 Evidence of host-microbe interactions in different clinical settings is rapidly increasing, including interactions of the microbiota with the central nervous system (CNS).3 While the interactions of the microbiota-gut-brain axis are multifactorial and have not yet been completely defined, the enteric nervous system (ENS) acts as a communication conduit between the gastro-intestinal (GI) microbiota and the CNS.4,5 The GI microbiota under extreme conditions (e.g., in a germ-free environment or during antibiotic treatment) affects the levels of various neurotrophins and monoamine neurotransmitters6 responsible for brain development and plasticity.7 Although evidence is accumulating, the role of the GI microbiota in brain disorders is still undefined. An interesting hypothesis concerns the role of the GI microbiota in the pathophysiology of autism spectrum disorders (ASDs).7
ASDs are a group of neurodevelopmental abnormalities that begin in early childhood (although the first diagnosis may sometimes occur later in life) and are characterized by problems in communication and social behavior. According to the 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), which are diagnostic criteria that have recently been released, the major ASD manifestations include impairments in social communication and behavioral problems such as fixated interests and repetitive behaviors.8 We include autism (AD), Asperger's syndrome and pervasive developmental disorder–not otherwise specified (PDD-NOS) under the umbrella of ASDs. In recent decades, the reported prevalence of ASDs has dramatically increased from 4.5 in 10,000 children in 1966 to 1 in 110 in 2006 and to 1 in 68 children in 2010 (http://www.cdc.gov/ncbddd/autism/data.html).
Research on ASDs is primarily focused on genetic associations, but recent evidence has suggested that other environmental factors, including pre- or postnatal exposure to chemicals and drugs, air pollution, stress, maternal infection, the GI microbiota and dietary factors, may play a role in the disease.9
We have recently assessed the gut microbiota and fecal volatile compounds of children who were referred for symptoms related to PDD-NOS or AD and compared them with those of healthy controls (HC). Our study revealed an imbalance in the fecal microbiota, which included the overgrowth of some organisms and the loss of others (dysbiosis) in children with PDD-NOS and, especially, AD compared to HC.10 Recently, other studies have reported similar findings, supporting a role for the GI microbiota in the pathogenesis of ASDs.1,11-14 GI disturbances (abdominal pain, diarrhea and bloating) and metabolic disorders typical of microbial dysbiosis are frequently described in infants with an ASD.11,15
Dysbiosis is often associated with a disruption of the mucosal barrier that is responsible for an alteration in the intestinal permeability leading to a “leaky gut” state. There are several reports showing increased gut permeability in ASD patients,16 although more convincing data are required.
Although the possible mechanisms are unknown, it has been suggested that some intestinal lesions that increase intestinal permeability to exogenous peptides of dietary origin or to neurotoxic peptides of bacterial origin may lead to the disruption of neuroregulatory mechanisms and normal brain development, thereby contributing to autistic symptoms.17,18 Indeed, the gut microbiome plays a crucial role in the bidirectional gut-brain axis; neural, endocrine and metabolic mechanisms are critical mediators of microbiome-CNS signaling and are involved in neuro-psychiatric disorders.4
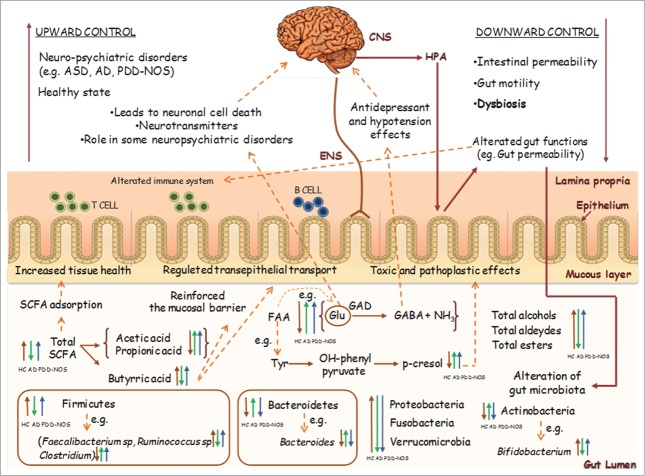
We hypothesize that alterations in the gut microbiota of children with PDD-NOS or AD may lead to an increased level of certain metabolites (e.g., Glu, propionic acid) that are known to play a role in the microbiota-gut-brain axis in children with an ASD. A model of the GI microbiota-gut-brain axis related to ASDs is shown in Figure 1. Dysbiosis, metabolomic profiling and potential new treatments for ASD patients are further discussed below.
Figure 1.
Schematic representation of gastro-intestinal (GI) microbiota-gut-brain axis in relation to autism spectrum disorders (ASD). Abbreviations: CNS, central nervous system; ENS, enteric nervous system; HPA, hypothalamus-pituitary-adrenal axis; SCFA, schort-chain fatty acid; FAA, free amino acids; GAD, glutamic acid decarboxylase; AD, autistic; PDD-NOS, Pervasive developmental disorder not otherwise specified; HC, healthy children.
Dysbiosis in ASDs
Dysbiosis has been demonstrated in ASD patients.1,11-14 This dysbiosis is characterized by alterations in the Firmicutes/Bacteroides ratio and the composition of the primary bacterial phyla (Firmicutes, Bacteroidetes, Fusobacteria and Verrucomicrobia). We recently demonstrated that the Firmicutes level is lowest in the fecal samples of children with AD and no significant differences are present between HC and children with PDD-NOS. Within the Firmicutes phylum, our study revealed that Clostridiaceae species are present at the highest level in children with AD. Among the primary bacterial groups, Clostridiaceae are able to synthesize certain metabolic products that are potentially toxic to humans, such as phenols, p-cresol and certain indole derivatives.19 Our findings support a previous hypothesis that Clostridium species are associated with ASD symptomology19-21 and that the spore-forming property of clostridia is one of the primary concerns related to the reoccurrence of ASD symptoms after oral vancomycin treatments.22 Faecalibacterium, which synthesizes short-chain fatty acids (SCFAs) with anti-inflammatory properties, is decreased in children with AD, relative to both PDD-NOS and HC. Roseburia intestinalis and Roseburia faecis increased in HC compared to PDD-NOS and AD children. On the contrary, other species of Roseburia sp. and Roseburia inulivorans are found at the higher level in AD, compared to PDD-NOS and, especially, in HC children.10 Species belonging to Roseburia are able to degrade starch and ferment other carbohydrates to synthesize SCFAs.23
Within lactic acid bacteria, Enterococcus genus is found at the highest levels in the fecal samples of children with PDD-NOS relative to both PDD-NOS and HC.10 Streptococcus sp and Streptococcus salivarius increased in HC compared to PDD-NOS and AD children. On the contrary, Streptococcus thermophilus is found at the higher level in AD and PDD-NOS compared to HC children.10 Lactobacilli species did not show significant variation among the different groups of children.
The fecal samples of children with AD have the highest levels of certain genera belonging to the phyla Bacteroidetes (Bacteroides, Barnesiella, Odoribacter, Parabacteroides, Prevotella and Alistipes), and Proteobacteria (e.g., Proteus, Shigellaand Parasutterella); by contrast, the Bifidobacterium species, belonging to the phylum Actinobacteria, are decreased.22,24 Finally, a novel mucin-degrading bacterium, Akkermansia muciniphila (phylum Verrucomicrobia), was found at a high level in children with AD by Finegold et al.19
Data regarding the gut microbiota are not always consistent, and some studies have described opposing results.24 Indeed, some authors have found other genera (Sutterella and Desulfovibrio) in association with ASD patients,19,25,26 and others have reported the absence of clinically meaningful differences in the intestinal microbiota composition of autistic patients.27,28
Further support for the microbial hypothesis and for a central role for the gut microbiota in AD derives from studies on autistic children who were treated with antibiotics; the results suggest that the clinical symptoms, regarding both gastrointestinal effects and cognitive skills, and the abnormal urinary secretion of certain chemical compounds may improve after antimicrobial treatment.14,22,29,30
The heterogeneity of the patients enrolled in the different studies in terms of age, symptoms, diet, pharmacological treatments (e.g., vancomycin, probiotics and nystatin), the presence of GI problems and family interactions and the children used as control groups limits the ability to compare and synthesize all the data to reach definitive conclusions.
Fecal metabolomic profiling in ASDs
The current hypothesis on the mechanism underlying the etiology of AD states that the disorder is most likely polygenic with a contribution from environmental factors that interact with the genetic factors to increase the risk of the disease.31 In this view, ASDs result from a combination of genetic, biological and environmental (pre- or postnatal) factors and their interactions, the effects of which might be reflected in the final metabolic pathways of the individual.
Metabolomics, which is among the classical “omics” disciplines, is an evolving research field that addresses the metabolic patterns of living systems. This field is commonly defined as the study of the full set of metabolites, which varies according to the physiological, developmental or pathological state of a cell, tissue, biofluid or organism. Metabolomics expresses a living system's activity at the functional level, downstream from gene expression (genomics) and protein synthesis (proteomics) and considers interactions with the environment. Small perturbations in the proteome can cause significant changes in the concentrations of several metabolites.
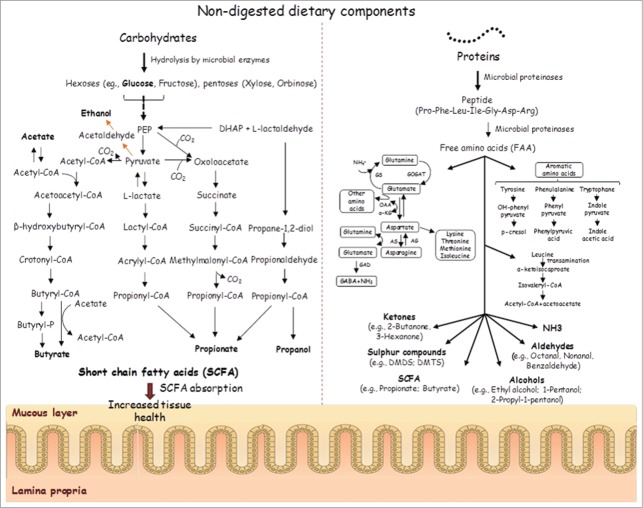
The relationship between ASDs and the metabolic profile has been widely investigated by measuring urinary amino acids and organic acids, the plasma amino acidic profile and the fecal metabolome.11 It has been estimated that 90% of children with an ASD eat only a small variety of foods with strong preferences for starches, snacks and processed foods and reject most fruits and vegetables.32,33 Dietary components are recognized as one of the major external modulators of the human GI microbiota,34,35 and the amount and type of substrate (non-digested dietary components) together with the composition of the gut microbiota affect the total metabolomic profile (Fig. 2). Because of the large metabolic capacity of the GI microbiota and its relatively high plasticity, bacteria play a key role in the modulation of the effects of foods on both health and disease. Based on their different dietetic preferences and microbial dysbiosis, a particular metabolomics profile could be hypothesized for ASD patients. We determined the concentration of total and individual free amino acids (FAA) in the fecal samples of children with PDD-NOS or AD and found the highest levels in children with AD. These metabolites, which are derived from the hydrolysis of proteins and peptides, correlate with the presence of proteolytic bacteria (e.g., Clostridium and Bacteroides) found in large amounts in children with AD along with some Lachnospiraceae genera (e.g., Roseburia and Dorea) that have a poor capacity to degrade FAA.36 In contrast, Clostridium bartlettii, which has a high catabolic activity toward FAA,36 was found at the highest levels in the fecal samples from HC. Some FAAs, especially Glu, also act as neurotransmitters in the CNS.37 In our study, Glu was found at the highest level in the fecal samples of children with AD; similar results have been reported by other authors.11 Glu plays a pivotal role in the pathophysiology of some neuropsychiatric disorders,37 and an excess of Glu may lead to neuronal cell death.
Figure 2.
Schematic representation of colonic fermentation of carbohydrates and proteins by microbial enzymes.
Our data indicate an alteration in the level of many volatile organic compounds (VOC), such as alcohols, aldehydes, esters, sulfur compounds, hydrocarbons, ketones, terpenes, indoles and furanones, in children with an ASD, especially in those with AD. Similar findings have been found for phenol compounds (e.g., phenol, 4-(1,1-dimethylethyl)-phenol, p-cresol). In our experience, children with an ASD have high levels of p-cresol, which is primarily synthesized by certain bacteria from the GI microbiota that are able to express synthetic enzymes not present in human cells.38 Postnatal exposure to abnormal concentrations of p-cresol and/or p-cresyl sulfate is considered to be a pathoplastic contributor to the severity of behavioral abnormalities and the cognitive impairment of children with an ASD.38
We have found that indole and 3-methylindole are increased in children with an ASD. Indole is a microbial metabolite of tryptophane that is synthesized by several commensal bacteria (e.g., Alistipes) that colonise the human GI tract and is a critical precursor of physiologically important molecules, such as serotonin and melatonin.
Short chain fatty acid (SCFA) profiling in ASDs
Consistent with a previous report,11 children with AD had significantly lower levels of SCFAs compared with HC with the exception of propionic and acetic acids. SCFAs represent the primary fuel for colonocytes and are involved in water and electrolyte absorption by the colonic mucosa.39 Butyric acid has specific effects on the regulation of transepithelial transport, positively modulating the inflammatory and oxidative states of the intestinal mucosa, reinforcing the mucosal barrier and modulating visceral sensitivity and motility. Although SCFAs do not belong to the classic neuroactive substances, these metabolites play a significant regulatory role in establishing the neurotransmitter phenotype after birth and in modulating catecholaminergic biosynthesis throughout the lifespan.40 Butyric acid also acts as a potent inhibitor of histone deacetylase (HDAC) activity,41 and in rats, propionic acid treatments result in ASD behaviors.42-44 Previously, several neurological effects of propionic acid in rats have been reported by Finegold et al.14,21
The correlations between metabolically active bacterial genera and the metabolomic data are described and reported in Figure 3. Bifidobacterium is negatively and positively correlated with propionic and butyric acids, respectively. Faecalibacterium, Ruminococcus and Eubacterium had a negative or no correlation with propionic acid, but the presence of these bacteria is positively correlated with butyric acid. Opposite correlations were found for the Clostridium and Roseburia genera. Many Clostridia species are able to produce propionic acid. Dominant Bacteroidetes and Proteobacteria genera are variously correlated with the SCFA level. Most consistently, Bacteroides (the dominant Bacteroidetes genera) is positively and negatively correlated with propionic and butyric acids, respectively.
Figure 3.
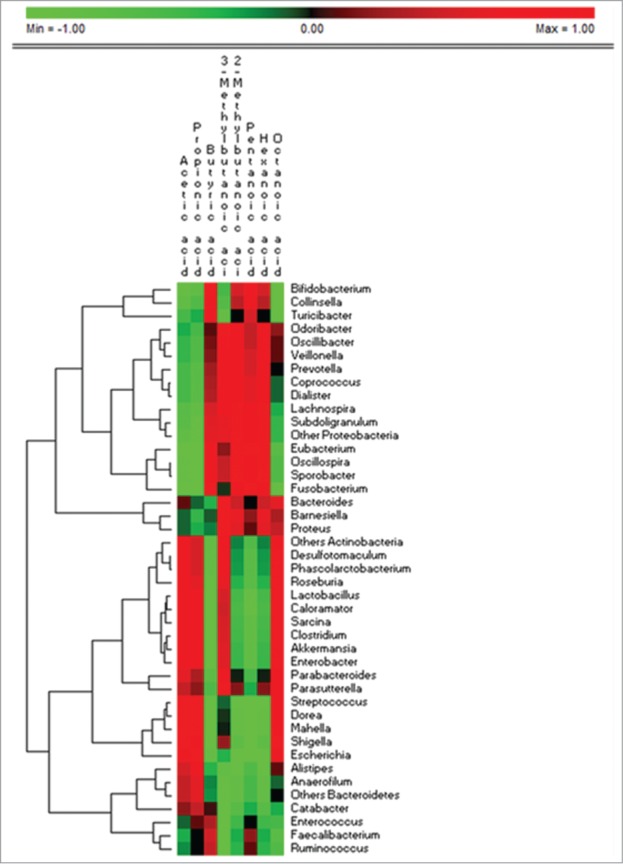
Permutation analysis of the correlation data between the active bacterial genera and short chain fatty acids found in feces of pervasive developmental disorder not otherwise specified, autistic and healthy children. The genera that showed values less than 0.1% of the total metabolically active bacterial were grouped together on the same phylum (others Actinobacteria, others Bacteroidetes, others Firmicutes, others Proteobacteria).
Urinary metabolomic profiling in ASD
Recently, we performed a Gas-chromatography mass spectrometry-solid-phase microextraction (GC-MS) quantification of urinary metabolites and analyzed a network model of the interactions. We found several abnormally increased or decreased substances in the urine of autistic subjects compared with normal controls. In particular, we found increased levels of 3-(3-hydroxyphenyl)-3-hydroxypropanoic acid, cis-aconitic acid, glycolic acid, 3,4-dihydroxybutyric acid, pyroglutamic acid and erythronic acid in the urine of children with an ASD, and some of these compounds have been previously linked to autistic disorders.45,46,47 Finally, we have demonstrated the increased excretion of tryptophan, a neurotransmitter precursor of serotonin, a brain neurotransmitter. The increased urination of tryptophan fragments correlates with increased tryptophan degradation, and this increase has been observed in psychiatric conditions such as depression, mental retardation and anxiety.45
Both clinical and pre-clinical studies provide promising evidence that indicates an important role for dietetic components and the gut microbiota in developing new therapeutic approaches to managing neurodevelopmental disorders. Furthermore, the metabolomic characterization of patients with ASDs and the identification of a metabolomics signature may lead to an innovative diagnostic strategy.
Specific treatments for ASDs
The Interagency Autism Coordinating Committee (IACC) notes the need to elucidate the role of the environment in the genesis of ASDs and aims to develop specific treatments (http://www.nimh.nih.gov/research-funding/scientific-meetings/recurring-meetings/iacc/strategic-plan/index.shtml).22
At present, there are no definitive treatments for this condition, and parents, often driven by the hope for a cure, look to complementary and alternative medicine (CAM)48,49 and are encouraged by the belief that this approach is risk-free. Among these alternative treatments are the use of vitamin and mineral supplements, secretin, and melatonin and the adoption of elimination diets, in particular of gluten50 and/or casein.
The rationale for adopting a gluten-free, casein-free diet is related to the release of peptides with opioid activity in the intestines, especially in presence of a leaky gut. If these peptides cross the blood-brain barrier and reach the CNS (in large amounts), brain function may be altered.51 However, there are several pieces of evidence against this theory, such as the low affinity of exorphins for opioid receptors, the presence of dietetic gluten/casein-derived peptides with antagonistic activity on opioid receptors and the failure to demonstrate abnormally high concentrations of opioid peptides in either the plasma or the nervous system of patients with an ASD.52 A recent systematic review concluded that the evidence to support a gluten-free, casein-free diet is limited and weak, considering that dietary restrictions might be responsible for further social withdrawal and integration, in addition to potential adverse clinical effects.53 Elimination diets for ASD patients should only be initiated after reaching a diagnosis of an adverse food reaction.
Probiotics can be useful for restoring the microbial balance in the intestine and ameliorating gastrointestinal symptoms. Some evidence has accumulated regarding the possible role of probiotics in modulating some neurological symptoms. Because ASD patients presented GI dysbiosis,19,20 which may exacerbate the disease,22 these patients could benefit from microbial ecosystem therapeutics (MET). However, before issuing probiotics to children with ASDs, the data should be confirmed in large, well-controlled, randomized trials. One key issue is the choice of probiotic strains, as the effects are highly strain-specific. For example, anxiety-like or depressive-like behavior in mice was increased after the administration of Campylobacter jejuni and decreased with the use of a Bifidobacterium strain.7 Furthermore, a pro/prebiotic-based therapy (e.g., Lactobacillus rhamnosus (JB-1), NCC4007, Bifidobacterium infantis, Bifidobacterium longum NCC3001, RO07, Lactobacillus helveticus R0052, Lactobacillus reuteri and Lactobacillus paracasei) was able to ameliorate the gastrointestinal symptoms that are frequently observed in children with ASDs.5,54 MET therapy, which employs whole bacterial communities derived directly from the human GI tract (transplantation), showed positive results for different GI diseases (e.g., Clostridium difficile infection).1 Nevertheless, no results are available for ASD patients.
Until clear clinical evidence of the efficacy of probiotics is obtained, the administration of probiotics and prebiotics should be considered an adjuvant therapy to the currently available conventional pharmacological approaches to encourage a healthier GI microbiota and metabolome in ASD patients.7 Future approaches with the appropriate categorisation of patients and controls, together with the application of state-of-the-art “omics” methods to identify the microbiota and the fecal, urinary, and plasma metabolomes, will help to reveal the underlying mechanisms controlling the deregulation of the microbiota-gut-brain axis in ASD patients and possibly the achievement of new therapeutic strategies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Petrof EO, Claud EC, Gloor GB, Allen-Vercoe E. Microbial ecosystems therapeutics: a new paradigm in medicine? Benef Microbes 2013; 4:53-65; PMID:23257018; http//dx.doi.org/ 10.3920/BM2012.0039 [DOI] [PubMed] [Google Scholar]
- 2. Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev 2010; 90:859-904; PMID:20664075; http://dx.doi.org/ 10.1152/physrev.00045.2009 [DOI] [PubMed] [Google Scholar]
- 3. Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour – epigenetic regulation of the gut–brain axis. Genes, Brain and Behavior 2014; 13:69-86; PMID:24286462; http://dx.doi.org/ 10.1111/gbb.12109 [DOI] [PubMed] [Google Scholar]
- 4. Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav. Immunology 2013; pii: S0889-1591(13)00600-4; PMID:24370461; http://dx.doi.org/ 10.1016/j.bbi.2013.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behaviour and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 2011; 108:16050-55; PMID:21876150; http://dx.doi.org/ 10.1073/pnas.1102999108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Desbonnet L, Clarke G, Shanahan F, Dinan TG, Cryan JF. Microbiota is essential for social development in the mouse. Mol Psychiatry 2014; 19:146-48; PMID:23689536; http://dx.doi.org/ 10.1038/mp.2013.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Theije CG, Wopereis H, Ramadan M, van Eijndthoven T, Lambert J, Knol J, Garssen J, Kraneveld AD, Oozeer R. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav Immun 2013; pii: S0889-1591(13)00590-4; PMID:24333160; http://dx.doi.org/ 10.1016/j.bbi.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 8. Grzadzinski R, Huerta M, Lord C. DSM-5 and autism spectrum disorders (ASDs): an opportunity for identifying ASD subtypes. Mol Autism 2013; 4:12; PMID:23675638; http://dx.doi.org/ 10.1186/2040-2392-4-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dietert RR, Dietert JM, DeWitt JC. Environmental risk factors for autism. Emerg Health Threats J 2011; 4:7111; PMID:24149029; http//dx.doi.org/ 10.3402/ehtj.v4i0.7111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, Cristofori F, Guerzoni ME, Gobbetti M, Francavilla R. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One 2013; 8:e76993; PMID:24130822; http://dx.doi.org/ 10.1371/journal.pone.0076993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism-comparisons to typical children and correlation with autism severity. BMC Gastroenterol 2011; 1:22; PMID:21410934; http//dx.doi.org/ 10.1186/1471-230X-11-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heberling CA, Dhurjati PS, Sasser M. Hypothesis for a systems connectivity model of autism spectrum disorder pathogenesis: links to gut bacteria, oxidative stress, and intestinal permeability. Med Hypotheses 2013; 80:264-70; PMID:23273906; http//dx.doi.org/ 10.1016/j.mehy.2012.11.044 [DOI] [PubMed] [Google Scholar]
- 13. Finegold SM, Downes J, Summanen PH. Microbiology of regressive autism. Anaerobe 2012; 18:260-262; PMID:22202440; http//dx.doi.org/ 10.1016/j.anaerobe.2011.12.018 [DOI] [PubMed] [Google Scholar]
- 14. Louis P. Does the human gut microbiota contribute to the etiology of autism spectrum disorders? Dig Dis Sci 2012; 57:1987-89; PMID:22736019; http//dx.doi.org/ 10.1007/s10620-012-2286-1 [DOI] [PubMed] [Google Scholar]
- 15. de Theije CG, Wu J, da Silva SL, Kamphuis PJ, Garssen J, Korte SM, Kraneveld AD. Pathways underlying the gut-to-brain connection in autism spectrum disorders as future targets for disease management. Eur J Pharmacol 2011; 668 Suppl. One: S70-80; PMID:21810417; http://dx.doi.org/ 10.1016/j.ejphar.2011.07.013 [DOI] [PubMed] [Google Scholar]
- 16. de Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P, Carteni M, De Rosa M, Francavilla R, Riegler G, et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr. 2010; 5:418-24; PMID:20683204; http://dx.doi.org/ 10.1097/MPG.0b013e3181dcc4a5 [DOI] [PubMed] [Google Scholar]
- 17. Banati M, Csecsei P, Koszegi E, Nielsen HH, Suto G, Bors L, Trauninger A, Csepany T, Rozsa C, Jakab G, et al. Antibody response against gastrointestinal antigens in demyelinating diseases of the central nervous system. Eur J Neurol 2013; 20:1492-95; PMID:23293933; http://dx.doi.org/ 10.1111/ene.12072 [DOI] [PubMed] [Google Scholar]
- 18. Severance EG, Gressitt KL, Stallings CR, Origoni AE, Khushalani S, Leweke FM, Dickerson FB, Yolken RH. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr Res 2013; 148:130-37; PMID:23746484; http://dx.doi.org/ 10.1016/j.schres.2013.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Finegold SM. Therapy and epidemiology of autism-clostridial spores as key elements. Med Hypotheses 2008; 70:508-11; PMID:17904761 [DOI] [PubMed] [Google Scholar]
- 20. Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, Summanen PH, Granpeesheh D, Dixon D, et al. Pyrosequencing study of fecal microbiota of autistic and control children. Anaerobe 2010; 16:444-53; PMID:20603222; http://dx.doi.org/ 10.1016/j.anaerobe.2010.06.008 [DOI] [PubMed] [Google Scholar]
- 21. Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol 2005; 54:987-91; PMID:16157555 [DOI] [PubMed] [Google Scholar]
- 22. Sandler RH, Finegold SM, Bolte ER, Buchanan CP, Maxwell AP, Väisänen ML, Nelson MN, Wexler HM. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol 2000; 15:429-35; PMID:10921511 [DOI] [PubMed] [Google Scholar]
- 23. Razacka R, Seidner DL. Nutrition in inflammatory bowel disease. Curr Opin Gastroenterol 2007; 23:400-05; PMID:17545776 [DOI] [PubMed] [Google Scholar]
- 24. Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl Environ Microbiol 2011; 77:6718-21; PMID:21784919; http://dx.doi.org/ 10.1128/AEM.05212-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Finegold SM. Desulfovibrio species are potentially important in regressive autism. Med Hypotheses 2011; 77:270-74; PMID:21592674; http://dx.doi.org/ 10.1016/j.mehy.2011.04.032 [DOI] [PubMed] [Google Scholar]
- 26. Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol Autism 2013; 4:42; PMID:24188502; http://dx.doi.org/ 10.1186/2040-2392-4-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gondalia SV, Palombo EA, Knowles SR, Austin DW. Faecal microbiota of individuals with autism spectrum disorder. E J Appl Physiol 2010; 6:24-29; http://dx.doi.org/ 10.7790/ejap.v6i2.213 [DOI] [Google Scholar]
- 28. Gondalia SV, Palombo EA, Knowles SR, Cox SB, Meyer D, Austin DW. Molecular characterisation of gastrointestinal microbiota of children with autism (with and without gastrointestinal dysfunction) and their neurotypical siblings. Autism Res 2012; 5:419-27; PMID:22997101; http://dx.doi.org/ 10.1002/aur.1253 [DOI] [PubMed] [Google Scholar]
- 29. Ait-Belgnaoui A, Durand H, Cartier C, Chaumaz G, Eutamene H, Ferrier L, Houdeau E, Fioramonti J, Bueno L, Theodorou V. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroendocrinology 2012; 37:1885-95; PMID:22541937; http://dx.doi.org/ 10.1016/j.psyneuen.2012.03.024 [DOI] [PubMed] [Google Scholar]
- 30. Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, Deng Y, Blennerhassett P, Macri J, McCoy KD, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011; 141:599-609, 609 e1-e3; PMID:21683077; http://dx.doi.org/ 10.1053/j.gastro.2011.04.052 [DOI] [PubMed] [Google Scholar]
- 31. Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 2011; 68:1095-102; PMID:21727249; http://dx.doi.org/ 10.1001/archgenpsychiatry.2011.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sharp WG, Jaquess DL, Luckens CT. Multi-method assessment of feeding problems among children with autism spectrum disorders. Res Autism Spectr Disord 2013; 7:56-65. [Google Scholar]
- 33. Zimmer MH, Hart LC, Manning-Courtney P, Murray DS, Bing NM, Summer S. Food variety as a predictor of nutritional status among children with autism. J Autism Dev Disord 2012; 42:549-56; PMID:21556968; http://dx.doi.org/ 10.1007/s10803-011-1268-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Salonen A, de Vos WM. Impact of diet on human intestinal microbiota and health. Annu Rev Food Sci Technol 2014; 5:6.1-6.24; PMID:24387608; http://dx.doi.org/ 10.1146/annurev-food-030212-182554 [DOI] [PubMed] [Google Scholar]
- 35. Francavilla R, Calasso M, Calace L, Siragusa S, Ndagijimana M, Vernocchi P, Brunetti L, Mancino G, Tedeschi G, Guerzoni E, et al. Effect of lactose on gut microbiota and metabolome of infants with cow's milk allergy. Pediatr Allergy Immunol 2012; 23:420-27; PMID:22435727; http://dx.doi.org/ 10.1111/j.1399-3038.2012.01286.x [DOI] [PubMed] [Google Scholar]
- 36. Wendy RR, Duncan SH, Scobbie L, Duncan G, Cantlay L, Calder AG, Anderson SE, Flint HJ. Major phenylpropanoid-derived metabolites in the human gut can arise from microbial fermentation of protein. Mol Nutr Food Res 2013; 57:523-35; PMID:23349065; http://dx.doi.org/ 10.1002/mnfr.201200594 [DOI] [PubMed] [Google Scholar]
- 37. Shimmura C, Suda S, Tsuchiya KJ, Hashimoto K, Ohno K, Matsuzaki H,, Iwata K, Matsumoto K, Wakuda T, Kameno Y, et al. Alteration of plasma glutamate and glutamine levels in children with high-functioning autism. PLoS One 2011; 6: e25340; PMID:21998651; http://dx.doi.org/ 10.1371/journal.pone.0025340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Persico AM, Napolioni V. Urinary p-cresol in autism spectrum disorder. Neurotoxicol Teratol 2012; 36:82-90; PMID:22975621; http://dx.doi.org/ 10.1016/j.ntt.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 39. Wall R, Ross PR, Shanahan F, Quigley EM, Dinan TG, Cryan JF, Fitzgerald GF, Stanton C. Influence of gut microbiota and manipulation by probiotics and prebiotics on host tissue fat: potential clinical implications. Lipid Technol 2012; 24:10; http://dx.doi.org/ 10.1002/lite.201200232 [DOI] [Google Scholar]
- 40. DeCastro M, Nankova BB, Shah P, Patel P, Mally PV, Mishra R, La Gamma EF. Short chain fatty acids regulate tyrosine hydroxylase gene expression through a cAMP-dependent signaling pathway. Brain Res Mol Brain Res. 2005; 142:28-38; PMID:16219387; http://dx.doi.org/ 10.1038/mp.2013.65 [DOI] [PubMed] [Google Scholar]
- 41. Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr 2003; 133:2485S-93S; PMID:12840228 [DOI] [PubMed] [Google Scholar]
- 42. MacFabe DF, Caina NE, Boona F, Ossenkopp KP, Cain DP. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: relevance to autism spectrum disorder. Behav Brain Res 2011; 217:47-54; PMID:20937326; http://dx.doi.org/ 10.1016/j.bbr.2010.10.005 [DOI] [PubMed] [Google Scholar]
- 43. Thomas RH, Meeking MM, Mepham JR, Tichenoff L, Possmayer F, Liu S. and MacFabe DF. The enteric bacterial metabolite propionic acid alters brain and plasma phospholipid molecular species: further development of a rodent model of autism spectrum disorders. J Neuroinflammation 2012; 9:153; PMID:22747852; http://dx.doi.org/ 10.1186/1742-2094-9-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. MacFabe DF. Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb Ecol Health Dis 2012; 23:19260; PMID:23990817; http://dx.doi.org/ 10.3402/mehd.v23i0.19260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Noto A, Fanos V, Barberini B, Grapov D, Fattuoni C, Zaffanello M, Casanova A, Fenu G, De Giacomo A, De Angelis M, et al. The urinary metabolomics profile of an Italian autistic children population and their unaffected siblings. J Matern Fetal Neonatal Med 2014; 27 Suppl 2:46-52 PMID:25284177; http://dx.doi.org/ 10.3109/14767058.2014.954784 [DOI] [PubMed] [Google Scholar]
- 46. Shaw W. Increased urinary excretion of a 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylalanine metabolite of Clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophrenia. Nutr Neurosci 2010; 13:135-143; PMID:20603222; [DOI] [PubMed] [Google Scholar]
- 47. Emond P, Mavel S, Aïdoud N, Nadal-Desbarats L, Montigny F, Bonnet-Brilhault F, Barthélémy C, Merten M, Sarda P, Laumonnier F, et al. GC-MS-based urine metabolic profiling of autism spectrum disorder. Anal Bioanal Chem 2013; 405:5291-300; PMID:23571465; http://dx.doi.org/ 10.1007/s00216-013-6934-x [DOI] [PubMed] [Google Scholar]
- 48. Golnik AE, Ireland M. Complementary alternative medicine for children with autism: a physician survey. J Autism Dev Disord 2009; 39:996-1005; PMID:19280328; http://dx.doi.org/ 10.1007/s10803-009-0714-7 [DOI] [PubMed] [Google Scholar]
- 49. Levy SE, Mandell DS, Merhar S, Ittenbach RF, Pinto-Martin JA. Use of complementary and alternative medicine among children recently diagnosed with autistic spectrum disorder. J Dev Behav Pediatr 2003; 24:418-23; PMID:14671475 [DOI] [PubMed] [Google Scholar]
- 50. Whiteley P, Haracopos D, Knivsberg AM, Reichelt KL, Parlar S, Jacobsen J, Seim A, Pedersen L, Schondel M, Shattock P. The ScanBrit randomised, controlled, single-blind study of a gluten- and casein-free dietary intervention for children with autism spectrum disorders. Nutr Neurosci 2010; 13:87-100; PMID:20406576; [DOI] [PubMed] [Google Scholar]
- 51. J Hanecka A, Staniszewska R, Gach K, Fichna J. Enzymatic degradation of endomorphins. Peptides. 2008; 29:2066-73; PMID:18718496; http://dx.doi.org/ 10.1016/j.peptides.2008.07.015 [DOI] [PubMed] [Google Scholar]
- 52. Lennernäs H. Intestinal permeability and its relevance for absorption and elimination. Xenobiotica. 2007; 37:1015-51; PMID:17968735 [DOI] [PubMed] [Google Scholar]
- 53. Marí-Bauset S, Zazpe I, Mari-Sanchis A, Llopis-González A, Morales-Suárez-Varela M. Evidence of the gluten-free and casein-free diet in autism spectrum disorders: a systematic review. J Child Neurol 2014; 29:1718-27 PMID:24789114; http://dx.doi.org/ 10.1177/0883073814531330 [DOI] [PubMed] [Google Scholar]
- 54. Saulnier DM, Ringel Y, Heyman MB, Foster JA, Bercik P, Shulman RJ, Versalovic J, Verdu EF, Dinan TG, Hecht G, et al. The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes 2013; 4:17-27; PMID:23202796; http://dx.doi.org/ 10.4161/gmic.22973 [DOI] [PMC free article] [PubMed] [Google Scholar]