Abstract
Shewanella oneidensis strain MR-1 is a dissimilatory metal-reducing bacterium frequently found in aquatic sediments. In the absence of oxygen, S. oneidensis can respire extracellular, insoluble oxidized metals, such as iron (hydr)oxides, making it intimately involved in environmental metal and nutrient cycling. The reduction of ferric iron (Fe3+) results in the production of ferrous iron (Fe2+) ions, which remain soluble under certain conditions and are toxic to cells at higher concentrations. We have identified an inner membrane protein in S. oneidensis, encoded by the gene SO_4475 and here called FeoE, which is important for survival during anaerobic iron respiration. FeoE, a member of the cation diffusion facilitator (CDF) protein family, functions to export excess Fe2+ from the MR-1 cytoplasm. Mutants lacking feoE exhibit an increased sensitivity to Fe2+. The export function of FeoE is specific for Fe2+, as an feoE mutant is equally sensitive to other metal ions known to be substrates of other CDF proteins (Cd2+, Co2+, Cu2+, Mn2+, Ni2+, or Zn2+). The substrate specificity of FeoE differs from that of FieF, the Escherichia coli homolog of FeoE, which has been reported to be a Cd2+/Zn2+ or Fe2+/Zn2+ exporter. A complemented feoE mutant has an increased growth rate in the presence of excess Fe2+ compared to that of the ΔfeoE mutant complemented with fieF. It is possible that FeoE has evolved to become an efficient and specific Fe2+ exporter in response to the high levels of iron often present in the types of environmental niches in which Shewanella species can be found.
INTRODUCTION
Shewanella oneidensis strain MR-1 is a versatile, facultatively anaerobic bacterium that lives in aquatic environments and is capable of respiring numerous organic and inorganic compounds in the absence of oxygen. The respiratory diversity of S. oneidensis has widespread effects on biogeochemical cycling (1) and has therefore been a focus for applications in biotechnology and bioremediation (2). Terminal electron acceptors that S. oneidensis can use, aside from oxygen, include dimethyl sulfoxide (DMSO), trimethylamine N-oxide, fumarate, nitrate, and sulfite (3–6), as well as oxidized metals, such as iron and manganese (hydr)oxides (3, 7), which are abundant in the types of sediments (8) in which Shewanella spp. are often found (1). The molecular mechanisms that allow dissimilatory metal-reducing bacteria to survive under iron-rich conditions, however, are not fully understood.
Respiration of ferric iron (Fe3+) results in the production of ferrous iron (Fe2+), which can remain as aqueous Fe2+ ions or become incorporated into solid-phase minerals (9, 10), depending on the environmental conditions. As iron respiration by S. oneidensis continues, the local concentration of aqueous Fe2+ may increase, and Fe2+ ions can be taken up by cells through transition metal ion uptake systems, primarily the iron transport complex FeoAB (11). At higher concentrations, however, Fe2+ is toxic to cells. Aerobically, Fe2+ toxicity is thought to be caused by oxidative damage from hydroxyl radicals produced through the Fenton reaction (12), but the cause of damage under anaerobic conditions is not well understood. Several possible causes of anaerobic Fe2+ toxicity have been proposed, such as the production of reactive nitrogen species (13) or inhibition of the FoF1 ATPase (14). Regardless of the basis for toxicity, microorganisms have evolved means of minimizing the cellular damage caused by high concentrations of Fe2+ and other metal ions.
One of the well-characterized mechanisms that microorganisms use to prevent metal toxicity is efflux via membrane transporters. Metal efflux proteins are widespread in all three domains of life and comprise multiple protein families and superfamilies. For example, the major facilitator family includes the tetracycline-metal ion transporter TetL in Bacillus subtilis (15) and the iron citrate exporter IceT in Salmonella enterica serovar Typhimurium (16). P-type ATPases, which couple the uptake or efflux of cations to ATP hydrolysis, include the cadmium exporter CadA in Staphylococcus aureus and B. subtilis (17, 18) and the copper transporter CopA in Escherichia coli (19). To date, however, no proteins mediating Fe2+ resistance have been described in S. oneidensis.
A transposon screen identified mutations in gene locus SO_4475 resulting in a strong growth defect during ferric citrate respiration but not during respiration of fumarate or DMSO (E. D. Brutinel and J. A. Gralnick, unpublished data). SO_4475 is predicted to encode a metal ion exporter in the cation diffusion facilitator (CDF) family, a group of inner membrane proteins that utilize proton motive force (PMF) to export a range of divalent metal cations (20, 21). The closest homolog of SO_4475 described in the literature, at an amino acid sequence similarity of 60.9% and identity of 47.7%, is the E. coli protein FieF (YiiP). FieF from E. coli has been reported to export Zn2+/Cd2+ by some researchers (22, 23) and Fe2+/Zn2+ by others (24); the protein encoded by SO_4475 was described as exporting Zn2+/Cd2+ (25), although Fe2+ transport was not evaluated in that study. Here we characterize SO_4475, which we name feoE (for ferrous iron export), and show physiological evidence demonstrating that the protein encoded by feoE exports excess Fe2+ from S. oneidensis and is important for survival under iron-reducing conditions.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. oneidensis strain MR-1 was originally isolated from Lake Oneida in New York State (3). The E. coli strains used for cloning (UQ950) and mating (WM3064) have been previously described (26). E. coli K-12 strain MG1655 was used for FieF analysis. The strains used for cloning, derivative strains of MR-1 and MG1655, and the plasmids used in this study are found in Table 1. Liquid overnight Luria-Bertani (LB) cultures supplemented with 50 μg/ml kanamycin, when appropriate, were inoculated with colonies isolated from freshly streaked −80°C stocks. All cultures were grown at 30°C (S. oneidensis) or 37°C (E. coli); liquid cultures were shaken at 250 rpm. Unless otherwise noted, all experiments using liquid and solid media were performed with LB; where indicated, Shewanella basal medium (SBM) (27) supplemented with 0.05% (wt/vol) Casamino Acids, 5 ml/liter vitamin solution (28), and 5 ml/liter mineral solution (29) was used as a defined minimal medium. Anaerobic cultures were flushed with nitrogen gas and supplemented with 20 mM sodium lactate and an electron acceptor, as indicated below. Results are reported as the means from three biological replicates ± 1 standard deviation. Data were statistically analyzed using analysis of variance.
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Descriptiona | Reference or source |
|---|---|---|
| Strains | ||
| JG274 | S. oneidensis MR-1, wild type | 3 |
| JG2989 | JG274 ΔfeoE | This work |
| JG168 | JG274 with empty pBBR1MCS-2, Kmr | 27 |
| JG2993 | JG2989 with empty pBBR1MCS-2, Kmr | This work |
| JG2780 | JG274 with pBBR1MCS-2::feoE, Kmr | This work |
| JG2994 | JG2989 with pBBR1MCS-2::feoE, Kmr | This work |
| JG2997 | JG2989 with pBBR1MCS-2::fieF, Kmr | This work |
| MG1655 | E. coli K-12, wild type | Arkady Khodursky, University of Minnesota |
| JG3304 | MG1655 ΔfieF | This work |
| JG3306 | JG3304 with empty pBBR1MCS-2, Kmr | This work |
| JG3307 | JG3304 with pBBR1MCS-2::fieF, Kmr | This work |
| JG3308 | JG3304 with pBBR1MCS-2::feoE, Kmr | This work |
| UQ950 | E. coli DH5α λ(pir) cloning host; F− Δ(argF-lac)169 ϕ80dlacZ58ΔM15 glnV44(AS) rfbD1 gyrA96 (Nalr) recA1 endA1 spoT1 thi-1 hsdR17 deoR λpir+ | 26 |
| WM3064 | E. coli conjugation strain; thrB1004 pro thi rpsL hsdS lacZΔM15 RP4-1360 Δ(araBAD)567 ΔdapA1341::[erm pir(wt)] | 26 |
| Plasmids | ||
| pSMV3 | Deletion vector, Kmr sacB | 31 |
| pBBR1MCS-2 | Broad-host-range cloning vector, Kmr | 30 |
| pBBR1MCS-2::feoE | SO_4475 (feoE), 48 bp upstream, 51 bp downstream, Kmr | This work |
| pBBR1MCS-2::fieF | b3915 (fieF), 76 bp upstream, 26 bp downstream, Kmr | This work |
AS, amber (UAG) suppressor; wt, wild type.
Plasmid and mutant construction.
The primers used to construct the plasmids are listed in Table 2. In-frame deletion of feoE from the MR-1 genome and fieF from the MG1655 genome was performed as previously described (26). Briefly, fragments 1 kb upstream and downstream of feoE (SO_4475) with flanking SacI and BamHI sites were fused via a SpeI restriction site and ligated into the suicide vector pSMV3, which has kanamycin resistance and sacB cassettes. Fragments 1 kb upstream and downstream of fieF (b3915) with flanking SpeI and BamHI restriction sites were fused via a SacII site and ligated into pSMV3. To make the feoE complementation vector, feoE was cloned from the S. oneidensis MR-1 genome with flanking BamHI and SpeI restriction sites and inserted into the pBBR1MCS-2 multiple-cloning site (30). To make the fieF complementation vector, fieF (b3915) was cloned from the E. coli MG1655 genome with flanking BamHI and SpeI restriction sites and inserted into the multiple-cloning site of pBBR1MCS-2.
TABLE 2.
Primers used for mutant construction and complementation in this work
| Primer | Sequence | Restriction site |
|---|---|---|
| 4475USF | GTACGGATCCGCAGAGCGCGTAACACTTC | BamHI |
| 4475USR | GTACACTAGTCCATTGTATATCAGCTTGGCG | SpeI |
| 4475DSF | GTACACTAGTGCGACACTGAATCGATTATTCAAC | SpeI |
| 4475DSR | GTACGAGCTCGCTCAGTCACAGCGGCATTAACAC | SacI |
| 4475CompF | GTACGGATCCCGCCAAGCTGATATACAATGG | BamHI |
| 4475CompR | GTACACTAGTGGCATAACCACTCCTTTGATTG | SpeI |
| ECfieFUSF | GATCACTAGTCGATACCATTTTTCTTCGGC | SpeI |
| ECfieFUSR | GATCCCGCGGCATAAATACTCCCGCTATCAAC | SacII |
| ECfieFDSF | GATCCCGCGGCGGTCTATGCTTTCATAATCAG | SacII |
| ECfieFDSR | GATCGGATCCCATACGGGAAGCCAGAATAC | BamHI |
| ECfiefF | GTACGGATCCCAATTTGCCTGCTGCTTAATGC | BamHI |
| ECfiefR | GTACACTAGTGCGGGTCTGGCTCTCTTTTATAC | SpeI |
Growth curves.
Overnight cultures of each strain were pelleted, washed once, and resuspended in fresh LB or SBM. For aerobic and anaerobic Fe3+ cultures, SBM was supplemented with 20 mM sodium lactate and 80 mM ferric citrate. The growth of anaerobic Fe3+ cultures was measured by periodically plating serial 1:10 dilutions of each culture onto LB plates and performing colony counts after 1 day of incubation. The growth of aerobic Fe3+ cultures was measured by taking the optical density at 600 nm (OD600). For cultures with divalent metals, LB was supplemented with 0.45 mM CdCl2; 0.8 mM CoCl2; 2.2 mM CuCl2; 20 mM sodium lactate, 40 mM sodium fumarate, and 1.0 mM, 2.5 mM, 3.5 mM, 5.0 mM, or 7.0 mM FeCl2; 20 mM sodium lactate, 40 mM sodium fumarate, and 8.0 mM MnCl2; 1.0 mM NiCl2; or 1.0 mM ZnCl2. Cultures with FeCl2 and MnCl2 were grown anaerobically to prevent the oxidation of Fe2+ to Fe3+ or Mn2+ to Mn4+. Growth was periodically measured by taking the OD600 using a spectrophotometer.
Iron citrate reduction assay.
Fe3+ respiration was measured using ferrozine assays as previously described (31). Briefly, overnight cultures of each strain were pelleted, washed once, resuspended in fresh SBM, and adjusted to an OD600 of 1.00. Thirty microliters of this suspension was inoculated into 270 μl SBM with 20 mM sodium lactate, 5 mM ferric citrate, 5 ml/liter vitamin solution, and 5 ml/liter mineral solution in anaerobic 96-well plates. Fe2+ production was monitored over time by determining the ferrozine absorbance at 542 nm (32).
Iron retention assay.
Overnight cultures of each strain were pelleted, washed once, and resuspended in fresh LB. Suspensions were inoculated into 5-ml anaerobic cultures of LB with 20 mM sodium lactate and 40 mM sodium fumarate at an OD600 of 0.05. Cultures were incubated at 30°C until the growth reached an OD600 of approximately 0.50. FeCl2 was spiked into each culture at a concentration of 2.5 mM, and the cultures were incubated at 30°C for 1 h. Each culture was pelleted, washed once, and resuspended in 5 ml fresh SBM. Cell suspensions were assayed for iron concentration via inductively coupled plasma mass spectrometry (ICP-MS) analysis by the Analytical Geochemistry Lab in the Department of Earth Sciences at the University of Minnesota. Iron concentrations were normalized to the final OD600 before harvesting.
RESULTS
The ΔfeoE mutant has decreased survival with ferric citrate as an electron acceptor.
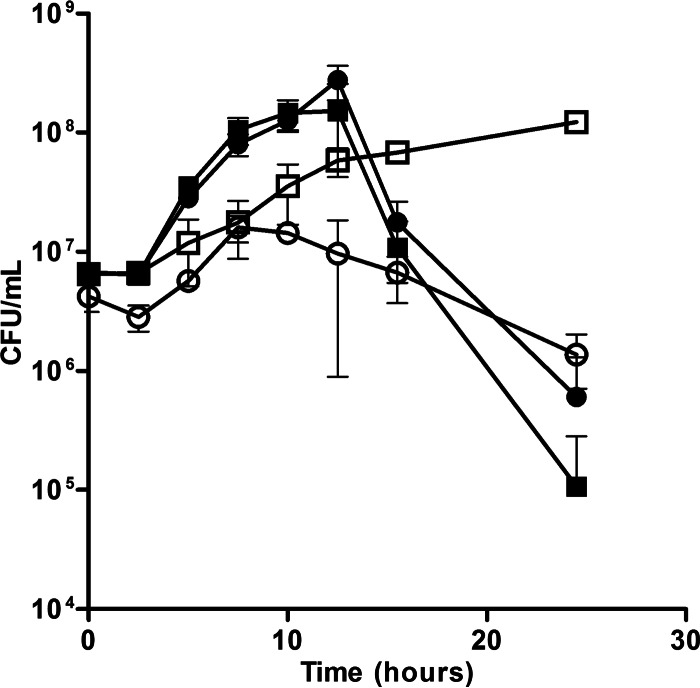
A transposon screen indicated that inactivation of feoE (SO_4475) in S. oneidensis caused a growth defect during anaerobic ferric citrate respiration but not during respiration of fumarate or DMSO (Brutinel and Gralnick, unpublished). These results indicate that the protein product of feoE is important for growth during Fe3+ respiration, rather than anaerobic growth in general. To confirm the results of the transposon screen, an in-frame deletion of feoE was made in S. oneidensis. No significant differences in the growth rate between the ΔfeoE mutant and the wild type were found in anaerobic cultures supplemented with 20 mM lactate and 40 mM fumarate (doubling times, 1.94 ± 0.23 and 1.91 ± 0.22 h, respectively) or 20 mM lactate and 40 mM DMSO (doubling times, 1.48 ± 0.16 and 1.45 ± 0.15 h, respectively). To evaluate the importance of feoE during respiration of Fe3+, growth was monitored in anaerobic cultures supplemented with 20 mM lactate and 80 mM ferric citrate. Deletion of feoE resulted in impaired growth over time during iron respiration compared to that of the wild type (Fig. 1). Complementation of the ΔfeoE mutant and the wild type with pBBR1MCS-2::feoE enhanced the log-phase growth rate of both strains over that of the wild type with the empty vector (doubling times, 1.57 ± 0.48, 1.48 ± 0.66, and 4.71 ± 1.20 h, respectively). The complemented strains also displayed steeper die-off than either strain MR-1 or the ΔfeoE mutant with the empty vector (Fig. 1). A similar growth impairment of the ΔfeoE mutant was observed in anaerobic SBM cultures supplemented with 20 mM lactate, 40 mM fumarate, and 1 mM ferric citrate (see Fig. S1 in the supplemental material).
FIG 1.
Anaerobic growth of the wild-type and ΔfeoE strains on ferric citrate. The rate of growth in SBM with 20 mM lactate and 80 mM ferric citrate over time was measured for the ΔfeoE mutant with empty pBBR1MCS-2 (○), MR-1 with empty pBBR1MCS-2 (□), the ΔfeoE mutant with pBBR1MCS-2::feoE (●), and MR-1 with pBBR1MCS-2::feoE (■). Growth was determined by counting the numbers of CFU per milliliter of culture medium.
To rule out the possibility that the growth defect seen for the ΔfeoE mutant during Fe3+ respiration was due to an increased sensitivity to citrate or soluble Fe3+, the aerobic growth of cultures supplemented with 20 mM lactate and 80 mM ferric citrate was evaluated. The growth rate of the ΔfeoE mutant showed no difference from that of the wild type during aerobic respiration in the presence of ferric citrate (doubling times, 1.16 ± 0.04 and 1.15 ± 0.04 h, respectively, during log phase).
Deletion of feoE does not impair ferric citrate respiration.
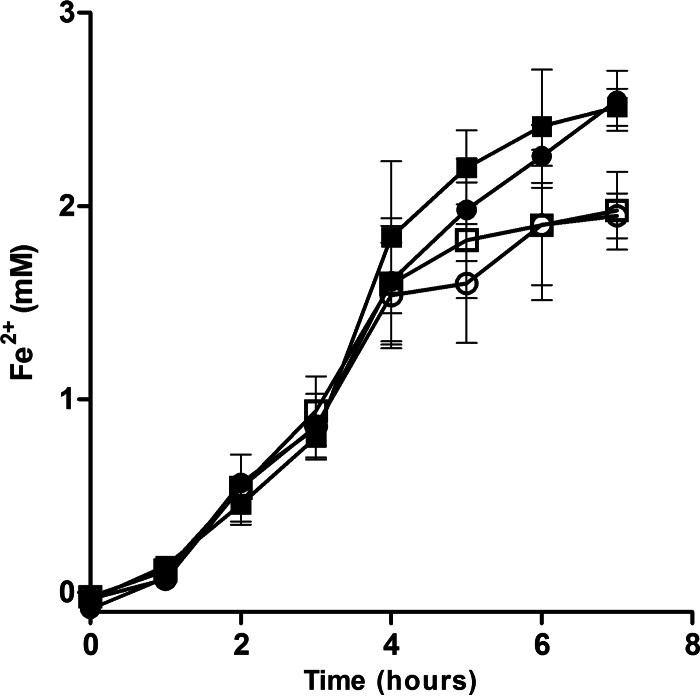
To determine whether the impaired growth of the ΔfeoE mutant during anaerobic Fe3+ respiration was due to a defect in the strain's ability to use Fe3+ as an electron acceptor, ferrozine assays were performed to measure the production of Fe2+ from the respiration of ferric citrate. No statistically significant difference (P > 0.05) in the initial rate of Fe2+ production was observed between the ΔfeoE mutant and MR-1 whether they were complemented with pBBR1MCS-2::feoE or with the empty vector (Fig. 2). Complementation of the ΔfeoE mutant and the wild type with pBBR1MCS-2::feoE led to the production of final concentrations of Fe2+ slightly higher than those produced by the strains complemented with the empty vector (P < 0.01; Fig. 2).
FIG 2.
Ferric citrate reduction by the wild-type and the ΔfeoE mutant strains. The rate of ferric citrate reduction was measured for the ΔfeoE mutant with empty pBBR1MCS-2 (○), MR-1 with empty pBBR1MCS-2 (□), the ΔfeoE mutant with pBBR1MCS-2::feoE (●), and MR-1 with pBBR1MCS-2::feoE (■).
ΔfeoE mutants exhibit greater sensitivity to Fe2+.
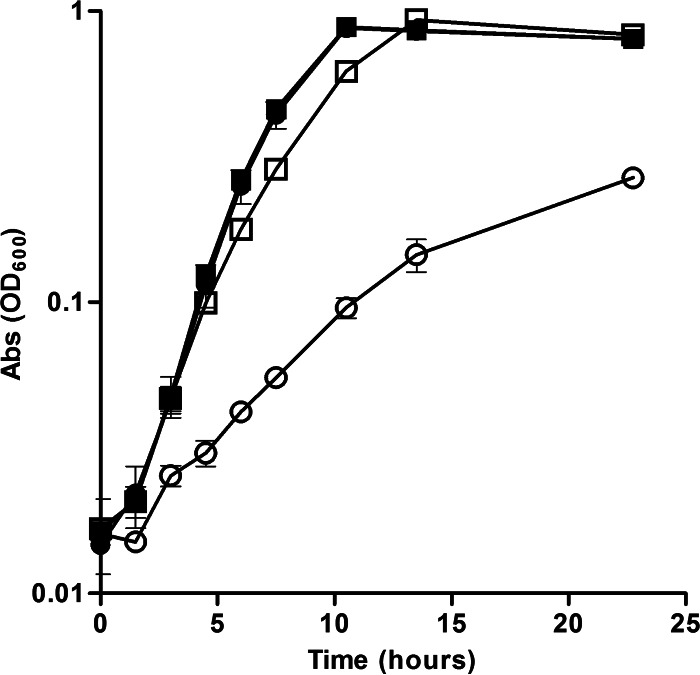
Because the ΔfeoE mutant was not defective in reducing Fe3+, we hypothesized that the growth defect displayed by the ΔfeoE mutant during Fe3+ respiration was due to increased sensitivity to Fe2+, the by-product of Fe3+ respiration. To determine if the ΔfeoE mutant is more sensitive to Fe2+ than the wild type, cultures of the ΔfeoE mutant and the wild type with pBBR1MCS-2 and pBBR1MCS-2::feoE were grown anaerobically in LB with 20 mM lactate, 40 mM fumarate, and 1 mM FeCl2. The ΔfeoE mutant displayed a greater sensitivity to Fe2+ than the parent strain, as indicated by a lower growth rate (Fig. 3). Complementation of the mutant with pBBR1MCS-2::feoE restored the rate of growth in the presence of Fe2+ to that of the wild type (Fig. 3). The average doubling times during log phase were 3.69 ± 0.14 h for the ΔfeoE mutant with the empty vector, 2.35 ± 0.16 h for the ΔfeoE mutant with pBBR1MCS-2::feoE, 2.36 ± 0.12 h for the wild type with the empty vector, and 2.41 ± 0.16 h for the wild type with pBBR1MCS-2::feoE.
FIG 3.
Growth of the wild-type and ΔfeoE strains in the presence of excess Fe2+. The rate of growth in anaerobic LB with 20 mM lactate, 40 mM fumarate, and 1 mM FeCl2 was measured for the ΔfeoE mutant with empty pBBR1MCS-2 (○), MR-1 with empty pBBR1MCS-2 (□), the ΔfeoE mutant with pBBR1MCS-2::feoE (●), and MR-1 with pBBR1MCS-2::feoE (■). Abs, absorbance.
Loss of feoE increases Fe2+ retention.
The protein product of feoE has been annotated as belonging to the CDF protein family, the members of which confer increased resistance to a variety of divalent metal ions via active export of the ions from a cell's cytoplasm (21). In order to determine whether the increased sensitivity to Fe2+ seen in the ΔfeoE mutant was due to an impaired ability to export Fe2+, iron retention assays were performed. The wild type and the ΔfeoE mutant carrying the empty vector and the pBBR1MCS-2::feoE vector were grown anaerobically with 20 mM lactate and 40 mM fumarate into log phase (OD600, approximately 0.5) and then spiked with 2.5 mM FeCl2. The concentration of 2.5 mM FeCl2 was chosen because the growth of both the ΔfeoE mutant and the wild type is decreased but not arrested at this concentration. After incubation with Fe2+ for 1 h, cells in each culture were harvested and analyzed by ICP-MS for total iron content. ΔfeoE mutant cells with the empty pBBR1MCS-2 vector retained a significantly larger amount of iron (P < 0.0001) than the ΔfeoE mutant with pBBR1MCS-2::feoE, the wild type with the empty vector, or the wild type with pBBR1MCS-2::feoE (218.0 ± 9.1, 148.9 ± 6.3, 141.3 ± 8.0, and 137.3 ± 7.8 ng · ml−1 · OD600−1 iron, respectively). Similar results were observed in experiments performed using SBM in place of LB (data not shown).
The export function of FeoE is specific for Fe2+.
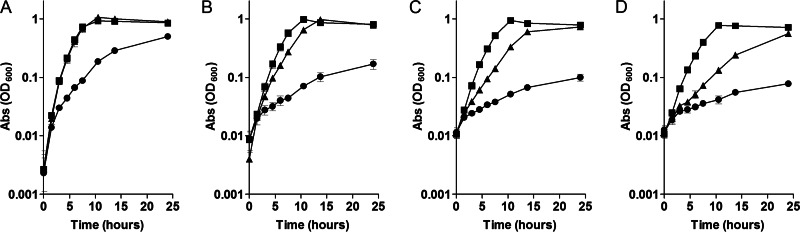
To determine the export specificity of FeoE, the sensitivity of the wild type and the ΔfeoE mutant to various divalent metals was tested. Cultures of the ΔfeoE mutant and the wild type were grown aerobically in LB with excess CdCl2, CoCl2, CuCl2, NiCl2, or ZnCl2 and anaerobically in LB with 20 mM lactate, 40 mM fumarate, and excess MnCl2. No difference in sensitivity to any of these divalent metals was observed between the ΔfeoE mutant and the wild type (Fig. 4). Similarly, no difference in sensitivity to any of these metals was seen in zone-of-inhibition assays performed anaerobically on tryptone medium plates using Noble agar as the solidifying agent (see Table S1 in the supplemental material).
FIG 4.
Growth of the wild type and the ΔfeoE mutant in the presence of divalent metals. Growth was measured over time in LB with 0 mM or 0.45 mM CdCl2 (A); 0 mM or 0.8 mM CoCl2 (B); 0 mM or 2.2 mM CuCl2 (C); 20 mM sodium lactate, 40 mM fumarate, and 0 mM or 8 mM MnCl2 (D); 0 mM or 1.0 mM NiCl2 (E); or 0 mM or 1.0 mM ZnCl2 (F). □, MR-1 with no metal added; ○, the ΔfeoE mutant with no metal added; ■, MR-1 with metal added; ●, the ΔfeoE mutant with metal added.
FeoE confers greater resistance to Fe2+ than the E. coli homolog FieF.
An earlier study indicated that E. coli FieF, the closest characterized homolog of FeoE, may export Fe2+ (25). In order to determine how the function of FeoE compares to that of FieF, a cross-complementation study was performed in which the ΔfeoE mutant was transformed with pBBR1MCS-2::fieF. Anaerobic cultures of the ΔfeoE mutant with the empty vector, pBBR1MCS-2::feoE, or pBBR1MCS-2::fieF were grown in LB with 20 mM lactate, 40 mM fumarate, and 1.0 mM, 2.5 mM, 3.5 mM, or 5.0 mM FeCl2. The growth rates of the ΔfeoE mutant complemented with pBBR1MCS-2::fieF were similar to those of the ΔfeoE mutant complemented with pBBR1MCS-2::feoE at 1.0 and 2.5 mM FeCl2, but at higher Fe2+ concentrations, the growth of the fieF-complemented mutant was considerably diminished compared to that of the mutant complemented with feoE (Fig. 5). Similarly, E. coli ΔfieF grown anaerobically in LB with 20 mM lactate, 40 mM fumarate, and 7 mM FeCl2 showed significantly impaired growth when complemented with pBBR1MCS-2::fieF compared with that when complemented with pBBR1MCS-2::feoE (P < 0.001; see Fig. S2 in the supplemental material).
FIG 5.
Growth of the ΔfeoE mutant complemented with feoE or fieF from E. coli in the presence of excess Fe2+. Growth in anaerobic LB with 20 mM lactate, 40 mM fumarate, and 1.0 mM (A), 2.5 mM (B), 3.5 mM (C), or 5.0 mM (D) FeCl2 was measured for the ΔfeoE mutant with empty pBBR1MCS-2 (●), pBBR1MCS-2::feoE (■), or pBBR1MCS-2::fieF (▲).
DISCUSSION
Efflux proteins, responsible for the maintenance of intracellular concentrations of various small molecules, are found among all domains of life. The actions of cation exporters allow cells to maintain subtoxic intracellular levels of heavy metals, most frequently, Cd2+, Co2+, Ni2+, Fe2+, and Zn2+ (33). Here we have characterized FeoE, a member of the CDF family found in S. oneidensis MR-1 and encoded by the gene locus SO_4475 which specifically exports Fe2+ and is important for survival during Fe3+ respiration.
FeoE is well conserved throughout the shewanellae, with closely related homologs being found in the other metal-reducing Shewanella spp., Shewanella sp. strains ANA-3, MR-4, and MR-7 and Shewanella putrefaciens CN-32 (92.0 to 94.3% identity), suggesting that Fe2+ efflux is an important strategy for these iron-respiring organisms. Interestingly, distantly related FeoE homologs can also be found in the metal-reducing Deltaproteobacteria species Geobacter metallireducens and Geobacter sulfurreducens, but the sequence similarity between these proteins and FeoE (23.1 to 25.6% identity) is too low to draw conclusions about function without additional experimentation.
Initial experiments indicated that deletion of SO_4475 (feoE) resulted in decreased cell density over time during growth with ferric citrate as a terminal electron acceptor (Fig. 1; see also Fig. S1 in the supplemental material) but not during respiration of DMSO or fumarate. Complementation of both the wild type and the ΔfeoE mutant with a plasmid carrying feoE conferred an increased rate of growth to each strain during ferric citrate respiration (Fig. 1), indicating that having multiple copies of the gene allows cells to minimize the inhibitory effects of increased Fe2+. Unexpectedly, both feoE-complemented strains displayed a rapid die-off after reaching stationary phase (Fig. 1). One possible explanation for this phenomenon is that production of excess FeoE is energetically taxing to cells. However, we think that a more likely explanation is that the initial expansion to a high cell density causes the feoE-complemented strains to run out of a limiting nutrient earlier than the strains complemented with an empty vector, given that the strains grow much faster with feoE constitutively expressed from a multicopy vector. Alternatively, the increased amounts of FeoE in cells could result in the nonspecific efflux of a trace metal(s) required for growth.
To confirm that the decrease in cell density seen for the ΔfeoE mutant during growth on ferric citrate (Fig. 1) was not due to a respiratory defect, production of Fe2+ from ferric citrate respiration by resting cells was measured. Both the ΔfeoE mutant with the empty vector and the ΔfeoE mutant with pBBR1MCS-2::feoE produced Fe2+ at the same rate as the corresponding wild-type strains (Fig. 2). Interestingly, the feoE-complemented wild-type and ΔfeoE strains produced slightly but statistically significant (P < 0.01) increased amounts of Fe2+ near the end of the assay (Fig. 2). It appears that, similar to the results seen in the ferric citrate growth curve (Fig. 1), production of more copies of the FeoE transporter allows feoE-complemented strains to minimize inhibition by Fe2+ and thus increase metabolic processes.
To determine whether the decline in cell density seen for the ΔfeoE mutant during ferric citrate respiration (Fig. 1) was due to an increased susceptibility to Fe2+ toxicity, growth curves were performed with 1 mM FeCl2. The feoE mutant with the empty vector displayed a notably lower rate of growth than the ΔfeoE mutant with pBBR1MCS-2::feoE, the wild type with an empty vector, or the wild type with pBBR1MCS-2::feoE, indicating that deletion of feoE caused greater sensitivity to Fe2+ (Fig. 3). Commensurate with the findings of the ferric citrate respiration assays (Fig. 1 and 2), both the wild type and the ΔfeoE mutant complemented with feoE displayed significant (P < 0.0001) increases in log-phase growth rate over that of the wild type with the empty vector in the presence of excess Fe2+ (Fig. 3), again suggesting that the enhanced activity of FeoE facilitates greater resistance to Fe2+.
To verify that the increased Fe2+ sensitivity of the ΔfeoE mutant was due to higher cellular concentrations of iron, analysis of the iron concentration in the wild type and the ΔfeoE mutant strains was performed. ΔfeoE mutant cells with the empty vector retained considerably more iron than the ΔfeoE mutant with pBBR1MCS-2::feoE, the wild type with the empty vector, or the wild type with pBBR1MCS-2::feoE. Greater iron retention by the ΔfeoE mutant indicates that the increased sensitivity of the ΔfeoE mutant to Fe2+ (Fig. 3) is due to the inability of the mutant to export excess Fe2+ from the cytoplasm through FeoE.
Previous studies have characterized E. coli FieF as being an Fe2+, Cd2+, and/or Zn2+ exporter (23, 24). FieF and FeoE have a 47.7% amino acid sequence identity, which is below the typical threshold for substrate specificity prediction (34, 35). In order to determine whether FeoE is responsible for the export of any other divalent metals known to be substrates of cation diffusion facilitators, growth curves in LB with or without an excess of Cd2+, Co2+, Cu2+, Mn2+, Ni2+, or Zn2+ were performed for the wild-type and ΔfeoE mutant S. oneidensis strains. No difference in sensitivity to any of these metals was seen between the two strains (Fig. 4; see Table S1 in the supplemental material), indicating that FeoE is specific for the export of Fe2+.
In order to compare the functionality of S. oneidensis FeoE to that of E. coli FieF, the growth rates of the respective mutants and complemented strains of each species were compared. The mutant strains complemented with pBBR1MCS-2::feoE and pBBR1MCS-2::fieF had similar growth rates at an Fe2+ concentration of 1.0 or 2.5 mM (Fig. 5), but a difference in the ability of each strain to resist Fe2+ toxicity emerged at higher concentrations (Fig. 5; see also Fig. S2 in the supplemental material). The strains carrying fieF had lower growth rates in the presence of higher Fe2+ concentrations, suggesting that FeoE activity results in more effective Fe2+ export. The vector and expression strategies used for fieF and feoE complementation were identical; therefore, expression levels should not influence the activity differences observed in vivo.
To determine a potential explanation for the difference in transport specificity and efficiency between FeoE and FieF, we compared the protein sequences of each. Structural studies of FieF have determined that metal binding sites A and B are responsible for Zn2+ transport, while binding site C is important for the structural integrity of the homodimer (25, 36). Despite an amino acid sequence identity between FeoE and FieF of 47.7%, the key metal-coordinating residues in the three metal binding sites, as well as those responsible for salt bridge formation (36), were conserved among all 47 E. coli and 29 Shewanella strains investigated (see Table S2 in the supplemental material), aside from one metal-binding residue at site 285 in E. coli M605. However, one difference between FieF and FeoE was found in a residue at the cytoplasmic end of transmembrane helix 2 (TM2). In all E. coli strains investigated, this residue is a glutamine at position 65, which forms a hydrogen bond with a zinc-coordinating histidine at position 75 in binding site B (36). The genomes of nearly all Shewanella spp. investigated encode a valine in place of a glutamine at this position. Shewanella denitrificans and Shewanella amazonensis encode an alanine and a threonine at this position, respectively. The replacement of glutamine, a polar residue, with a hydrophobic one, such as valine, could alter the conformation of binding site B, thereby changing the metal coordination geometry. Additionally, two residues important for dimerization in E. coli, an aspartic acid at position 69 and a serine at position 70, also located at the base of TM2 (36), are poorly conserved among the shewanellae. Any of these three substitutions could also affect the orientation of TM2 and therefore change the coordination geometry of metal binding site A. Alternatively, one or more of these substitutions could influence the hinge architecture at the base of TM2, affecting the conformational change that occurs to facilitate cation exchange (25). Currently there is no high-resolution structural information available for FeoE. Further investigation would be needed to determine if these residues affect transport specificity and efficiency.
The difference in Fe2+ transport efficiency between FieF and FeoE should not be surprising, considering the environmental conditions in which their respective species are found: the primary environmental niche of E. coli is the mammalian and avian intestinal tract (37, 38), where microorganisms must frequently scavenge for adequate iron rather than mitigate the toxic effects of high iron concentrations (39). Meanwhile, Shewanella spp. thrive in the oxic/anoxic transition zones of sediments, often rich in iron and manganese cycling between their oxidized and reduced states (1, 40), causing continual shifting between aerobic and anaerobic respiratory strategies in the cells. Retaining a low concentration of intracellular iron would become especially important upon a return to oxygen respiration, in order to minimize the production of damaging reactive oxygen species. S. oneidensis has therefore likely evolved a greater Fe2+ export efficiency by FeoE, affording it better survival in iron-rich, redox-active environments.
Supplementary Material
ACKNOWLEDGMENTS
We thank Rick A. Knurr in the Department of Earth Sciences at the University of Minnesota for ICP-MS analysis.
This work was supported by the Office of Naval Research (N000141310552). B.D.B. was supported by the University of Minnesota Biotechnology Training Grant Program through the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02835-15.
REFERENCES
- 1.Nealson KH, Scott J. 2006. Ecophysiology of the genus Shewanella, p 1133–1151. In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (ed), The prokaryotes, 3rd ed Springer Science, New York, NY. [Google Scholar]
- 2.Gralnick JA, Hau HH. 2007. Ecology and biotechnology of the genus Shewanella. Annu Rev Microbiol 61:237–258. doi: 10.1146/annurev.micro.61.080706.093257. [DOI] [PubMed] [Google Scholar]
- 3.Myers CR, Nealson KH. 1988. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240:1319–1321. doi: 10.1126/science.240.4857.1319. [DOI] [PubMed] [Google Scholar]
- 4.Myers JM, Myers CR. 2001. Role for outer membrane cytochromes OmcA and OmcB of Shewanella putrefaciens MR-1 in reduction of manganese dioxide. Appl Environ Microbiol 67:260–269. doi: 10.1128/AEM.67.1.260-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samuelsson MO. 1985. Dissimilatory nitrate reduction to nitrate, nitrous oxide, and ammonium by Pseudomonas putrefaciens. Appl Environ Microbiol 50:812–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shirodkar S, Reed S, Romine M, Saffarini D. 2011. The octahaem SirA catalyses dissimilatory sulfite reduction in Shewanella oneidensis MR-1. Environ Microbiol 13:108–115. doi: 10.1111/j.1462-2920.2010.02313.x. [DOI] [PubMed] [Google Scholar]
- 7.Kostka JE, Nealson KH. 1995. Dissolution and reduction of magnetite by bacteria. Environ Sci Technol 29:2535–2540. doi: 10.1021/es00010a012. [DOI] [PubMed] [Google Scholar]
- 8.Canfield DE. 1989. Reactive iron in marine sediments. Geochim Cosmochim Acta 53:619–632. doi: 10.1016/0016-7037(89)90005-7. [DOI] [PubMed] [Google Scholar]
- 9.O'Reilly SE, Watkins J, Furukawa Y. 2005. Secondary mineral formation associated with respiration of nontronite, NAu-1 by iron reducing bacteria. Geochem Trans 6:67–76. doi: 10.1186/1467-4866-6-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blöthe M, Roden EE. 2009. Microbial iron redox cycling in a circumneutral-pH groundwater seep. Appl Environ Microbiol 75:468–473. doi: 10.1128/AEM.01817-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kammler M, Schön C, Hantke K. 1993. Characterization of the ferrous iron uptake system of Escherichia coli. J Bacteriol 175:6212–6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imlay JA, Chin SM, Linn S. 1988. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 240:640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 13.Carlson HK, Clark IC, Melnyk RA, Coates JD. 2012. Toward a mechanistic understanding of anaerobic nitrate-dependent iron oxidation: balancing electron uptake and detoxification. Front Microbiol 3:57. doi: 10.3389/fmicb.2012.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunning JC, Ma Y, Marquis RE. 1998. Anaerobic killing of oral streptococci by reduced, transition metal cations. Appl Environ Microbiol 64:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin J, Guffanti AA, Bechhofer DH, Krulwich TA. 2002. Tet(L) and Tet(K) tetracycline-divalent metal/H+ antiporters: characterization of multiple catalytic modes and a mutagenesis approach to differences in their efflux substrate and coupling ion preferences. J Bacteriol 184:4722–4732. doi: 10.1128/JB.184.17.4722-4732.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frawley ER, Crouch M-LV, Bingham-Ramos LK, Robbins HF, Wang W, Wright GD, Fang FC. 2013. Iron and citrate export by a major facilitator superfamily pump regulates metabolism and stress resistance in Salmonella Typhimurium. Proc Natl Acad Sci U S A 110:12054–12059. doi: 10.1073/pnas.1218274110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nucifora G, Chu L, Misra TK, Silver S. 1989. Cadmium resistance from Staphylococcus aureus plasmid, p1258 cadA results from a cadmium-efflux ATPase. Proc Natl Acad Sci U S A 86:3544–3548. doi: 10.1073/pnas.86.10.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai KJ, Yoon KP, Lynn AR. 1992. ATP-dependent cadmium transport by the cadA cadmium resistance determinant in everted membrane vesicles of Bacillus subtilis. J Bacteriol 174:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan B, Rosen BP. 2002. Biochemical characterization of CopA, the Escherichia coli Cu(I)-translocating P-type ATPase. J Biol Chem 277:46987–46992. doi: 10.1074/jbc.M208490200. [DOI] [PubMed] [Google Scholar]
- 20.Nies DH, Silver S. 1995. Ion efflux systems involved in bacterial metal resistances. J Ind Microbiol 14:186–199. doi: 10.1007/BF01569902. [DOI] [PubMed] [Google Scholar]
- 21.Paulsen IT, Saier MH. 1997. A novel family of ubiquitous heavy metal ion transport proteins. J Membr Biol 156:99–103. doi: 10.1007/s002329900192. [DOI] [PubMed] [Google Scholar]
- 22.Chao Y, Fu D. 2004. Thermodynamic studies of the mechanism of metal binding to the Escherichia coli zinc transporter YiiP. J Biol Chem 279:17173–17180. doi: 10.1074/jbc.M400208200. [DOI] [PubMed] [Google Scholar]
- 23.Wei Y, Fu D. 2005. Selective metal binding to a membrane-embedded aspartate in the Escherichia coli metal transporter YiiP (FieF). J Biol Chem 280:33716–33724. doi: 10.1074/jbc.M506107200. [DOI] [PubMed] [Google Scholar]
- 24.Grass G, Otto M, Fricke B, Haney CJ, Rensing C, Nies DH, Munkelt D. 2005. FieF (YiiP) from Escherichia coli mediates decreased cellular accumulation of iron and relieves iron stress. Arch Microbiol 183:9–18. doi: 10.1007/s00203-004-0739-4. [DOI] [PubMed] [Google Scholar]
- 25.Coudray N, Valvo S, Hu M, Lasala R, Kim C, Vink M, Zhou M, Provasi D, Filizola M, Tao J, Fang J, Penczek PA, Ubarretxena-Belandia I, Stokes DL. 2013. Inward-facing conformation of the zinc transporter YiiP revealed by cryoelectron microscopy. Proc Natl Acad Sci U S A 110:2140–2145. doi: 10.1073/pnas.1215455110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saltikov CW, Newman DK. 2003. Genetic identification of a respiratory arsenate reductase. Proc Natl Acad Sci U S A 100:10983–10988. doi: 10.1073/pnas.1834303100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hau HH, Gilbert A, Coursolle D, Gralnick JA. 2008. Mechanism and consequences of anaerobic respiration of cobalt by Shewanella oneidensis strain MR-1. Appl Environ Microbiol 74:6880–6886. doi: 10.1128/AEM.00840-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS. 1979. Methanogens: reevaluation of a unique biological group. Microbiol Rev 43:260–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR. 2008. Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci U S A 105:3968–3973. doi: 10.1073/pnas.0710525105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 31.Coursolle D, Gralnick JA. 2010. Modularity of the Mtr respiratory pathway of Shewanella oneidensis strain MR-1. Mol Microbiol 77:995–1008. doi: 10.1111/j.1365-2958.2010.07266.x. [DOI] [PubMed] [Google Scholar]
- 32.Stookey LL. 1970. Ferrozine—a new spectrophotometric reagent for iron. Anal Chem 42:779–781. doi: 10.1021/ac60289a016. [DOI] [Google Scholar]
- 33.Cubillas C, Vinuesa P, Tabche ML, Garcia-de los Santos A. 2013. Phylogenomic analysis of cation diffusion facilitator proteins uncovers Ni2+/Co2+ transporters. Metallomics 5:1634–1643. doi: 10.1039/c3mt00204g. [DOI] [PubMed] [Google Scholar]
- 34.Tian W, Skolnick J. 2003. How well is enzyme function conserved as a function of pairwise sequence identity? J Mol Biol 333:863–882. doi: 10.1016/j.jmb.2003.08.057. [DOI] [PubMed] [Google Scholar]
- 35.Rost B. 2002. Enzyme function less conserved than anticipated. J Mol Biol 318:595–608. doi: 10.1016/S0022-2836(02)00016-5. [DOI] [PubMed] [Google Scholar]
- 36.Lu M, Chai J, Fu D. 2009. Structural basis for auto-regulation of the zinc transporter YiiP. Nat Struct Mol Biol 16:1063–1067. doi: 10.1038/nsmb.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon DM, Cowling A. 2003. The distribution and genetic structure of Escherichia coli in Australian vertebrates: host and geographic effects. Microbiology 149:3575–3586. doi: 10.1099/mic.0.26486-0. [DOI] [PubMed] [Google Scholar]
- 38.Ishii S, Hansen DL, Hicks RE, Sadowsky MJ. 2007. Beach sand and sediments are temporal sinks and sources of Escherichia coli in Lake Superior. Environ Sci Technol 41:2203–2209. doi: 10.1021/es0623156. [DOI] [PubMed] [Google Scholar]
- 39.Raymond KN, Dertz EA, Kim SS. 2003. Enterobactin: an archetype for microbial iron transport. Proc Natl Acad Sci U S A 100:3584–3588. doi: 10.1073/pnas.0630018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DiChristina TJ, DeLong EF. 1993. Design and application of rRNA-targeted oligonucleotide probes for the dissimilatory iron- and manganese-reducing bacterium Shewanella putrefaciens. Appl Environ Microbiol 59:4152–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.