Abstract
Rhodococcus erythropolis BG43 is able to degrade the Pseudomonas aeruginosa quorum sensing signal molecules PQS (Pseudomonas quinolone signal) [2-heptyl-3-hydroxy-4(1H)-quinolone] and HHQ [2-heptyl-4(1H)-quinolone] to anthranilic acid. Based on the hypothesis that degradation of HHQ might involve hydroxylation to PQS followed by dioxygenolytic cleavage of the heterocyclic ring and hydrolysis of the resulting N-octanoylanthranilate, the genome was searched for corresponding candidate genes. Two gene clusters, aqdA1B1C1 and aqdA2B2C2, each predicted to code for a hydrolase, a flavin monooxygenase, and a dioxygenase related to 1H-3-hydroxy-4-oxoquinaldine 2,4-dioxygenase, were identified on circular plasmid pRLCBG43 of strain BG43. Transcription of all genes was upregulated by PQS, suggesting that both gene clusters code for alkylquinolone-specific catabolic enzymes. An aqdR gene encoding a putative transcriptional regulator, which was also inducible by PQS, is located adjacent to the aqdA2B2C2 cluster. Expression of aqdA2B2C2 in Escherichia coli conferred the ability to degrade HHQ and PQS to anthranilic acid; however, for E. coli transformed with aqdA1B1C1, only PQS degradation was observed. Purification of the recombinant AqdC1 protein verified that it catalyzes the cleavage of PQS to form N-octanoylanthranilic acid and carbon monoxide and revealed apparent Km and kcat values for PQS of ∼27 μM and 21 s−1, respectively. Heterologous expression of the PQS dioxygenase gene aqdC1 or aqdC2 in P. aeruginosa PAO1 quenched the production of the virulence factors pyocyanin and rhamnolipid and reduced the synthesis of the siderophore pyoverdine. Thus, the toolbox of quorum-quenching enzymes is expanded by new PQS dioxygenases.
INTRODUCTION
A wide variety of bacteria employ quorum sensing (QS) systems to communicate and coordinate their behavior according to their cell density by sensing self-generated small signal molecules (1). QS systems allow bacteria to act cooperatively in processes such as biofilm formation or pathogenesis. The opportunistic pathogen Pseudomonas aeruginosa uses a complex QS network comprising several interconnected signaling circuits to regulate group motility, biofilm maturation, and a battery of virulence factors (for a recent review, see reference 2). The Las and Rhl circuits use the signal molecules N-3-oxo-dodecanoyl homoserine lactone (3OC12-HSL) and N-butanoyl homoserine lactone (C4-HSL), respectively, whereas the Pqs circuit employs the alkylquinolone (AQ) signals 2-heptyl-3-hydroxy-4(1H)-quinolone (Pseudomonas quinolone signal [PQS]) and 2-heptyl-4(1H)-quinolone (HHQ), which act as coinducers of the transcriptional regulator PqsR (3–6). The PqsR-AQ complex mainly activates the transcription of the pqsABCDE operon coding for the enzymes of AQ biosynthesis (7, 8); moreover, PqsE, via unknown mechanisms, modulates the expression of large arrays of target genes (9–11). PqsE was termed the “PQS response protein,” because its disruption negatively affected the production of PQS-mediated exoproducts (3, 9, 12, 13). AQ signaling is involved in biofilm development and the regulation of many virulence factors, including the synthesis of LasB elastase, siderophores, rhamnolipid biosurfactants, the redox-active phenazine pigment pyocyanin, and the cytotoxic lectin and adhesin LecA (reviewed in references 14 and 15). Moreover, PQS induces membrane vesicle formation (16), acts as a ferric iron chelator (4, 17) and prooxidant (18), and exerts host immune-modulatory and proapoptotic activities (19–22).
Antimicrobials aimed at inhibiting virulence (rather than acting in a bactericidal or bacteriostatic manner) are thought to impose less selective pressure for the development of resistance than current antibiotics. Approaches for targeting virulence include inhibition of bacterial adhesion to host cells, inhibition of toxin delivery or toxin function, and disruption of regulatory mechanisms, such as QS circuits, that control virulence gene expression (23). QS interference can be achieved by inhibition of either signal molecule synthesis or signal perception or by inactivation of the signals by enzymatic degradation or modification. N-Acyl homoserine lactone (AHL) lactonases or acylases, when expressed in P. aeruginosa PAO1, were indeed observed to affect motility, biofilm formation, and the production of virulence factors (24–29) and to improve the survival rate in a mouse model of acute pneumonia (30). In an acute lethal model of P. aeruginosa pneumonia in rats, early intratracheal treatment with the engineered thermostable Sulfolobus solfataricus AHL lactonase SsoPox-I significantly reduced lung damage and mortality (31).
Whereas AHL signals are used by many Gram-negative bacteria, AQ signaling appears to be restricted to comparatively few species, such as Burkholderia pseudomallei, which mainly uses 3-methylated AQs (32, 33), and P. aeruginosa. PQS signaling has so far been observed only for the P. aeruginosa QS system and therefore constitutes an attractive target to specifically disarm this pathogen. So far, only one enzyme was described to catalyze the inactivation of PQS (34). The natural substrate of this enzyme, a dioxygenase termed Hod from Arthrobacter sp. strain Rue61a, is 3-hydroxy-2-methyl-4(1H)-quinolone, an intermediate in the degradation of 2-methylquinoline which is utilized as a carbon source by this bacterial strain (35, 36). Hod prefers substrates with short-chain alkyl substituents, and the activity of Hod towards PQS is very low and considered fortuitous (32).
Recently, we isolated Rhodococcus erythropolis strain BG43, which is capable of degrading HHQ and PQS to anthranilic acid (37, 38). In the present study, we identified two sets of PQS-inducible genes located on a plasmid of strain BG43, which code for the enzymes of the degradation pathway. Moreover, we demonstrate that the expression of genes coding for PQS-cleaving dioxygenases in P. aeruginosa quenches the production of the virulence factors pyocyanin, rhamnolipid, and pyoverdine.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
All bacterial strains and plasmids are listed in Table 1. R. erythropolis BG43 (DSM 46869) as well as recombinant P. aeruginosa PAO1 strains were cultivated in lysogeny broth (LB) (41) at 30°C and 37°C, respectively. Recombinant P. aeruginosa PAO1 strains were grown in the presence of 400 μg/ml carbenicillin. Recombinant Escherichia coli strains were cultivated in either KG medium, modified as described previously (42), or LB, both supplemented with 100 μg/ml ampicillin.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Reference(s) or source |
|---|---|---|
| Strains | ||
| R. erythropolis BG43 DSM 46869 | AQ degrader | 37, 38 |
| E. coli DH5α | F− endAI hsdRJ7(rK− mK+) supE44 thi-1 λ− recA1 gyrA96 relA1 deoR Δ(lacZYA-argF)U169 ϕ80lacZΔM15 | 39 |
| E. coli BL21(DE3) | F− ompT gal dcm lon hsdSB(rB− mB−) λ(DE3[lacI lacUV5-T7 gene 1 ind1 sam7 nin5]) | 40 |
| P. aeruginosa PAO1 | Wild type, Nottingham strain | Holloway collection |
| Plasmids | ||
| pET22b(+) | Expression plasmid, T7 promoter; Ampr | Novagen |
| pET22-aqdC1 | aqdC1 gene inserted into the NdeI/XhoI site of pET22b(+) | This study |
| pET22-aqdA1B1C1 | aqdA1B1C1 gene cluster inserted into the NdeI/XhoI site of pET22b(+) | This study |
| pET22-aqdA2B2C2 | aqdA2B2C2 gene cluster inserted into the NdeI/XhoI site of pET22b(+) | This study |
| pME6032(Ap) | pVS1-p15A shuttle plasmid, lacIq-Ptac expression vector; Ampr | M. Fletcher, unpublished data |
| pME-aqdC1 | aqdC1 gene inserted into the SacI/XhoI site of pME6032(Ap) | This study |
| pME-aqdC2 | aqdC2 gene inserted into the EcoRI/XhoI site of pME6032(Ap) | This study |
Phylogenetic analysis.
Protein alignment was carried out with the BLASTP 2.2.31 program (43). A phylogenetic tree was generated from protein sequences of AqdC1, AqdC2, and representative (putative) dioxygenases of the α/β-hydrolase fold family by using the Fast Minimum Evolution algorithm (44).
DNA techniques.
Plasmid isolation, PCR purification, and gel extraction were carried out with the innuPREP plasmid minikit, innuPREP DOUBLEpure kit, and innuPREP Gelextraction kit (Analytik Jena) according to the manufacturer's instructions. Genomic DNA of R. erythropolis BG43 was extracted with the innuSPEED Bacteria/Fungi DNA kit (Analytik Jena). Q5 polymerase (NEB) and GoTaq G2 polymerase (Promega Corporation) were used for PCR. Competent E. coli DH5α and P. aeruginosa PAO1 cells were prepared as described previously by Hanahan (45) and Choi et al. (46), respectively. P. aeruginosa PAO1 was transformed by electroporation as described previously by Iwasaki et al. (47), and cell suspensions were regenerated in SB medium (48) for 2 h at 37°C with shaking. Standard protocols (41) were applied for agarose gel electrophoresis, DNA restriction digestion, and ligation. Restriction enzymes were purchased from Thermo Scientific. Oligonucleotide synthesis was carried out by Eurofins MWG Operon, and sequencing was performed by GATC Biotech.
RNA isolation and reverse transcriptase PCR.
R. erythropolis BG43 was grown in LB for 24 h. A total of 20 μM PQS or an appropriate amount of methanol as a control was added 2 h before harvesting of the cells by centrifugation. Cell pellets were washed in 5 ml ice-cold TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]) and stored at −80°C. For RNA isolation, pellets were thawed and resuspended in 1 ml ice-cold TE buffer supplemented with 10 mg/ml lysozyme, and cells were disrupted by using 300-mg glass beads (diameter, 150 to 212 μm; Sigma-Aldrich) in a Mikro-Dismembrator S instrument (Sartorius) at 2,900 rpm for 5 min. Afterwards, RNA was isolated with the innuPREP RNA minikit (Analytik Jena) according to the manufacturer's instructions. The remaining DNA impurities were digested with RNase-free DNase I in the presence of the RiboLock inhibitor (Thermo Scientific). Subsequently, RNA was purified again by using the same kit. Synthesis of first-strand DNA was carried out at 43°C with 1 μg RNA and a random hexamer primer with the RevertAid H Minus first-strand cDNA synthesis kit (Thermo Scientific). Primers for quantitative reverse transcriptase PCR (qRT-PCR) analysis were designed with Primer Express 3.0 (Applied Biosystems) and exhibited melting temperatures of 62°C ± 1°C. The amplified DNA fragments had a size of 55 to 60 bp. Power SYBR green PCR master mix (Applied Biosystems) was used for qRT-PCR on a StepOne real-time PCR system (Applied Biosystems). Each reaction mixture (20 μl) contained 200 ng cDNA and 250 nM each primer. The following PCR conditions were applied: an initial step at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. Melting curve analysis was performed at 95°C for 15 s, 60°C for 1 min, and 95°C for 15 s. Data were analyzed by the comparative threshold cycle (CT) method (49), with gyrB as the internal control gene. The fold change in gene expression, 2−ΔΔCT, is defined as follows: 2−ΔΔCT = , where sample A is from PQS-treated cells and sample B is the untreated control.
Cloning of aqdC1, aqdA1B1C1, and aqdA2B2C2 in E. coli.
To construct the expression plasmids pET22-aqdC1, pET22-aqdA1B1C1, and pET22-aqdA2B2C2, aqdC1 and both gene clusters were amplified from genomic DNA of R. erythropolis BG43 by using primer pairs aqdC1-Nde-for/aqdC1-Xho-rev, aqdA1-Nde-for/aqdC1-Xho-rev, and aqdA2-Nde-for/aqdC2-Xho-rev, respectively (Table 2). The digested PCR products were ligated into the NdeI/XhoI sites of pET22b(+), and E. coli DH5α was transformed with the constructs. After verification of the gene sequences, E. coli BL21(DE3) was transformed with pET22-aqdC1, pET22-aqdA1B1C1, and pET22-aqdA2B2C2.
TABLE 2.
Primers used in this study
| Primer designation | Sequence (5′→3′) | Application |
|---|---|---|
| aqdA1-qRT-for | CCGACGGTGCTGTTTTCG | qRT-PCR |
| aqdA1-qRT-rev | ATGCTGTGCTCGGGAGTCA | |
| aqdB1-qRT-for | TCGACGAGTACGTGACGTTCTACT | qRT-PCR |
| aqdB1-qRT-rev | CGCTGCACGGAGTGTACAGA | |
| aqdC1-qRT-for | TGGAGTCCCGGGTCGAA | qRT-PCR |
| aqdC1-qRT-rev | CGACCGTGCCCACATGT | |
| aqdR-qRT-for | GCATCGCGACCTTGAGATTT | qRT-PCR |
| aqdR-qRT-rev | CGGCGTTTGTGCGAATTT | |
| aqdA2-qRT-for | TCCCGAGCAATCATTCAAAGT | qRT-PCR |
| aqdA2-qRT-rev | GCCTGCTCAACGCTATGGA | |
| aqdB2-qRT-for | ATGGAACTGTCCGAGGCATT | qRT-PCR |
| aqdB2-qRT-rev | TCGATCCCGGGTCGTGTA | |
| aqdC2-qRT-for | CTTCGTTCCCGTTGCACAT | qRT-PCR |
| aqdC2-qRT-rev | AGCTCGTCGGCAAGTTGAA | |
| gyrB-qRT-for | AGGTCGCAATGCAGTGGAA | qRT-PCR |
| gyrB-qRT-rev | TGATCGTGTTGGCGAAGGT | |
| aqdC1-Sac-for | GCTAGAGCTCATGCACCATCACCATCACCATAATATGCCTCAGCTATCGACGATCC | Amplification of aqdC1 |
| aqdC1-Xho-rev | TATACTCGAGTCATCGCATCGCTCCTGCCTGG | |
| aqdC2-Eco-for | TATAGAATTCATGCATCACCATCACCATCACACCGCATTGATGACGCTCAATGG | Amplification of aqdC2 |
| aqdC2-Xho-rev | GCTACTCGAGTCATGTGGACTCGTTTAC | |
| aqdC1-Nde-for | GCGCCATATGCACCATCACCATCACCATAATATGCCTCAGCTATCGACGATCC | Amplification of aqdC1 with aqdC1-Xho-rev |
| aqdA1-Nde-for | GCTACATATGACGGCAAACGGTGACGTCCG | Amplification of aqdA1B1C1 with aqdC1-Xho-rev |
| aqdA2-Nde-for | GCTACATATGTTCCAAACTGTGACGGC | Amplification of aqdA2B2C2 with aqdC2-Xho-rev |
AQ conversion by recombinant E. coli BL21(DE3) strains.
E. coli BL21(DE3)[pET22-aqdA1B1C1], E. coli BL21(DE3)[pET22-aqdA2B2C2], and E. coli BL21(DE3)[pET22b(+)] were grown in LB with 100 μg/ml ampicillin at 37°C to an optical density at 600 nm (OD600) of 0.5 before gene expression was induced with 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and cultures were grown for a further 20 h at 30°C. Cells were pelleted by centrifugation (5,000 × g for 10 min at 4°C), washed twice with phosphate-buffered saline (PBS), resuspended in modified KG medium (42), and diluted to an OD600 of 2.5. After the addition of 0.5 mM IPTG, 100 μg/ml ampicillin, and 20 μM PQS or HHQ, cultures were incubated at 30°C with vigorous shaking. Samples were taken at different time points, and AQs were extracted three times with ethyl acetate (acidified with 1 ml/liter acetic acid). The organic phases of each sample were combined, dried to completion, and redissolved in 100% methanol.
Cloning and heterologous expression of aqdC1 and aqdC2 in P. aeruginosa.
For cloning of the aqdC1 and aqdC2 genes in pME6032(Ap), the genes were amplified by PCR using primer sets aqdC1-Sac-for/aqdC1-Xho-rev and aqdC2-Eco-for/aqdC2-Xho-rev (Table 2), introducing the sequence of a hexahistidine tag. The PCR products were digested with either SacI and XhoI (aqdC1) or EcoRI and XhoI (aqdC2) and ligated into appropriately digested pME6032(Ap). The resulting plasmids, pME-aqdC1 and pME-aqdC2, were established in E. coli DH5α and sequenced. Subsequently, P. aeruginosa PAO1 was transformed with the plasmids. Overnight-grown cultures of P. aeruginosa PAO1 harboring either pME-aqdC1, pME-aqdC2, or pME6032(Ap), as an empty vector control, were used to inoculate 50 ml LB medium supplemented with 400 μg/ml carbenicillin to an OD600 of 0.05 and cultivated at 37°C for 2 h before gene transcription was induced by the addition of 0.5 mM IPTG. Culture samples were taken at 8 h and 24 h postinduction (p.i.), and their OD600 was measured. Samples were either extracted with acidified ethyl acetate for quantification of PQS concentrations or centrifuged for determination of the levels of extracellular virulence factors in the supernatants.
Preparation of cell extracts.
Cells of recombinant E. coli and P. aeruginosa strains, grown in LB, were harvested 20 h (E. coli) and 8 h (P. aeruginosa) after the induction of gene expression by centrifugation at 9,000 × g for 20 min at 4°C. Cell pellets were resuspended in 50 mM potassium phosphate buffer (pH 7.5), disrupted by sonication at 4°C, and centrifuged at 20,000 × g at 4°C for 30 min. The supernatants (termed crude extracts) were used for AQ conversion and enzyme assays.
Protein analysis and enzyme assays.
Protein concentrations were estimated by using the method of Zor and Selinger (50). Proteins separated in denaturing polyacrylamide gels were transferred onto a polyvinylidene fluoride membrane by electroblotting. Immunodetection of His6-tagged AqdC1 and AqdC2 was performed with the SuperSignal Western blot enhancer (Thermo Scientific) according to the manufacturer's instructions, and relative amounts of AqdC proteins on Western blots were quantified by using ImageJ 1.48v software. PQS dioxygenase activity was determined spectrophotometrically at 30°C, measuring PQS at 337 nm. The assay buffer (50 mM potassium phosphate, pH 7.5) was supplemented with 50 mM arginine, 50 mM glutamate, and 10% dimethyl sulfoxide (DMSO) in order to increase the solubility of PQS, which was added to a concentration of up to 20 μM. The molar extinction coefficient of PQS at 337 nm in this assay solution is 9.28 × 103 M−1 cm−1. The enzyme-catalyzed formation of carbon monoxide from PQS was detected spectrophotometrically by using a hemoglobin assay described previously by Klendshoj et al. (51).
AQ conversion by crude extracts of recombinant E. coli BL21(DE3) strains.
Crude extracts of E. coli BL21(DE3) containing pET22-aqdA1B1C1, pET22-aqdA2B2C2, or pET22b(+) were applied to Zeba Spin desalting columns (7,000-molecular-weight cutoff; Thermo Scientific) to remove salts and small molecules. The protein concentrations were set to 1 mg/ml, and either 20 μM PQS or 20 μM HHQ with 500 μM NADH was added to the extracts. The samples were incubated at 30°C at 1,000 rpm, and extraction of AQs was carried out with ethyl acetate (acidified with 1 ml/liter acetic acid) at different time points. Organic phases were dried to completion, redissolved in methanol, and analyzed by high-performance liquid chromatography (HPLC).
Determination of P. aeruginosa virulence factors.
Rhamnolipid levels were measured by using the orcinol method, as described previously by Wilhelm et al. (52), using rhamnose standards with defined concentrations and assuming that 1 μg rhamnose corresponds to 2.5 μg rhamnolipid (53). Pyocyanin content was quantified spectrophotometrically at 520 nm after extraction, as described previously by Essar et al. (54). The siderophore pyoverdine was determined by measuring the absorption of supernatants at 405 nm. Elastolytic activity in supernatants was measured with the Elastin Congo red assay (55).
Heterologous production and purification of the PQS dioxygenase AqdC1.
Recombinant E. coli BL21(DE3)[pET22-aqdC1] was grown in 20 ml LB with 100 μg/ml ampicillin at 37°C until an OD600 of 3 was reached. The culture was used to inoculate 750 ml medium and was then further cultivated at 30°C. The transcription of aqdC1 was induced with 0.5 mM IPTG at an OD600 of 0.5, and cells were grown at 25°C for an additional 16 h. Cells were harvested by centrifugation and stored at −80°C. Recombinant AqdC1 was purified from cell extract supernatants by affinity chromatography on Ni2+-nitrilotriacetate (Ni-NTA) agarose as described previously for the His6-tagged Hod protein by Beermann et al. (56).
Analytical methods.
For analysis of extracts from PQS conversion, HPLC was performed on a 250- by 4-mm Eurospher II RP-18 column at 35°C by using a linear gradient of 80% (vol/vol) methanol in water to 100% methanol at a flow rate of 0.5 ml/min. Extracts from HHQ conversion were separated with a linear gradient of 15% (vol/vol) methanol in water to 100% methanol within 40 min. Eluents were acidified by the addition of 1 g/liter citric acid. Light absorption spectra of the eluted compounds were recorded with a diode array detector (L-2450 LaChrome Elite; Merck Hitachi). For calibration, PQS, HHQ, and anthranilic acid (all purchased from Sigma-Aldrich) were used as reference compounds.
RESULTS
Genes predicted to be involved in AQ degradation by R. erythropolis BG43 and PQS-dependent transcription.
Based on in vivo and in vitro biotransformation experiments, we previously proposed a pathway for AQ conversion by R. erythropolis BG43 involving HHQ hydroxylation to PQS by a monooxygenase, followed by cleavage of the heterocyclic ring and alkyl chain removal to form anthranilic acid (37). These reactions were tentatively assumed to proceed analogously to steps of the 2-methylquinoline degradation pathway of Arthrobacter sp. Rue61a, which involves the hydroxylation of 2-methyl-4(1H)-quinolone to 3-hydroxy-2-methyl-4(1H)-quinolone, followed by 2,4-dioxygenolytic cleavage of the quinolone ring to carbon monoxide and N-acetylanthranilic acid and amide hydrolysis of the latter to release anthranilic acid. Therefore, the genome of strain BG43 (GenBank accession numbers CP011295.1 to CP011298.1) (38) was screened for gene clusters comprising putative monooxygenase, dioxygenase, and hydrolase genes.
Two gene clusters, each coding for a predicted hydrolase, a flavin-dependent monooxygenase, and an enzyme related to 3-hydroxy-2-methyl-4(1H)-quinolone 2,4-dioxygenase, Hod, of Arthrobacter sp. Rue61a, were identified on circular plasmid pRLCBG43 of strain BG43 (GenBank accession number CP011296.1). The genes, corresponding to the locus tags XU06_RS29630 to XU06_RS29640 and XU06_RS29730 to XU06_RS29740, were termed aqdA1B1C1 and aqdA2B2C2, respectively (aqd for alkylquinolone degradation). A gene coding for a putative transcriptional regulator with a helix-turn-helix HTH_XRE (xenobiotic-responsive element) family-like motif (amino acids [aa] 10 to 48), termed aqdR (XU06_RS29725), is located adjacent to the aqdA2B2C2 cluster (Fig. 1A and B). The qRT-PCR analysis of RNA from R. erythropolis BG43 grown in the presence or absence of PQS revealed that transcript levels of the aqdABC genes were increased between 3-fold and 17-fold if PQS was added to cultures. The inducing effect was more pronounced for aqdA2B2C2 expression than for transcription of the first gene cluster. Expression of aqdR was upregulated 3.1-fold by PQS, supporting a putative regulatory role in AQ catabolism and an autoregulatory function (Fig. 1C).
FIG 1.
Genetic organization and PQS-dependent expression of genes encoding proteins involved in AQ degradation. (A and B) Genetic organization of the aqdA1B1C1 (A) and aqdR-aqdA2B2C2 (B) gene clusters of R. erythropolis BG43. The aqdA1 and aqdA2 genes code for putative hydrolases, aqdB1 and aqdB2 code for putative monooxygenases, aqdC1 and aqdC2 code for putative dioxygenases, and aqdR codes for a predicted transcriptional regulator. (C) Relative expression levels of the aqdA1B1C1, aqdA2B2C2, and aqdR genes determined by qRT-PCR. The mRNA levels of R. erythropolis BG43 cells grown in the absence of PQS (“noninduced cells”) and cells spiked with PQS 2 h before harvesting were analyzed by the comparative CT method (49), with gyrB as an internal control gene. The mRNA levels of noninduced cells were set to 1. Data are from three independent cultures, each analyzed in quadruplicate. The error bars represent standard deviations. For primers, see Table 2.
AqdA1, which has a predicted α/β-hydrolase fold, and AqdA2, a member of the carboxylesterase type B family (COG2272), share only 22% sequence identity and do not exhibit significant similarity to the N-acetylanthranilate amide hydrolase of Arthrobacter sp. Rue61a (GenBank accession number AFR34518.1). In contrast, the sequences of the predicted monooxygenases AqdB1 and AqdB2 are 58% and 44% identical, respectively, to the monooxygenase of the 2-methylquinoline degradation pathway (accession number AFR34516.1). The AqdB1 and AqdB2 sequences show <30% identity to PqsH, which catalyzes the hydroxylation of HHQ to PQS in P. aeruginosa (accession number NP_251277.1). The AqdC1 and AqdC2 proteins, like the dioxygenase Hod, are members of the α/β-hydrolase fold family. The so-called “catalytic triad” of the α/β-hydrolases, consisting of a nucleophilic residue, an acidic residue, and an invariant histidine, is conserved in Hod as well as in AqdC1. However, the conserved serine, which in Hod is important for substrate binding (36, 57), is exchanged to alanine in AqdC2.
The aqd1 and aqd2 gene clusters presumably have different phylogenetic origins. Whereas proteins with significant sequence similarity (>30% identity) to the AqdA1B1C1 proteins can be found among actinobacteria as well as alpha- and gammaproteobacteria, proteins similar to those encoded by the aqd2 cluster are confined mainly to members of the actinobacteria. A dendrogram of AqdC1, AqC2, and other representative putative dioxygenases of the α/β-hydrolase family illustrates the relatedness of AqdC1 to Hod from Arthrobacter sp. Rue61a and to proteins from different proteobacteria and the close clustering of AqdC2 with putative dioxygenases from specific actinobacteria (Fig. 2). Actually, the entire aqdR-aqdA2B2C2 cluster is conserved in Streptomyces bingchenggensis BCW-1 (SBI_08685 to SBI_08688 genes), Mycobacterium fortuitum DSM 46621 (MFORT_16219 to MFORT_16204), Mycobacterium mageritense DSM 44476 (BN978_00714 to BN978_00717), and many strains of Mycobacterium abscessus (MAB_03000c to MAB_0303c) as well as in the clinical isolates Nocardia farcinica IFM 10152 (nfa52220 to nfa52190) and Nocardia cyriacigeorgica GUH-2 (NOCYR_5041 to NOCYR_5044).
FIG 2.
Dendrogram of AqdC1, AqdC2, and similar representative proteins from different organisms, generated with the Fast Minimum Evolution algorithm (44). GenBank accession numbers and the corresponding locus tags are as follows: WP_011555318.1 for Myxococcus xanthus MXAN_RS25980, WP_033653009.1 for Serratia marcescens DH21_RS02255, WP_036842662.1 for Photorhabdus temperata PTE_RS01805, WP_012093671.1 for Ochrobactrum anthropi OANT_RS22650, WP_016491460.1 for Pseudomonas resinovorans PCA10_RS07555, AKE01130.1 for R. erythropolis BG43 AqdC1 XU06_29640, WP_043472130.1 for Arthrobacter sp. Rue61a ARUE_RS22835, AKE01142.2 for R. erythropolis BG43 AqdC2 XU06_29740, WP_043492345.1 for Streptomyces bingchenggensis SBI_RS42735, WP_036434328.1 for Mycobacterium mageritense BN978_RS03495, EJZ13104.1 for Mycobacterium fortuitum MFORT_16204, WP_016343652.1 for Mycobacterium abscessus MASS_RS23870, WP_011211753.1 for Nocardia farcinica NFA_RS25885, and WP_036540657.1 for Nocardia cyriacigeorgica RJ05_RS23465.
Heterologous expression of aqdA1B1C1 and aqdA2B2C2 in E. coli.
To test whether the identified gene clusters are indeed responsible for AQ degradation, aqdA1B1C1 and aqdA2B2C2 were heterologously expressed in E. coli. As illustrated in Fig. 3A and B, cell suspensions of E. coli BL21(DE3)[pET22-aqdA1B1C1] and E. coli BL21(DE3)[pET22-aqdA2B2C2] degraded 20 μM PQS to anthranilic acid within 300 min and 180 min, respectively. The HPLC elution profiles of the culture extracts indicated that small amounts of N-octanoylanthranilic acid are formed transiently (data not shown), supporting our assumption that cleavage and further degradation of PQS proceed analogously to the reactions of the 2-methylquinoline degradation pathway. In crude extracts of the recombinant E. coli strains, specific activities of 29.9 ± 3.0 and 21.4 ± 3.6 nmol min−1 (mg protein)−1 were measured for the PQS-converting enzymes AqdC1 and AqdC2, respectively. Crude extracts of E. coli containing the empty vector pET22b(+) did not transform PQS.
FIG 3.
Degradation of AQs by cell suspensions of recombinant E. coli strains (OD600 of 2.5) harboring aqdABC genes. (A) PQS conversion by E. coli BL21(DE3)[pET22-aqdA1B1C1]; (B) PQS conversion by E. coli BL21(DE3)[pET22-aqdA2B2C2]; (C) HHQ conversion by E. coli BL21(DE3)[pET22-aqdA2B2C2]. Squares, PQS; circles, anthranilic acid; triangles, HHQ. Filled symbols indicate substrates added to cultures, and open symbols indicate intermediates or products formed. Error bars represent standard deviations from three independent experiments. E. coli BL21(DE3) containing the empty pET22b(+) vector did not transform PQS and HHQ (data not shown).
The addition of 20 μM HHQ to cell suspensions of the recombinant E. coli strain expressing aqdA2B2C2 led to the conversion of HHQ to PQS within 30 min, which was then further degraded to anthranilic acid (Fig. 3C). However, E. coli[pET22-aqdA1B1C1] was not able to transform HHQ. It remains unclear so far whether HHQ is not a substrate of the putative monooxygenase AqdB1 or whether the E. coli host is not able to synthesize AqdB1 in its native, catalytically competent form.
Purification and activity of the PQS dioxygenase AqdC1.
AqdC1, purified as a His6-tagged protein from E. coli[pET22b-aqdC1], showed a specific activity of 15.3 ± 1.5 μmol min−1 mg−1 under the conditions of the spectrophotometric enzyme assay. For comparison, Hod from Arthrobacter sp. Rue61a converts PQS with a specific activity of ∼0.2 μmol min−1 mg−1. The activity of AqdC1 toward 3-hydroxy-2-methyl-4(1H)-quinolone, the physiological substrate of Hod, was 1.3 μmol min−1 mg−1, indicating that, contrary to Hod, AqdC1 shows a preference for 2-alkyl-3-hydroxy-4(1H)-quinolones with longer alkyl chains. Consumption of O2 in the AqdC1-catalyzed PQS cleavage reaction was verified by use of a Clark-type oxygen electrode, and release of carbon monoxide was proven by spectrophotometric detection of CO-hemoglobin, supporting the proposed 2,4-dioxygenolytic cleavage. The Km value of AqdC1 for PQS of 27 μM ± 7 μM is in the range of or a bit higher than PQS concentrations reported previously for P. aeruginosa culture supernatants (12, 58). The kcat value of 20.5 ± 1.6 s−1 is within the range observed for many metabolic enzymes (10 s−1 to up to 3,000 s−1) (59) and in the same order of magnitude as those of some other ring cleavage dioxygenases; for example, a kcat of 38 s−1 was determined for the 2,4-dioxygenase Hod and its physiological substrate 3-hydroxy-2-methyl-4(1H)-quinolone (57). Thus, the catalytic competence of AqdC1 should suffice to convert PQS at physiological concentrations. Unfortunately, when producing AqdC2 in E. coli (as a Strep- or His6-tagged protein, as a fusion protein with the maltose binding protein, or by using chaperone-expressing plasmids pBB540 and pBB542 [60] in combination with the His6-AqdC2-encoding plasmid), the cell extracts contained mainly insoluble protein under all conditions tested, precluding a comparative characterization of the two proteins.
Expression of genes coding for PQS dioxygenases in P. aeruginosa PAO1 and quenching of virulence factor production.
The possibility of affecting P. aeruginosa virulence by quenching PQS-dependent quorum sensing was assessed by expression of the aqdC genes in P. aeruginosa PAO1. The growth curves (measurement of OD600) of IPTG-induced as well as noninduced cultures of P. aeruginosa PAO1 containing either pME-aqdC1, pME-aqdC2, or the empty vector pME6032(Ap) were virtually superimposable, indicating that the expression of aqdC does not affect growth (data not shown). PQS concentrations in cultures of P. aeruginosa PAO1[pME-aqdC1] and P. aeruginosa PAO1[pME-aqdC2], determined 8 h after induction of aqdC expression, were decreased 2.6-fold and 5-fold, respectively, compared to PQS levels in P. aeruginosa PAO1[pME6032(Ap)] cultures. Thus, both PQS dioxygenases appear to be functional in P. aeruginosa.
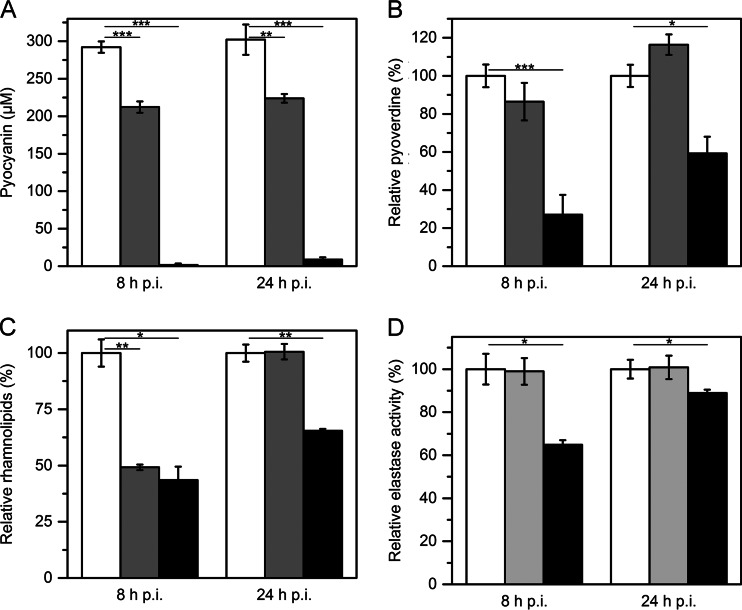
During infection, P. aeruginosa PAO1 produces a variety of virulence factors, like the redox-active phenazine pigment pyocyanin, which is required for full virulence (61). Besides generating reactive oxygen species and interfering with multiple functions of eukaryotic cells, pyocyanin acts as another signaling molecule of P. aeruginosa, controlling a small number of genes during stationary phase, including the efflux pump genes mexGHI-opmD (62). Expression of aqdC1 in P. aeruginosa PAO1 led to 28% and 26% reductions of pyocyanin concentrations in supernatants 8 h and 24 h after induction of aqdC1 expression, respectively, whereas the production of pyocyanin was nearly abolished in cultures expressing the second PQS dioxygenase gene aqdC2 (Fig. 4A).
FIG 4.
Presence of virulence factors in P. aeruginosa cultures expressing either aqdC1 or aqdC2 8 and 24 h after induction of gene expression. White, gray, and black bars indicate levels of the respective factors in P. aeruginosa PAO1[pME6032(Ap)], P. aeruginosa PAO1[pME-aqdC1], and P. aeruginosa PAO1[pME-aqdC2], respectively. Error bars indicate standard deviations from three independent experiments. Statistical analyses were performed with Student's t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Pyoverdine is the major siderophore secreted by P. aeruginosa under conditions of iron limitation. It is also part of a signaling cascade influencing the production of the virulence factor exotoxin A, the PrpL endoprotease, and pyoverdine itself (63). Determination of the levels of this siderophore showed that 8 and 24 h after induction of aqdC2 expression, pyoverdine contents were reduced by 73% and 41%, respectively. In contrast, the presence of AqdC1 did not significantly affect the pyoverdine amount in the cultures (Fig. 4B).
Rhamnolipids of P. aeruginosa act as biosurfactants, enhance the solubility of PQS in aqueous solutions (64), and also modulate the swarming motility of P. aeruginosa (65). Furthermore, rhamnolipids are considered to impair normal tracheal ciliary function (66). The presence of AqdC1 led to a 51% reduction of the rhamnolipid content 8 h after induction of gene expression, whereas after 24 h, there were no differences noticed between the empty vector control and the aqdC1-expressing P. aeruginosa strain. However, in P. aeruginosa harboring pME-aqdC2, rhamnolipids were reduced to 43% (8 h p.i.) and 65% (24 h p.i.) of the levels observed for the empty vector control (Fig. 4C).
The elastase LasB, a major virulence factor, causes tissue damage and degrades several substrates, like elastin, immunoglobulins, or cytokines (67). The activity of extracellular elastase was affected only in P. aeruginosa PAO1[pME-aqdC2] (reduction by 35% at 8 h p.i. in comparison to the control) but not in cultures expressing aqdC1 (Fig. 4D).
Since the expression of aqdC1 in P. aeruginosa PAO1 had less impact on the production of all tested virulence factors than did the expression of aqdC2, synthesis of AqdC proteins in the recombinant P. aeruginosa strains was checked by disrupting cell samples (set to the same OD600), separation of the resulting extracts by gel electrophoresis, and subsequent immunodetection of the His6-tagged AqdC proteins by Western blotting. The protein intensities suggested that extracts of cells grown at 37°C, harvested 8 h and 24 h after the induction of gene expression, contained ∼1.7-fold and 6.1-fold less AqdC1, respectively, than did the corresponding extracts from cells grown at 30°C. In contrast, the intensities of the AqdC2 protein on the Western blots were very similar, irrespective of the growth temperature. The PQS dioxygenolytic activity of crude extracts of P. aeruginosa PAO1[pME-aqdC1] grown at 37°C and harvested at 8 h p.i. was 0.13 ± 0.01 μmol min−1 mg−1, while extracts from cells cultivated at 30°C showed an ∼1.7-fold-higher activity. In comparison, the PQS dioxygenase activity in crude extracts of P. aeruginosa expressing the aqdC2 gene was ∼0.1 μmol min−1 mg−1, irrespective of the cultivation temperature. Incubation of purified AqdC1 protein at 37°C for 1 h led to a 2-fold decrease of enzymatic activity, supporting the notion that AqdC1 is instable at elevated temperatures. This may restrict its quenching effect in P. aeruginosa, which was grown at 37°C for analysis of virulence factor production.
DISCUSSION
R. erythropolis BG43 is able to degrade the quorum sensing signal molecules HHQ and PQS from P. aeruginosa (37). Genome sequence analysis revealed that circular plasmid pRLCBG43 of strain BG43 harbors two sets of genes, aqdA1B1C1 and aqdA2B2C2, possibly coding for enzymes mediating HHQ hydroxylation to PQS and the degradation of PQS to anthranilate. The complete aqdR-aqdA2B2C2 region is conserved in several Mycobacterium and Nocardia spp.; however, it is not a general characteristic of the Corynebacteriales; for example, it was not identified in Corynebacterium spp. or in members of the Mycobacterium tuberculosis and Mycobacterium avium complexes. Since P. aeruginosa and M. abscessus, an emerging pathogen in cases of cystic fibrosis (68), may coexist in the lungs of cystic fibrosis patients, the presence of genes that possibly mediate QS signal interference in M. abscessus opens up new perspectives for studying the interaction of the two species.
The inducibility of all genes by PQS suggested that in R. erythropolis BG43, both sets of genes are involved in alkylquinolone catabolism. Indeed, the expression of aqdA1B1C1 or aqdA2B2C2 in E. coli conferred the ability to degrade PQS to anthranilic acid. Figure 5 summarizes the steps of the HHQ and PQS degradation pathway and the involvement of the individual Aqd enzymes, as deduced from this study.
FIG 5.
HHQ and PQS degradation pathway in R. erythropolis BG43.
Because the use of PQS as a QS signaling molecule has so far been observed exclusively for P. aeruginosa, PQS is an attractive target to specifically attenuate this pathogen. Expression of the PQS dioxygenase gene aqdC2 in P. aeruginosa PAO1 indeed had a significant impact on the production of the virulence factors pyocyanin, rhamnolipid, and pyoverdine. Reduction of PQS levels by the activity of AqdC2 should predominantly lead to decreased expression of the pqsABCDE operon, which codes for the AQ biosynthetic enzymes (7, 8, 13). The corresponding decrease in the level of PqsE, which has a major regulatory role for the production of PQS-mediated products (3, 9, 12, 13), may be a major cause for the observed reduction of pyocyanin and rhamnolipid concentrations.
PQS, but not HHQ, positively controls the transcription of the small regulatory RNA RsmZ (14) via an unknown mechanism. RsmZ acts by sequestering the RsmA protein (69, 70), which, through modulation of the levels of the second messenger cyclic di-GMP, inhibits the production of the siderophore pyoverdine (71). Thus, an increase in the level of free RsmA, resulting from a decrease in the level of RsmZ at low PQS concentrations, could in part account for the observed quenching of pyoverdine production by aqdC2 expression in P. aeruginosa. However, because PQS acts as a chelator of Fe(III), depletion of PQS in the extracellular milieu should increase the bioavailability of iron, resulting in the downregulation of iron-responsive genes through regulatory cascades governed by the ferric uptake regulator Fur. Among the genes repressed by Fur-Fe(II) complexes, the extracytoplasmic function (ECF) sigma factor PvdS is required for transcription of the pyoverdine biosynthesis genes (72). Thus, downregulation of pyoverdine biosynthesis by the activity of AqdC2 may be due largely to the abolishment of the PQS-induced iron starvation response.
The comparatively weak reduction of LasB activity by aqdC2 expression in strain PAO1 presumably is due to the direct effect of LasR/3OC12-HSL (and also RhlR/C4-HSL) on lasB transcription (73, 74). In line with the important role of LasR/3OC12-HSL in the control of lasB, Hraiech et al. (31) recently observed that the engineered AHL lactonase SsoPox-I, which is active toward the 3OC12-HSL signal, efficiently quenched lasB transcription as well as the proteolytic activity of P. aeruginosa PAO1.
Overall, the partial quenching (to different extents) of the production of virulence factors associated with the AQ regulon in P. aeruginosa[pME-aqdC2] may also be due to the presence of HHQ, which is not degraded by AqdC2 and which also acts as a coinducer of PqsR to activate the transcription of pqsABCDE (6, 75, 76). The resulting maintenance, albeit at a reduced level, of HHQ biosynthesis by PqsABCDE feeds PQS formation by the HHQ monooxygenase PqsH, whose synthesis is regulated by the las system. Thus, for efficient QS interference, the activity of the PQS-cleaving enzyme has to outcompete continuing PQS biosynthesis.
The AqdC proteins are the first enzymes identified to specifically cleave PQS. Nevertheless, their efficacy for reducing the virulence of P. aeruginosa has to be validated in future studies. Moreover, a possible use of enzymes as therapeutic tools requires tolerance of the protein by the infected host as well as high stability of the enzyme against physical and chemical factors and proteases or protection of the enzyme by immobilization or encapsulation.
ACKNOWLEDGMENTS
We thank Bianca Müsker for construction and analysis of recombinant E. coli strains harboring aqdC2 expression plasmids, Heiko Niewerth for help with RNA extraction, and Almut Kappius for expert technical assistance. We acknowledge Paul Williams, Matthew Fletcher, and Stephan Heeb (Nottingham, United Kingdom) for kindly providing pME6032(Ap) and Bernd Bukau (Heidelberg, Germany) for plasmids pBB540 and pBB542.
This work was supported by the Deutsche Forschungsgemeinschaft in the framework of Graduate School GRK1409 (Molecular Interactions of Pathogens with Biotic and Abiotic Surfaces).
REFERENCES
- 1.Williams P, Winzer K, Chan WC, Cámara M. 2007. Look who's talking: communication and quorum sensing in the bacterial world. Philos Trans R Soc Lond B Biol Sci 362:1119–1134. doi: 10.1098/rstb.2007.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jimenez PN, Koch G, Thompson JA, Xavier KB, Cool RH, Quax WJ. 2012. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev 76:46–65. doi: 10.1128/MMBR.05007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Déziel E, Gopalan S, Tampakaki AP, Lépine F, Padfield KE, Saucier M, Xiao G, Rahme LG. 2005. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-L-homoserine lactones. Mol Microbiol 55:998–1014. [DOI] [PubMed] [Google Scholar]
- 4.Diggle SP, Matthijs S, Wright VJ, Fletcher MP, Chhabra SR, Lamont IL, Kong X, Hider RC, Cornelis P, Cámara M, Williams P. 2007. The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem Biol 14:87–96. doi: 10.1016/j.chembiol.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Wade DS, Calfee MW, Rocha ER, Ling EA, Engstrom E, Coleman JP, Pesci EC. 2005. Regulation of Pseudomonas quinolone signal synthesis in Pseudomonas aeruginosa. J Bacteriol 187:4372–4380. doi: 10.1128/JB.187.13.4372-4380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao G, Déziel E, He J, Lépine F, Lesic B, Castonguay MH, Milot S, Tampakaki AP, Stachel SE, Rahme LG. 2006. MvfR, a key Pseudomonas aeruginosa pathogenicity LTTR-class regulatory protein, has dual ligands. Mol Microbiol 62:1689–1699. doi: 10.1111/j.1365-2958.2006.05462.x. [DOI] [PubMed] [Google Scholar]
- 7.Dulcey CE, Dekimpe V, Fauvelle DA, Milot S, Groleau MC, Doucet N, Rahme LG, Lépine F, Déziel E. 2013. The end of an old hypothesis: the Pseudomonas signaling molecules 4-hydroxy-2-alkylquinolines derive from fatty acids, not 3-ketofatty acids. Chem Biol 20:1481–1491. doi: 10.1016/j.chembiol.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drees SL, Fetzner S. 2015. PqsE of Pseudomonas aeruginosa acts as pathway-specific thioesterase in the biosynthesis of alkylquinolone signaling molecules. Chem Biol 22:611–618. doi: 10.1016/j.chembiol.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Farrow JM III, Sund ZM, Ellison ML, Wade DS, Coleman JP, Pesci EC. 2008. PqsE functions independently of PqsR-Pseudomonas quinolone signal and enhances the rhl quorum-sensing system. J Bacteriol 190:7043–7051. doi: 10.1128/JB.00753-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hazan R, He J, Xiao G, Dekimpe V, Apidianakis Y, Lesic B, Astrakas C, Déziel E, Lépine F, Rahme LG. 2010. Homeostatic interplay between bacterial cell-cell signaling and iron in virulence. PLoS Pathog 6:e1000810. doi: 10.1371/journal.ppat.1000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rampioni G, Pustelny C, Fletcher MP, Wright VJ, Bruce M, Rumbaugh KP, Heeb S, Cámara M, Williams P. 2010. Transcriptomic analysis reveals a global alkyl-quinolone-independent regulatory role for PqsE in facilitating the environmental adaptation of Pseudomonas aeruginosa to plant and animal hosts. Environ Microbiol 12:1659–1673. doi: 10.1111/j.1462-2920.2010.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diggle SP, Winzer K, Chhabra SR, Worrall KE, Cámara M, Williams P. 2003. The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol Microbiol 50:29–43. doi: 10.1046/j.1365-2958.2003.03672.x. [DOI] [PubMed] [Google Scholar]
- 13.Gallagher LA, McKnight SL, Kuznetsova MS, Pesci EC, Manoil C. 2002. Functions required for extracellular quinolone signaling by Pseudomonas aeruginosa. J Bacteriol 184:6472–6480. doi: 10.1128/JB.184.23.6472-6480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heeb S, Fletcher MP, Chhabra SR, Diggle SP, Williams P, Cámara M. 2011. Quinolones: from antibiotics to autoinducers. FEMS Microbiol Rev 35:247–274. doi: 10.1111/j.1574-6976.2010.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huse H, Whiteley M. 2011. 4-Quinolones: smart phones of the microbial world. Chem Rev 111:152–159. doi: 10.1021/cr100063u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mashburn LM, Whiteley M. 2005. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature 437:422–425. doi: 10.1038/nature03925. [DOI] [PubMed] [Google Scholar]
- 17.Bredenbruch F, Geffers R, Nimtz M, Buer J, Häussler S. 2006. The Pseudomonas aeruginosa quinolone signal (PQS) has iron chelating activity. Environ Microbiol 8:1318–1329. doi: 10.1111/j.1462-2920.2006.01025.x. [DOI] [PubMed] [Google Scholar]
- 18.Häussler S, Becker T. 2008. The Pseudomonas quinolone signal (PQS) balances life and death in Pseudomonas aeruginosa populations. PLoS Pathog 4:e1000166. doi: 10.1371/journal.ppat.1000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooi DS, Bycroft BW, Chhabra SR, Williams P, Pritchard DI. 2004. Differential immune modulatory activity of Pseudomonas aeruginosa quorum-sensing signal molecules. Infect Immun 72:6463–6470. doi: 10.1128/IAI.72.11.6463-6470.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skindersoe ME, Zeuthen LH, Brix S, Fink LN, Lazenby J, Whittall C, Williams P, Diggle SP, Froekiaer H, Cooley M, Givskov M. 2009. Pseudomonas aeruginosa quorum-sensing signal molecules interfere with dendritic cell-induced T-cell proliferation. FEMS Immunol Med Microbiol 55:335–345. doi: 10.1111/j.1574-695X.2008.00533.x. [DOI] [PubMed] [Google Scholar]
- 21.Hänsch GM, Prior B, Brenner-Weiss G, Obst U, Overhage J. 2014. The Pseudomonas quinolone signal (PQS) stimulates chemotaxis of polymorphonuclear neutrophils. J Appl Biomater Funct Mater 12:21–26. doi: 10.5301/jabfm.5000204. [DOI] [PubMed] [Google Scholar]
- 22.Holban AM, Bleotu C, Chifiriuc MC, Bezirtzoglou E, Lazar V. 2014. Role of Pseudomonas aeruginosa quorum sensing (QS) molecules on the viability and cytokine profile of human mesenchymal stem cells. Virulence 5:303–310. doi: 10.4161/viru.27571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clatworthy AE, Pierson E, Hung DT. 2007. Targeting virulence: a new paradigm for antimicrobial therapy. Nat Chem Biol 3:541–548. doi: 10.1038/nchembio.2007.24. [DOI] [PubMed] [Google Scholar]
- 24.Lin YH, Xu JL, Hu J, Wang LH, Ong SL, Leadbetter JR, Zhang LH. 2003. Acyl-homoserine lactone acylase from Ralstonia strain XJ12B represents a novel and potent class of quorum-quenching enzymes. Mol Microbiol 47:849–860. doi: 10.1046/j.1365-2958.2003.03351.x. [DOI] [PubMed] [Google Scholar]
- 25.Sio CF, Otten LG, Cool RH, Diggle SP, Braun PG, Bos R, Daykin M, Cámara M, Williams P, Quax WJ. 2006. Quorum quenching by an N-acyl-homoserine lactone acylase from Pseudomonas aeruginosa PAO1. Infect Immun 74:1673–1682. doi: 10.1128/IAI.74.3.1673-1682.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahjudi M, Papaioannou E, Hendrawati O, van Assen AH, van Merkerk R, Cool RH, Poelarends GJ, Quax WJ. 2011. PA0305 of Pseudomonas aeruginosa is a quorum quenching acylhomoserine lactone acylase belonging to the Ntn hydrolase superfamily. Microbiology 157:2042–2055. doi: 10.1099/mic.0.043935-0. [DOI] [PubMed] [Google Scholar]
- 27.Bijtenhoorn P, Schipper C, Hornung C, Quitschau M, Grond S, Weiland N, Streit WR. 2011. BpiB05, a novel metagenome-derived hydrolase acting on N-acylhomoserine lactones. J Biotechnol 155:86–94. doi: 10.1016/j.jbiotec.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Krysciak D, Schmeisser C, Preuss S, Riethausen J, Quitschau M, Grond S, Streit WR. 2011. Involvement of multiple loci in quorum quenching of autoinducer I molecules in the nitrogen-fixing symbiont Rhizobium (Sinorhizobium) sp. strain NGR234. Appl Environ Microbiol 77:5089–5099. doi: 10.1128/AEM.00112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schipper C, Hornung C, Bijtenhoorn P, Quitschau M, Grond S, Streit WR. 2009. Metagenome-derived clones encoding two novel lactonase family proteins involved in biofilm inhibition in Pseudomonas aeruginosa. Appl Environ Microbiol 75:224–233. doi: 10.1128/AEM.01389-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Migiyama Y, Kaneko Y, Yanagihara K, Morohoshi T, Morinaga Y, Nakamura S, Miyazaki T, Hasegawa H, Izumikawa K, Kakeya H, Kohrogi H, Kohno S. 2013. Efficacy of AiiM, an N-acylhomoserine lactonase, against Pseudomonas aeruginosa in a mouse model of acute pneumonia. Antimicrob Agents Chemother 57:3653–3658. doi: 10.1128/AAC.00456-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hraiech S, Hiblot J, Lafleur J, Lepidi H, Papazian L, Rolain JM, Raoult D, Elias M, Silby MW, Bzdrenga J, Bregeon F, Chabriere E. 2014. Inhaled lactonase reduces Pseudomonas aeruginosa quorum sensing and mortality in rat pneumonia. PLoS One 9:e107125. doi: 10.1371/journal.pone.0107125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diggle SP, Lumjiaktase P, Dipilato F, Winzer K, Kunakorn M, Barrett DA, Chhabra SR, Cámara M, Williams P. 2006. Functional genetic analysis reveals a 2-alkyl-4-quinolone signaling system in the human pathogen Burkholderia pseudomallei and related bacteria. Chem Biol 13:701–710. doi: 10.1016/j.chembiol.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Vial L, Lépine F, Milot S, Groleau MC, Dekimpe V, Woods DE, Déziel E. 2008. Burkholderia pseudomallei, B. thailandensis, and B. ambifaria produce 4-hydroxy-2-alkylquinoline analogues with a methyl group at the 3 position that is required for quorum-sensing regulation. J Bacteriol 190:5339–5352. doi: 10.1128/JB.00400-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pustelny C, Albers A, Büldt-Karentzopoulos K, Parschat K, Chhabra SR, Cámara M, Williams P, Fetzner S. 2009. Dioxygenase-mediated quenching of quinolone dependent quorum sensing in Pseudomonas aeruginosa. Chem Biol 16:1259–1267. doi: 10.1016/j.chembiol.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Niewerth H, Schuldes J, Parschat K, Kiefer P, Vorholt JA, Daniel R, Fetzner S. 2012. Complete genome sequence and metabolic potential of the quinaldine-degrading bacterium Arthrobacter sp. Rue61a. BMC Genomics 13:534. doi: 10.1186/1471-2164-13-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thierbach S, Bui N, Zapp J, Chhabra SR, Kappl R, Fetzner S. 2014. Substrate assisted O2 activation in a cofactor-independent dioxygenase. Chem Biol 21:217–225. doi: 10.1016/j.chembiol.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Müller C, Birmes FS, Niewerth H, Fetzner S. 2014. Conversion of the Pseudomonas aeruginosa quinolone signal (PQS) and related alkylhydroxyquinolines by Rhodococcus sp. strain BG43. Appl Environ Microbiol 80:7266–7274. doi: 10.1128/AEM.02342-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rückert C, Birmes FS, Müller C, Niewerth H, Winkler A, Fetzner S, Kalinowski J. 2015. Complete genome sequence of Rhodococcus erythropolis BG43 (DSM 46869), a degrader of Pseudomonas aeruginosa quorum sensing signal molecules. J Biotechnol 211:99–100. doi: 10.1016/j.jbiotec.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Grant SG, Jessee J, Bloom FR, Hanahan D. 1990. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc Natl Acad Sci U S A 87:4645–4649. doi: 10.1073/pnas.87.12.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Studier FW. 2005. Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 42.Soh EY, Chhabra SR, Halliday N, Heeb S, Müller C, Birmes FS, Fetzner S, Cámara M, Chan KG, Williams P. 25 March 2015. Biotic inactivation of the Pseudomonas aeruginosa quorum sensing signal molecule PQS. Environ Microbiol doi: 10.1111/1462-2920.12857. [DOI] [PubMed] [Google Scholar]
- 43.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–33402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desper R, Gascuel O. 2002. Fast and accurate phylogeny reconstruction algorithms based on the minimum-evolution principle. J Comput Biol 9:687–705. doi: 10.1089/106652702761034136. [DOI] [PubMed] [Google Scholar]
- 45.Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580. doi: 10.1016/S0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 46.Choi KH, Kumar A, Schweizer HP. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J Microbiol Methods 64:391–397. doi: 10.1016/j.mimet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Iwasaki K, Uchiyama H, Yagi O, Kurabayashi T, Ishizuka K, Takamura Y. 1994. Transformation of Pseudomonas putida by electroporation. Biosci Biotechnol Biochem 58:851–854. doi: 10.1271/bbb.58.851. [DOI] [PubMed] [Google Scholar]
- 48.Gartemann KH, Eichenlaub R. 2001. Isolation and characterization of IS1409, an insertion element of 4-chlorobenzoate-degrading Arthrobacter sp. strain TM1, and development of a system for transposon mutagenesis. J Bacteriol 183:3729–3736. doi: 10.1128/JB.183.12.3729-3736.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 50.Zor T, Selinger Z. 1996. Linearization of the Bradford protein assay increases its sensitivity: theoretical and experimental studies. Anal Biochem 236:302–308. doi: 10.1006/abio.1996.0171. [DOI] [PubMed] [Google Scholar]
- 51.Klendshoj NC, Feldstein M, Sprague AL. 1950. The spectrophotometric determination of carbon monoxide. J Biol Chem 183:297–303. [Google Scholar]
- 52.Wilhelm S, Gdynia A, Tielen P, Rosenau F, Jaeger KE. 2007. The autotransporter esterase EstA of Pseudomonas aeruginosa is required for rhamnolipid production, cell motility, and biofilm formation. J Bacteriol 189:6695–6703. doi: 10.1128/JB.00023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ochsner UA, Fiechter A, Reiser J. 1994. Isolation, characterization, and expression in Escherichia coli of the Pseudomonas aeruginosa rhlAB genes encoding a rhamnosyltransferase involved in rhamnolipid biosurfactant synthesis. J Biol Chem 269:19787–19795. [PubMed] [Google Scholar]
- 54.Essar DW, Eberly L, Hadero A, Crawford IP. 1990. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol 172:884–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toder DS, Gambello MJ, Iglewski BH. 1991. Pseudomonas aeruginosa LasA: a second elastase under the transcriptional control of lasR. Mol Microbiol 5:2003–2010. doi: 10.1111/j.1365-2958.1991.tb00822.x. [DOI] [PubMed] [Google Scholar]
- 56.Beermann B, Guddorf J, Boehm K, Albers A, Kolkenbrock S, Fetzner S, Hinz HJ. 2007. Stability, unfolding, and structural changes of cofactor-free 1H-3-hydroxy-4-oxoquinaldine 2,4-dioxygenase. Biochemistry 46:4241–4249. doi: 10.1021/bi0622423. [DOI] [PubMed] [Google Scholar]
- 57.Steiner RA, Janssen HJ, Roversi P, Oakley AJ, Fetzner S. 2010. Structural basis for cofactor-independent dioxygenation of N-heteroaromatic compounds at the alpha/beta-hydrolase fold. Proc Natl Acad Sci U S A 107:657–662. doi: 10.1073/pnas.0909033107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lépine F, Déziel E, Milot S, Rahme LG. 2003. A stable isotope dilution assay for the quantification of the Pseudomonas quinolone signal in Pseudomonas aeruginosa cultures. Biochim Biophys Acta 1622:36–41. doi: 10.1016/S0304-4165(03)00103-X. [DOI] [PubMed] [Google Scholar]
- 59.Traut TW. 2008. Allosteric regulatory enzymes, p 29–48. Springer, New York, NY. [Google Scholar]
- 60.de Marco A, Deuerling E, Mogk A, Tomoyasu T, Bukau B. 2007. Chaperone-based procedure to increase yields of soluble recombinant proteins produced in E. coli. BMC Biotechnol 7:32. doi: 10.1186/1472-6750-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lau GW, Hassett DJ, Ran H, Kong F. 2004. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol Med 10:599–606. doi: 10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Dietrich LE, Price-Whelan A, Petersen A, Whiteley M, Newman DK. 2006. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol Microbiol 61:1308–1321. doi: 10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- 63.Lamont IL, Beare PA, Ochsner U, Vasil AI, Vasil ML. 2002. Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 99:7072–7077. doi: 10.1073/pnas.092016999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calfee MW, Shelton JG, McCubrey JA, Pesci EC. 2005. Solubility and bioactivity of the Pseudomonas quinolone signal are increased by a Pseudomonas aeruginosa-produced surfactant. Infect Immun 73:878–882. doi: 10.1128/IAI.73.2.878-882.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caiazza NC, Shanks RM, O'Toole GA. 2005. Rhamnolipids modulate swarming motility patterns of Pseudomonas aeruginosa. J Bacteriol 187:7351–7361. doi: 10.1128/JB.187.21.7351-7361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Read RC, Roberts P, Munro N, Rutman A, Hastie A, Shryock T, Hall R, McDonald-Gibson W, Lund V, Taylor G. 1992. Effect of Pseudomonas aeruginosa rhamnolipids on mucociliary transport and ciliary beating. J Appl Physiol 72:2271–2277. [DOI] [PubMed] [Google Scholar]
- 67.Wretlind B, Pavlovskis OR. 1983. Pseudomonas aeruginosa elastase and its role in Pseudomonas infections. Rev Infect Dis 5:998–1004. [DOI] [PubMed] [Google Scholar]
- 68.Qvist T, Pressler T, Høiby N, Katzenstein TL. 2014. Shifting paradigms of nontuberculous mycobacteria in cystic fibrosis. Respir Res 15:41. doi: 10.1186/1465-9921-15-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kay E, Humair B, Dénervaud V, Riedel K, Spahr S, Eberl L, Valverde C, Haas D. 2006. Two GacA-dependent small RNAs modulate the quorum-sensing response in Pseudomonas aeruginosa. J Bacteriol 188:6026–6033. doi: 10.1128/JB.00409-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sonnleitner E, Haas D. 2011. Small RNAs as regulators of primary and secondary metabolism in Pseudomonas species. Appl Microbiol Biotechnol 91:63–79. doi: 10.1007/s00253-011-3332-1. [DOI] [PubMed] [Google Scholar]
- 71.Frangipani E, Visaggio D, Heeb S, Kaever V, Cámara M, Visca P, Imperi F. 2014. The Gac/Rsm and cyclic-di-GMP signalling networks coordinately regulate iron uptake in Pseudomonas aeruginosa. Environ Microbiol 16:676–688. doi: 10.1111/1462-2920.12164. [DOI] [PubMed] [Google Scholar]
- 72.Cornelis P, Matthijs S, Van Oeffelen L. 2009. Iron uptake regulation in Pseudomonas aeruginosa. Biometals 22:15–22. doi: 10.1007/s10534-008-9193-0. [DOI] [PubMed] [Google Scholar]
- 73.Schuster M, Urbaowski ML, Greenberg EP. 2004. Promoter specificity in Pseudomonas aeruginosa quorum sensing revealed by DNA binding of purified LasR. Proc Natl Acad Sci U S A 101:15833–15839. doi: 10.1073/pnas.0407229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schuster M, Greenberg EP. 2007. Early activation of quorum sensing in Pseudomonas aeruginosa reveals the architecture of a complex regulon. BMC Genomics 8:287. doi: 10.1186/1471-2164-8-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Déziel E, Lépine F, Milot S, He J, Mindrinos MN, Tompkins RG, Rahme LG. 2004. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc Natl Acad Sci U S A 101:1339–1344. doi: 10.1073/pnas.0307694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Müller C, Fetzner S. 2013. A Pseudomonas putida bioreporter for the detection of enzymes active on 2-alkyl-4(1H)-quinolone signalling molecules. Appl Microbiol Biotechnol 97:751–760. doi: 10.1007/s00253-012-4236-4. [DOI] [PubMed] [Google Scholar]