Abstract
Loss of ordered molecular structure in proteins is known to increase their adhesion to surfaces. The aim of this work was to study the stability of norovirus secondary and tertiary structures and its implications for viral adhesion to fresh foods and agrifood surfaces. The pH, ionic strength, and temperature conditions studied correspond to those prevalent in the principal vehicles of viral transmission (vomit and feces) and in the food processing and handling environment (pasteurization and refrigeration). The structures of virus-like particles representing GI.1, GII.4, and feline calicivirus (FCV) were studied using circular dichroism and intrinsic UV fluorescence. The particles were remarkably stable under most of the conditions. However, heating to 65°C caused losses of β-strand structure, notably in GI.1 and FCV, while at 75°C the α-helix content of GII.4 and FCV decreased and tertiary structures unfolded in all three cases. Combining temperature with pH or ionic strength caused variable losses of structure depending on the particle type. Regardless of pH, heating to pasteurization temperatures or higher would be required to increase GII.4 and FCV adhesion, while either low or high temperatures would favor GI.1 adhesion. Regardless of temperature, increased ionic strength would increase GII.4 adhesion but would decrease GI.1 adhesion. FCV adsorption would be greater at refrigeration, pasteurization, or high temperature combined with a low salt concentration or at a higher NaCl concentration regardless of temperature. Norovirus adhesion mediated by hydrophobic interaction may depend on hydrophobic residues normally exposed on the capsid surface at pH 3, pH 8, physiological ionic strength, and low temperature, while at pasteurization temperatures it may rely more on buried hydrophobic residues exposed upon structural rearrangement.
INTRODUCTION
Noroviruses are the main cause of acute nonbacterial gastroenteritis in the United States, accounting for nearly 58% of all food-borne illnesses reported in 2011 (1). Most European countries experienced repeated outbreaks of norovirus gastroenteritis during the period 2002 to 2006 (2). Multiple outbreaks were also reported in Canada (3) and numerous other countries throughout the world (4). Noroviruses are classified in the calicivirus family (5). Human illness usually involves genogroup I or II. The former is frequently involved in transmission via shellfish (6), while the latter is transmitted person to person (7). The norovirus structure comprises a single positive strand of RNA with an icosahedral nonenveloped capsid about 28 to 35 nm in diameter (8).
The infectiousness of noroviruses is strongly correlated with their capacity to adhere to food preparation or processing surface materials, such as stainless steel (9), and to remain infectious over time. Biophysical and biochemical parameters such as pH tolerance, isoelectric pH, ionic strength, temperature tolerance, and electrostatic/hydrophobic interactions are reportedly important factors for adhesion to food surfaces (6) and inert surfaces (10). Increased ionic strength may increase adhesion by strengthening van der Waals attraction or hydrophobic interaction (11, 12). Low pH reportedly favors adhesion, while high pH favors detachment (10). In studies of human norovirus GI.1, da Silva et al. observed inconsistent attachment to silica below the isoelectric pH but a clear decrease in attachment above the isoelectric pH (13). Other biomaterials known to behave in this manner include bovine serum albumin, gamma globulin, and fibrinogen (14) and cells of bacteria such as Staphylococcus aureus (15). The effect of temperature is less clear. While no data on noroviruses or viruses in general have been published, proteins such as bovine serum albumin have been shown to adsorb to polymer surfaces by two types of mechanisms. In the case of type 1, the attraction is somewhat hydrophilic, and adhesion generally decreases with increasing temperature. Type 2 involves firm hydrophobic attraction, and adhesion usually increases with increasing temperature (14).
Hydrophobic interactions are thought to be the main contributor to the strength of adhesive interactions between proteins and surfaces in aqueous media (16–22). These interactions involve loss of hydration layers and agglomeration of hydrophobic groups. Shedding of the hydration layer due to movement of hydrophobic groups (proteins and surfaces) is entropy driven (21). Structural rearrangements involving losses of secondary structure and globular conformation have been shown to expose internal hydrophobic groups within protein molecules and to increase entropy and interaction with surfaces (22–24). The secondary structure content, especially the α-helix content of proteins such as bovine serum albumin, chymotrypsin, cutinase, lysozyme, α-lactalbumin, and human plasma albumin, reportedly differs between the adsorbed and nonadsorbed states (16–22). However, no similar studies of norovirus structure/adhesion relationships have been published.
In our investigation of norovirus zeta potential and aggregation, we found that viral particles adsorb maximally at pH 4 (near their isoelectric point) to hydrophobic surfaces such as lettuce leaves and common inert surfaces (polystyrene, polyethylene, polypropylene, etc.) in the food sector (25). Virus adhesion to hydrophobic surfaces appeared to be positively correlated with increasing ionic strength and temperature. The objective of the present study was to evaluate the stability of norovirus secondary and tertiary structures under different biophysical and biochemical conditions encountered in various environments, including food manufacturing and food services. Virus-like particles (VLPs) representing human noroviruses and feline calicivirus (FCV) were exposed to acidic, neutral, or slightly basic pH, ionic strengths of 0.0, 0.1, or 0.25 M NaCl, and refrigeration, room, or pasteurization temperatures and to certain combinations thereof. Structural changes in viral capsids were monitored using circular dichroism (CD) and UV fluorescence spectroscopic techniques.
MATERIALS AND METHODS
VLP production and purification.
Virus-like particles (VLPs) of norovirus GI.1 and GII.4 and feline calicivirus (FCV) were produced using a baculovirus expression vector system (26), purified as described by Huhti et al. (27), and concentrated by ultrafiltration using an Amicon-100 filter (molecular weight cuttoff [MWCO], 100,000; Millipore). Purified VLPs were then resuspended in 0.2-μm-filtered high-pressure liquid chromatography (HPLC) water (for GI.1) or phosphate-buffered saline (PBS) (pH 7.4) (for GII.4 and FCV) to obtain a stock solution whose concentration was measured as absorption at 280 nm using a NanoDrop instrument (ND-1000; NanoDrop, Wilmington, DE, USA). The VLP suspensions were kept at 4°C until use.
Treatment of virus-like particles under different pH, ionic strength, and temperature conditions.
The pH range of 3 to 8 was selected because it is representative of the vehicle (vomit or feces) via which noroviruses are most often transmitted. Neutral pH was taken as a control condition. This range may be covered using 20 mM citrate phosphate buffer, which contributes very little to ionic strength. Physiological ionic strength could thus be set using 0.1 to 0.25 M NaCl, with deionized water taken as the control condition. Finally, the temperatures 4, 22, 65, and 75°C correspond, respectively, to refrigeration, room (control), and slow and fast pasteurization temperatures and hence to food storage, handling, and processing conditions. Combined treatments were investigated over a temperature range of 4 to 90°C coupled with pH range or ionic strength range. All buffers or NaCl solutions as well as deionized water were filtered before use (Phenex RC 4-mm [0.2-μm] syringe filter; Phenomenex).
CD.
VLP secondary structure was monitored by far-UV circular dichroism (CD) using a Jasco-815 spectropolarimeter (JASCO International Co., Ltd., USA) equipped with a water bath and a Peltier temperature controller. Samples were diluted to the appropriate concentration (0.22 mg · ml−1) in prefiltered buffer (pH 3 to 8) or ionic strength solution and refiltered before analysis. The sample volume was 140 μl, and the path length was 1 mm. Quartz cuvettes were used. The desired temperature (4, 22, 65, or 75°C) was selected using the spectropolarimeter software. Each spectrum was a mean of 10 scans from 260 to 190 nm. Blanks were made for all physicochemical conditions and subtracted from measured values. Measurement parameters were “standard” sensitivity, 0.5-nm resolution, 100 nm · s−1 scanning speed, 1-nm bandwidth, 1-s response time, and 1-s digital integration time, and results or ellipticities (θ) were displayed in m degree (y axis of plots, machine unit). Assays for spectral deconvolutions (i.e., secondary structure determination) were conducted first on DICHROWEB (28, 29), followed by routine deconvolution on the CDPro analysis program of the Jasco spectral analysis software. Algorithms used for deconvolution were CONTIN (30) and CDSSTR (31), both modified by Sreerama and Woody for CDPro analysis program use (32). The SP48 protein set was used, and only deconvolutions with root mean square deviation (RSMD) of <0.250 were considered conclusive.
Near-UV intrinsic fluorescence measurements.
Tertiary structural stability was analyzed in terms of intrinsic UV using a spectrofluorometer. Excitation was set for tryptophan residues, for which the emission wavelength is more meaningful than the fluorescence amplitude. When a tryptophan residue is exposed, its fluorescence peak is at 350 nm, while the peak for an embedded residue is at 330 nm (33). Based on tryptophan exposure, the relative unfolding or refolding of a protein can be monitored. Samples were diluted at the appropriate concentration (0.055 mg · ml−1) in prefiltered buffer (pH 3, 7, and 8 or ionic strengths 0, 0.1, and 0.25 M) and refiltered as described above before measurements. The sample volume was 300 μl. Quartz cuvettes were used. The desired temperature (4, 22, 65, and 75°C) was selected via the spectrofluorometer software and maintained by a temperature-controlled circulating water bath. The excitation wavelength was 280 nm, and emission was measured between 305 and 450 nm. The excitation and emission slits were set at 5; the excitation and emission filters were on “auto” and “open,” respectively; the axis minimum and maximum were 0.000 and 50,000, respectively; the emission photomultiplier tube voltage was 800 V; and the detector voltage was “high” and the speed “medium.” The threshold was 50, and the curve was smoothed using the Savitzky-Golay filter set at 9. Blanks were made for all physicochemical solutions and subtracted from measured values. The results presented here are means of three measurements.
RESULTS
Effect of pH on VLP secondary structure.
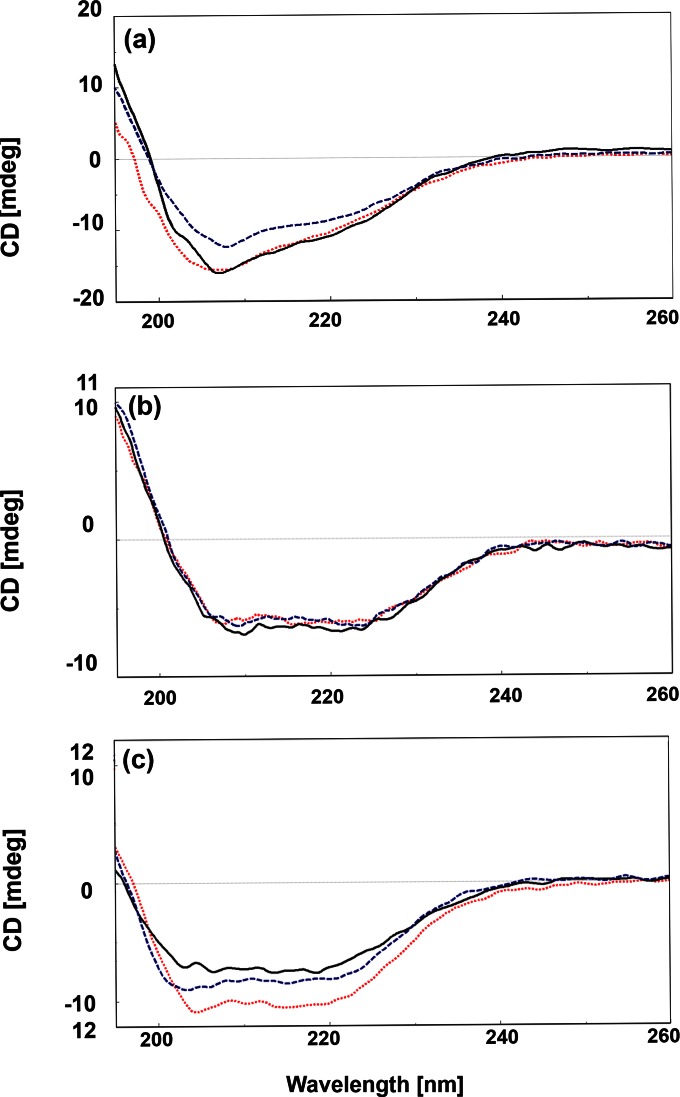
Figure 1 shows the effect of pH (3, 7, and 8) on VLP secondary structure at 22°C based on circular dichroism spectroscopy. The predicted secondary structure distribution based on data deconvolution is shown in Table 1. The unordered structure of GI.1 increased from 36.3% at pH 7 to 40% at pH 3, while α-helix and β-strand structures decreased, respectively, by 2.9% and 1.3%. The effect of increasing the pH to 8 was smaller. In comparison, GII.4 was stable, as shown in Fig. 1b and Table 1. In the case of FCV, a conspicuous shift from β-strand (−8.3%) to α-helix (+10.7%) occurred at pH 3, while pH 8 did not disrupt the secondary structure nearly as much, as shown in Fig. 1c.
FIG 1.
Circular dichroism spectra of virus-like particles of GI.1 (a), GII.4 (b), and FCV (c) at pH 3 (dotted lines), pH 7 (solid lines), and pH 8 (dashed lines).
TABLE 1.
Effect of pH on the distribution of secondary structures in VLPs, based on circular dichroism spectroscopy
| VLP | pH | Secondary structure type (%) |
|||
|---|---|---|---|---|---|
| α-Helix | β-Strand | Turns | Unordered | ||
| GI.1 | 3 | 5.0 | 34.7 | 20.4 | 40.0 |
| 7 | 8.1 | 36.0 | 19.3 | 36.3 | |
| 8 | 6.2 | 37.7 | 19.1 | 37.2 | |
| GII.4 | 3 | 7.2 | 35.7 | 19.5 | 36.8 |
| 7 | 7.7 | 35.2 | 20.2 | 36.9 | |
| 8 | 7.1 | 35.7 | 20.4 | 36.8 | |
| FCV | 3 | 15.7 | 24.9 | 20.8 | 38.5 |
| 7 | 5.0 | 33.2 | 19.9 | 40.7 | |
| 8 | 7.2 | 32.4 | 18.4 | 41.6 | |
Effect of ionic strength on VLP secondary structure.
Changes in VLP structure at different ionic strengths are summarized in Table 2. In the case of GI.1, both 0.1 M and 0.25 M NaCl induced conspicuous shifts from β-strand to α-helix and unordered structures (respectively, −13.0% and −13.7%, +9.5% and +8.4%, and +2.0% and +3.5%), while turns remained relatively unchanged compared to the control (deionized water). In comparison, GII.4 retained its α-helix and turn structures as ionic strength was increased to 0.1 or 0.25 M, while β-strands apparently shifted to unstructured forms. In the case of FCV, β-strands decreased from 38.3% to 27.4% and 23.6%, respectively, as the ionic strength increased from 0 to 0.1 and 0.25 M NaCl. A 3.4 to 5% decrease in turn content was also observed. Meanwhile, α-helix and unordered structures increased, respectively, by 10.3% and 11.1% and by 4.2% and 8.8% in response to these increases in ionic strength.
TABLE 2.
Effect of ionic strength ([NaCl]) on the distribution of secondary structures in VLPs, based on circular dichroism spectroscopy
| VLP | Ionic strength (M) | Secondary structure type (%) |
|||
|---|---|---|---|---|---|
| α-Helix | β-Strand | Turns | Unordered | ||
| GI.1 | 0.00 | 6.4 | 39.8 | 18.9 | 35.4 |
| 0.10 | 15.9 | 26.8 | 19.4 | 37.4 | |
| 0.25 | 14.8 | 26.1 | 19.9 | 38.9 | |
| GII.4 | 0.00 | 7.5 | 36.1 | 19.9 | 37.0 |
| 0.10 | 6.7 | 33.2 | 22.9 | 37.7 | |
| 0.25 | 6.3 | 30.0 | 20.7 | 41.9 | |
| FCV | 0.00 | 5.8 | 38.3 | 23.1 | 32.1 |
| 0.10 | 16.1 | 27.4 | 19.7 | 36.3 | |
| 0.25 | 16.9 | 23.6 | 18.1 | 40.9 | |
Effect of temperature on VLP secondary structure.
The effect of temperatures ranging from 4°C to 75°C on VLP secondary structure at neutral pH is summarized in Table 3. No VLP underwent any significant shift in structure at 4°C relative to the corresponding control held at room temperature (22°C). Heating to 65°C altered the β-strand content of GI.1, causing the helix content to increase from 8.1% to 16.1%, while all ordered structures shifted slightly to unordered structure in GII.4, and the β-strand content of FCV dropped by 9.9% in favor of unordered structure (helix and turn contents remained relatively stable). Further heating to 75°C caused additional shifts of secondary structure to unordered in both GI.1 and GII.4 (from 36.9% to 45.1% in the latter case), mostly at the expense of the α-helix content. In the case of FCV, this shift occurred at the expense of the α-helix (−3.3%), β-strand (−6.3%), and turn (−4.5%) structures. Beta-strands withstood the increase from 65°C to 75°C better in GII.4.
TABLE 3.
Effect of temperature on the distribution of secondary structures in VLPs, based on circular dichroism spectroscopy
| VLP | Temp (°C) | Secondary structure type (%) |
|||
|---|---|---|---|---|---|
| α-Helix | β-Strand | Turns | Unordered | ||
| GI.1 | 4 | 7.7 | 35.3 | 19.4 | 37.0 |
| 22 | 8.1 | 36.0 | 19.3 | 36.3 | |
| 65 | 16.1 | 26.3 | 19.5 | 37.7 | |
| 75 | 7.0 | 28.0 | 21.4 | 43.4 | |
| GII.4 | 4 | 8.2 | 35.4 | 20.3 | 36.0 |
| 22 | 7.7 | 35.2 | 20.2 | 36.9 | |
| 65 | 6.3 | 34.0 | 18.4 | 41.3 | |
| 75 | 0.7 | 33.6 | 20.2 | 45.1 | |
| FCV | 4 | 7.1 | 29.5 | 22.2 | 40.5 |
| 22 | 5.0 | 33.2 | 19.9 | 40.7 | |
| 65 | 5.2 | 23.3 | 22.3 | 49.0 | |
| 75 | 1.7 | 26.9 | 15.4 | 55.1 | |
Combined effects of temperature with pH or ionic strength on VLP secondary structure.
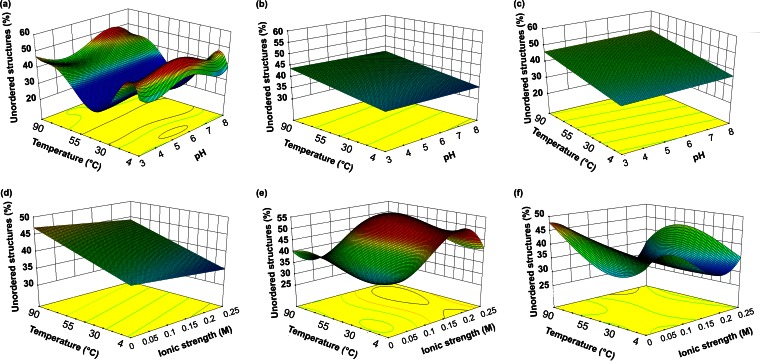
Figure 2 shows the response plot of VLP secondary structures for combined treatments (temperature with pH and temperature with ionic strength). The loss of orderly secondary structure in GI.1 fluctuated considerably in temperature/pH combined treatments (Fig. 2a), with maxima observed at T < 12.6°C/pH 5 to 6 and T > 72.8°C/pH 6 to 8. The greatest losses were observed over the temperature range of 29.8 to 55.6°C regardless of pH. In contrast, GII.4 (Fig. 2b) and FCV (Fig. 2c) presented flat loss profiles, with a minimum at 4°C regardless of pH and maxima at 90°C at pH 3 and pH 8.
FIG 2.
Response plot for the combined effects of temperature and pH (a, b, and c) or ionic strength (d, e, and f) on the unordered structure content of virus-like particles representing GI.1 (a and d), GII.4 (b and e), and FCV (c and f).
In addition, temperature and ionic strength had the opposite effects on GI.1 VLP secondary structure (Fig. 2d). While increasing temperature brought losses of order starting at 15°C, increasing ionic strength appeared to bring a gain independent of temperature. Similarly, loss in the case of FCV was minimal over the 0.1 to 0.2 M range regardless of temperature (Fig. 2f). Loss was maximal at 0.25 M NaCl regardless of temperature or at 4°C or high temperatures (64.2 to 90°C) combined with low ionic strength (0 to 0.05 M). In contrast, increasing ionic strength disrupted GII.4 VLP secondary structure (Fig. 2e), with maximal loss in the 0.1 to 0.2 M range regardless of temperature.
Effects of pH, ionic strength, and temperature on the stability of VLP tertiary structure.
Unfolding of VLP tertiary structure as a function of pH, ionic strength, or temperature was monitored as tryptophan fluorescence (Table 4). Neither pH nor ionic strength produced a variation of more than about 1 nm at 22°C. Decreases of 0.98 and 0.68 nm at pH 3 and increases of 1.02 and 1 at pH 8 were noted, respectively, for GI.1 and FCV, while increases of approximately 1 and 0.07 nm, respectively, at pH 3 and pH 8 were noted for GII.4. A small increase (2.34 nm) was noted for GI.1 at 4°C. In contrast, heating to 65°C produced increases of 2.66 nm and 3.32 nm in the cases of GI.1 and FCV, respectively, and heating to 75°C produced increases of 6.34, 6.32, and 5.32 nm, respectively, for GI.1, GII.4 and FCV.
TABLE 4.
Effect of pH, ionic strength, and temperature on the stability of virus-like particle tertiary structure, as monitored by UV fluorescence emission
| Condition | Tryptophan emission peak (nm) |
||
|---|---|---|---|
| GI.1 | GII.4 | FCV | |
| pH | |||
| 3 | 331.4 | 329.7 | 329.0 |
| 7 | 332.4 | 328.7 | 329.7 |
| 8 | 333.4 | 329.4 | 330.7 |
| Ionic strength (M) | |||
| 0 | 332.05 | 331.0 | 330.0 |
| 0.1 | 333.0 | 330.4 | 330.0 |
| 0.25 | 332.4 | 329.0 | 331.0 |
| Temp (°C) | |||
| 4 | 334.7 | 328.7 | 330.3 |
| 22 | 332.4 | 328.7 | 329.7 |
| 65 | 335.0 | 330.0 | 333.0 |
| 75 | 338.7 | 335.0 | 335.0 |
DISCUSSION
In this study, the effects of pH, ionic strength and temperature on the secondary and tertiary structures of virus-like particles representing noroviruses GI.1 and GII.4 and feline calicivirus were observed using circular dichroism and fluorescence spectroscopy. The experimental treatments were selected in order to produce conditions prevalent in the most likely vehicles of norovirus transmission (vomit and feces) and in the environment that the virus would have to withstand (pasteurization and refrigeration) in order to remain infectious. At neutral pH, the three VLPs, but especially GI.1 and GII.4, had similar proportions of the four types of secondary structure. The measured percentages of α-helices and β-strands (i.e., 8% and 35%, respectively) were in good agreement with the theoretical calculations and similar to levels reported by Ausar et al. for Norwalk VLPs (34).
All three VLPs were shown to undergo secondary and tertiary structural changes induced by changes in pH, ionic strength, and temperature. While acidic pH affected secondary structures of FCV (loss of β-strands) more than those of GI.1 (small losses of α-helices and β-strands), slightly basic pH did not induce much change in either VLP. Ausar et al. (34) reported that pH alone had no significant effect on Norwalk virus secondary structure other than a slight decrease in α-helix content concomitant with a slight increase in unordered structure at pH 8. In our study, FCV underwent a conspicuous shift from β to α at pH 3 but no net loss of ordered structure. These relative stabilities were reflected in the UV fluorescence results, which indicated no significant variation (±1 nm) in tryptophan emission wavelength and suggest that acidic and slightly basic conditions have minimal impact on the tertiary structures of these VLPs. This further suggests that the usual vehicles of human norovirus transmission do not promote viral adhesion to hydrophobic surfaces (e.g., lettuce, polypropylene, polyethylene, or polystyrene), since exposure of buried hydrophobic residues, loss of the hydration layer, structural rearrangement, and increased entropy do not result from a simple pH effect. While Girard et al. did not find any influence of pH on human norovirus adhesion to stainless steel (9), Vega et al. reported that FCV was more adherent to lettuce at pH 5 to 8 (35). In addition, Ausar et al. (34) did report changes in Norwalk virus tertiary structure due to pH variations and considered these to be sufficient to expose buried tryptophan residues particles to the solvent.
Variation of ionic strength caused conspicuous shifts from β-strand to α-helix, concomitant with slight losses of ordered secondary structure in GI.1 and FCV and barely perceptible losses of both structures in GII.4 particles. Increased ionic strength promoted helix formation in GI.1 and FCV at the expense of β-strands, a phenomenon likely due to a decrease in the dielectric constant of the solvent (36) favoring intrapeptide hydrogen bonding (33). Intrapeptide hydrogen bonds are features of helical conformations, while intermolecular hydrogen bonds are associated with strand-like conformations (33, 37). Trifluoroethanol (TFE) reportedly decreases the dielectric constant and promotes α-helix formation at the expense of both unordered and β-strand structures (33, 37). In contrast, increasing the ionic strength did not favor helical conformation in GII.4, which might be due to the observed overall stability of this VLP. In addition, changes in tryptophan emission wavelength at different ionic strengths were negligible and nonlinear, suggesting that VLP tertiary structures did not unfold to any appreciable degree, as was the case for the pH effect. A report by da Silva et al. (13) showed increased stability of GII.4 VLPs at higher concentrations of NaCl. Under the ionic strengths conditions tested in the present study, structural rearrangement, though considerable, was not expected to allow internal hydrophobic residues as large as tryptophan to disrupt the surface hydration layer and generate sufficient entropy to allow VLP adhesion to hydrophobic surfaces such as polystyrene, polyethylene, lettuce, etc. This might occur nevertheless, since da Silva et al. (13) reported positive correlations between ionic strength and adhesion of GI.1 and GII.4 VLPs to such surfaces at pH 8.
None of the three VLPs were sensitive to refrigeration at neutral pH, since their secondary structures remained comparable to that of the control (room temperature). However, heating to 65°C altered β-strands in both GI.1 and FCV, while these structures remained stable in GII.4. Although the helix remained relatively stable in GII.4 and FCV, a noticeable increase in helix content was observed in the case of GI.1, similar to that reported by Ausar et al. for Norwalk virus at 63°C (and at pH 3, 7, and 8) but attributed to the inherent inaccuracy of the structural evaluation (34). The observed drop in α-helix content in all three VLPs at 75°C is obviously consistent with the reported unfolding effect of temperature on this structure (33). The stability of β-strands is consistent with a previous report by Barrow et al. (37), who demonstrated that β-sheets are not sensitive to temperature and often increase to some extent. The secondary structures of the tested VLPs were generally more sensitive to heat than to pH or ionic strength. In addition, fluorescence UV results showed the largest variation in tryptophan emission wavelength, with positive shifts in the range of 5.32 to 6.34 nm for all VLPs at 75°C. Ausar et al. (34) found that the secondary, tertiary, and quaternary structures of norovirus VLPs are altered above 60°C. It therefore appears likely that heat would increase the adhesion of noroviruses to hydrophobic materials or food surfaces, and this adhesion is expected to be endothermic, in view of the conclusions of Dillman and Miller (1973) based on bovine serum albumin adhesion assays (14).
The combination of temperature with pH or ionic strength induced a broader range of changes to VLP secondary structure. Temperatures above 72.8°C combined with acidic pH brought the greatest loss of ordered structure for GII.4 particles, while GI.1 and FCV were more unstructured at pH 6 to 8. Heating to pasteurization temperatures or higher would be required to increase GII.4 or FCV adhesion to hydrophobic surfaces, while either refrigeration or pasteurization temperatures would favor GI.1 adhesion. Temperature and ionic strength had negatively correlated effects on GI.1 VLP structure. Regardless of the temperature, increased ionic strength caused a loss of ordered structure in GII.4 VLP but a gain in the case of GI.1. FCV lost ordered structure at low ionic strength (0 to 0.05 M) at refrigeration temperature, pasteurization temperature, and above and at high ionic strength (0.25 M) regardless of temperature. Under these conditions, subsequent loss of the hydration layer is expected to increase particle adhesion to hydrophobic surfaces. NaCl is the most common ingredient in prepared foods (e.g., sauces and condiments) and has been reported to favor virus adhesion by reinforcing hydrophobic or van der Waals attractions (11, 12). Combinations of low or high temperature with low or high ionic strength appear to increase VLP adhesion to hydrophobic surfaces, in particular that of GII.4. Human noroviruses GI and GII have been detected in berries after heating (heat drying treatment) to 80°C and 120°C, respectively (38). Although the authors of that study stated clearly that the particles detected were not infective (38), the finding that heating to pasteurization temperatures or higher may enhance VLP adhesion should be taken seriously in the food processing and food services sectors. In fact, native noroviruses can resist temperatures from freezing to 60°C and can persist on various surrounding surfaces, seafood, fresh foods, fruits, and vegetables (39). They are reportedly more heat resistant than poliovirus (40, 45), which may withstand 30 min of steaming (94°C) when buried in oysters (41). Any precautions for noroviruses should be extended to enteric viruses such as hepatitis A virus (HAV), which also has been found to resist inactivation by heat drying on berries at 100°C for 20 min (38). Furthermore, it is not yet certain that pasteurization inactivates all enteric viruses, including noroviruses and HAV (8, 42). Butot et al. reported that while blanching at 95°C considerably reduced the 50% tissue culture infective dose (TCID50) of HAV and FCV on various herbs (basil, chives, mint, and parsley), its efficacy for human norovirus GI and GII depended on the variety of the herb (38). The VLPs used in this study consisted of VP1 monomers. Norovirus VLPs are reportedly identical morphologically and antigenically to the native virus (26). However, they may be less stable because they lack the VP2 unit, which increases the stability of the major component VP1 by preventing disassembly and resisting attack by proteases (43). VLPs have been found to tolerate pH 3 but not pH 10 for 10 min (26). They are sensitive to temperatures above 60°C, and damage undergone at temperatures above 65°C has been found to be irreversible (34). They also have been found to be slightly more sensitive to physical agents such as gamma radiation than is murine norovirus 1 (MNV-1) (a well-known surrogate for human norovirus) and are believed to be inherently less stable than native human norovirus (44). However, the study of VLP stability may shed light on native norovirus stability and prove helpful in setting effective inactivation measures to limit the outbreak and spread of noroviruses.
VLP secondary and tertiary structural stability under the different pH and ionic strength conditions tested does not appear to be a major contributor to VLP adhesion. Under these conditions, a loss of hydration layer would rely on the exposed hydrophobic residues rather than the buried ones upon denaturation (structural rearrangement). In contrast, heat treatments, including pasteurization, are expected to expose buried hydrophobic residues that are likely to contribute to particles adhesion to hydrophobic material through structural rearrangement. Refrigeration would contribute to adhesion essentially under low- and high-ionic-strength conditions but to a lesser extent than would high temperatures. Since adhesion is a very complex phenomenon resulting from other intrinsic properties related to viral proteins and sorbent surfaces (food or inert surfaces), adhesion assays would be useful to clarify the role of structure and surface properties in the adhesion phenomenon. Of course, the actual infectiousness of viruses that become adherent as a result of structural rearrangements needs to be determined as well.
ACKNOWLEDGMENTS
This study was funded by a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC). Idrissa Samandoulgou was financially supported by a scholarship from Programme Canadien de Bourses de la Francophonie (PCBF).
We thank Ahmed Gomaa for advice on circular dichroism data deconvolution.
REFERENCES
- 1.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M-A, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroneman A, Harris J, Vennema H, Duizer E, van Duynhoven Y, Gray J, Iturriza M, Böttiger B, Falkenhorst G, Johnsen C, von Bonsdorff C-H, Maunula L, Kuusi M, Pothier P, Gallay A, Schreier E, Koch J, Szücs G, Reuter G, Krisztalovics K, Lynch M, McKeown P, Foley B, Coughlan S, Ruggeri FM, Di Bartolo I, Vainio K, Isakbaeva E, Poljsak-Prijatelj M, Hocevar Grom G, Bosch A, Buesa J, Sanchez Fauquier A, Hernandéz-Pezzi G, Hedlund K-O, Koopmans M. 2007. Data quality of 5 years of central norovirus outbreak reporting in the European network for food-borne viruses. J Public Health (Oxf) 30:82–90. [DOI] [PubMed] [Google Scholar]
- 3.Institut National de Santé Publique du Québec. 2011. Cas d'infection à Caliciviridae incluant le norovirus. Stat Anal Lab Santé Publ Québec 10:1–12. [Google Scholar]
- 4.Patel MM, Widdowson M-A, Glass RI, Akazawa K, Vinjé J, Parashar UD. 2008. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis 14:1224–1231. doi: 10.3201/eid1408.071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green KY, Ando T, Balayan MS, Berke T, Clarke N, Estes MK, Matson DO, Nakata S, Neill JD, Studdert MJ, Thiel H-J. 2000. Taxonomy of the caliciviruses. J Infect Dis 181:322–330. [DOI] [PubMed] [Google Scholar]
- 6.Le Guyader FS, Loisy F, Atmar RL, Hutson AM, Estes MK, Ruvoën-Clouet N, Pommepuy M, Le Pendu J. 2006. Norwalk virus-specific binding to oyster digestive tissues. Emerg Infect Dis 6:931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siebenga JJ, Vennema H, Zheng D-P, Vinje J, Lee BE, Pang X-L, Ho ECM, Lim W, Choudekar A, Broor S, Halperin T, Rasool BGN, Hewitt J, Greening GE, Jin M, Duan Z-J, Lucero Y, O'Ryan M, Hoehne M, Schreier E, Ratcliff RM, White PA, Iritani N, Reuter G, Koopmans M. 2009. Norovirus illness is a global problem: emergence and pread of norovirus GII.4 variants, 2001-2007. J Infect Dis 200:802–812. doi: 10.1086/605127. [DOI] [PubMed] [Google Scholar]
- 8.Greening GE. 2006. Human and animal viruses in food (including taxonomy of enteric viruses), p 5–42. In Goyal MS. (ed), Viruses in foods. Springer, New York, NY. [Google Scholar]
- 9.Girard M, Ngazoa S, Mattison K, Jean J. 2010. Attachment of noroviruses to stainless steel and their inactivation, using household disinfectants. J Food Prot 73:400–404. [DOI] [PubMed] [Google Scholar]
- 10.Gerba C. 1984. Applied and theoretical aspects of virus adsorption to surfaces. Adv Appl Microbiol 30:133–168. doi: 10.1016/S0065-2164(08)70054-6. [DOI] [PubMed] [Google Scholar]
- 11.Bitton G, Pancorbo O, Gifford GE. 1976. Factors affecting the adsorption of poliovirus to magnetite in water and wastewater. Water Res 10:978–980. [Google Scholar]
- 12.Farrah SR, Bitton G, Hoffmann EM, Lanni O, Pancorbo OC, Lutrick MC, Bertrand JE. 1981. Survival of enteroviruses and coliform bacteria in a sludge lagoon. Appl Environ Microbiol 41:459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.da Silva AK, Kavanagh OV, Estes MK, Elimelech M. 2011. Adsorption and aggregation properties of norovirus GI and GII virus-like particles demonstrate differing responses to solution chemistry. Environ Sci Technol 45:520–526. doi: 10.1021/es102368d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dillman WJ Jr, Miller IF. 1973. On the adsorption of serum proteins on polymer membrane surfaces. J Colloid Interface Sci 44:221–241. doi: 10.1016/0021-9797(73)90215-4. [DOI] [Google Scholar]
- 15.Mafu AA, Plumety C, Deschenes L, Goulet J. 2011. Adhesion of pathogenic bacteria to food contact surfaces: influence of pH of culture. Int J Microbiol 2011:1–9. doi: 10.1155/2011/972494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roach P, Farrar D, Perry CC. 2004. Interpretation of protein adsorption: surface-induced conformational changes. ACS Photonics 127:8168–8173. [DOI] [PubMed] [Google Scholar]
- 17.Norde W, Zoungrana T. 1998. Surface-induced changes in the structure and activity of enzymes physically immobilized at solid/liquid interfaces. Biotechnol Appl Biochem 28:133–143. [PubMed] [Google Scholar]
- 18.Haynes CA, Norde W. 1994. Globular proteins at solid/liquid interfaces. Colloids Surf B Biointerfaces 2:517–556. doi: 10.1016/0927-7765(94)80066-9. [DOI] [Google Scholar]
- 19.Norde W. 1994. Protein adsorption at solid surfaces: a thermodynamic approach. Pure Appl Chem 66:491–496. [Google Scholar]
- 20.Norde W, Macritchie F, Nowicka G, Lyklema J. 1986. Protein adsorption at solid-liquid interfaces: reversibility and conformation aspects. J Colloid Interface Sci 112:447–456. doi: 10.1016/0021-9797(86)90113-X. [DOI] [Google Scholar]
- 21.Norde W. 1986. Adsorption of proteins from solution at the solid-liquid interface. Adv Colloid Interface Sci 25:267–340. doi: 10.1016/0001-8686(86)80012-4. [DOI] [PubMed] [Google Scholar]
- 22.Soderquist ME, Walton AG. 1980. Structural changes in proteins adsorbed on polymer surfaces. J Colloid Interface Sci 75:386–397. doi: 10.1016/0021-9797(80)90463-4. [DOI] [Google Scholar]
- 23.Norde W, Lyklema J. 1989. Protein adsorption and bacterial adhesion to solid surfaces: a colloid-chemical approach. Colloid Surf 38:1–13. doi: 10.1016/0166-6622(89)80138-6. [DOI] [Google Scholar]
- 24.Norde W, Lyklema J. 1979. Thermodynamics of protein adsorption. Theory with special reference to the adsorption of human plasma albumin and bovine pancreas ribonuclease at polystyrene surfaces. J Colloid Interface Sci 71:350–366. [Google Scholar]
- 25.Samandoulgou I, Fliss I, Jean J. 2015. Zeta potential and aggregation of virus-like-particle of human norovirus and feline calicivirus under different physicochemical conditions. Food Environ Virol 7:249–260. doi: 10.1007/s12560-015-9198-0. [DOI] [PubMed] [Google Scholar]
- 26.Jiang X, Wang M, Graham DY, Estes MK. 1992. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol 66:6527–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huhti L, Blazevic V, Nurminen K, Koho T, Hytönen VP, Vesikari T. 2010. A comparison of methods for purification and concentration of norovirus GII-4 capsid virus-like particles. Arch Virol 155:1855–1858. doi: 10.1007/s00705-010-0768-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitmore L, Wallace BA. 2004. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acid Res 32:668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitmore L, Wallace BA. 2008. Protein secondary structure analyses from circular dichroism spectroscopy: methods and reference databases. Biopolymers 89:392–400. doi: 10.1002/bip.20853. [DOI] [PubMed] [Google Scholar]
- 30.Provencher SW, Glöckner J. 1981. Estimation of globular protein secondary structure from circular dichroism. Biochemistry 20:33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- 31.Johnson WC., Jr 1999. Analyzing protein circular dichroism spectra for accurate secondary structures. Proteins 35:307–312. [PubMed] [Google Scholar]
- 32.Sreerama N, Woody RW. 2000. Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal Biochem 287:252–260. doi: 10.1006/abio.2000.4880. [DOI] [PubMed] [Google Scholar]
- 33.Creighton TE. 2010. Biophysical chemistry of nucleic acids and proteins. Helvetian Press, New York, NY. [Google Scholar]
- 34.Ausar SF, Foubert TR, Hudson MH, Vedvick TS, Middaugh CR. 2006. Conformational stability and disassembly of Norwalk virus-like particles: effect of pH and temperature. J Biol Chem 281:19478–19488. doi: 10.1074/jbc.M603313200. [DOI] [PubMed] [Google Scholar]
- 35.Vega E, Smith J, Garlamd J, Matos A, Pillai SD. 2005. Variability of virus attachment patterns to butterhead lettuce. J Food Prot 68:2112–2117. [DOI] [PubMed] [Google Scholar]
- 36.Levy A, Andelman D, Orland H. 2012. The dielectric constant of ionic solutions: a field-theory approach. Phys Rev Lett 108:227801. doi: 10.1103/PhysRevLett.108.227801. [DOI] [PubMed] [Google Scholar]
- 37.Barrow CJ, Yasuda A, Kenny PTM, Zagorski MG. 1992. Solution conformations and aggregational properties of synthetic amyloid P-peptides of Alzheimer's disease. J Mol Biol 225:1075–1093. doi: 10.1016/0022-2836(92)90106-T. [DOI] [PubMed] [Google Scholar]
- 38.Butot S, Putallaz T, Amoroso R, Sánchez G. 2009. Inactivation of enteric viruses in minimally processed berries and herbs. Appl Environ Microbiol 75:4155–4161. doi: 10.1128/AEM.00182-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glass RI, Estes MK. 2009. Norovirus gastroenteritis. N Engl J Med 361:1776–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dolin R, Blacklow NR, DuPont H, Buscho RF, Wyatt RG, Kasel JA, Hornick R, Chanock RM. 1972. Biological properties of Norwalk agent of acute infectious nonbacterial gastroenteritis. Proc Soc Exp Biol Med 140:578–583. doi: 10.3181/00379727-140-36508. [DOI] [PubMed] [Google Scholar]
- 41.DiGirolamo R, Liston J, Matches JR. 1970. Survival of virus in chilled, frozen, and processed oysters. Appl Microbiol 20:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Appleton H. 2000. Control of foodborne viruses. Br Med Bull 56:172–183. doi: 10.1258/0007142001902879. [DOI] [PubMed] [Google Scholar]
- 43.Bertolotti-Ciarlet A, Crawford SE, Hutson AM, Estes MK. 2003. The 3′ end of Norwalk virus mRNA contains determinants that regulate the expression and stability of the viral capsid protein VP1: a novel function for the VP2 protein. J Virol 77:11603–11615. doi: 10.1128/JVI.77.21.11603-11615.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng K, Divers E, Ma Y, Li J. 2011. Inactivation of a human norovirus surrogate, human norovirus virus-like particles, and vesicular stomatitis virus by gamma irradiation. Appl Environ Microbiol 77:3507–3517. doi: 10.1128/AEM.00081-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirneisen KA, Black EP, Cascarino JL, Fino VR, Hoover DG, Kniel KE. 2010. Viral inactivation in foods: a review of traditional and novel food processing technologies. Compr Rev Food Sci Food Saf 9:3–20. doi: 10.1111/j.1541-4337.2009.00092.x. [DOI] [PubMed] [Google Scholar]