Abstract
The biosynthesis of the lantibiotics subtilin and nisin is regulated by autoinduction via two-component systems. Although subtilin is structurally closely related to nisin and contains the same lanthionine ring structure, both lantibiotics specifically autoinduce their biosynthesis. Subtilin and also the subtilin-like lantibiotics entianin and ericin autoinduce the two-component system SpaRK of Bacillus subtilis, whereas the biosynthesis of nisin is autoinduced via the two-component system NisRK of Lactococcus lactis. Autoinduction is highly specific for the respective lantibiotic and therefore of major importance for the functional expression of genetically engineered subtilin-like lantibiotics. To identify the structural features required for subtilin autoinduction, subtilin-nisin hybrids and specific point mutations of amino acid position 1 were generated. For subtilin autoinduction, the N-terminal tryptophan is the most important for full SpaK activation. The failure of subtilin to autoinduce the histidine kinase NisK mainly depends on the N-terminal tryptophan, as its single exchange to the aliphatic amino acid residues isoleucine, leucine, and valine provided NisK autoinduction. In addition, the production of subtilin variants which did not autoinduce their own biosynthesis could be rescued upon heterologous coexpression in B. subtilis DSM15029 by the autoinducing subtilin-like lantibiotic entianin.
INTRODUCTION
Lantibiotics are ribosomally synthesized and posttranslationally modified peptides with antimicrobial activity (1, 2). Members of the class I linear lantibiotics are the subtilin-like lantibiotics, produced by several Bacillus subtilis strains, and nisin, produced by various Lactococcus lactis strains (3–6). They exhibit strong antimicrobial activity against multiresistant pathogens, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) (3, 7). The presence of the modified amino acids meso-lanthionine and 3-methyllanthionine are name giving for lantibiotics (lanthionine-containing antibiotics) (1). The lantibiotic producers B. subtilis and L. lactis share a similar composition of genes within the lantibiotic gene cluster. The genes lanBTC code for a modification and transport machinery forming a multimeric synthetase complex (8, 9). This complex catalyzes the dehydration of serine and threonine residues (lanB) followed by a nucleophilic intramolecular addition of neighboring cysteine residues (lanC) (10, 11). The genes lanIFEG code for a self-immunity system consisting of an immunity protein and an ABC transporter (12–15). The genes lanRK code for a two-component system composed of a histidine kinase and a response regulator (12, 16).
Subtilin and nisin act as autoinducers for their own biosynthesis via a two-component system (TCS) (17–19). Upon sensing the extracellular lantibiotic subtilin or nisin, the respective histidine kinase gets phosphorylated. The phosphate group is transferred to the response regulator, which in turn induces the transcription of the operons within the lantibiotic cluster. In the case of subtilin, the SpaRK TCS induces the operons spaBTC and spaIFEG and the subtilin structural gene spaS itself (18); in the case of nisin, the NisRK TCS induces the operons nisABTC and nisIFEG (20). In L. lactis, the expression of NisRK is constitutive (20), and nisin biosynthesis is induced with increasing extracellular nisin concentrations. In B. subtilis, subtilin biosynthesis is under dual control (18). In addition to the regulation by SpaRK, biosynthesis is growth phase dependent (21), since the expression of SpaRK is under the control of the major transition state regulator AbrB and the alternative sigma factor H (22, 23). Additionally, as described previously, expression can be influenced by the glucose concentration independently of the growth phase (24).
The subtilin-like lantibiotics (subtilin, ericin S, and entianin) as well as nisin A from L. lactis share a highly similar ring structure. Subtilin-like lantibiotics are closely related and can activate SpaK (25) but cannot activate NisK. Conversely, nisin A is the activator of the NisRK TCS but cannot activate the subtilin TCS, SpaRK (19, 26). Recently, we showed that the N-terminal entianin fragment from amino acids 1 to 20 is sufficient to activate SpaK and that nisin quenches subtilin SpaK activation (25).
Due to the increasing number of multiresistant pathogenic bacteria, there is a strong need for the development of new antibiotics. Lantibiotics such as nisin and subtilin provide excellent tools to genetically engineer new lantibiotics, as they are ribosomally synthesized and the structural genes can be easily modified genetically. Whereas many such mutations have been generated for nisin in recent years, there were no attempts to engineer subtilin-like lantibiotics (19, 27–32). Knowing the structural features of the class I lantibiotics subtilin and nisin needed for autoinduction is an important prerequisite for genetic engineering and the improvement of their antimicrobial activities.
Here we describe an in vivo expression system that enables the expression of subtilin variants and subtilin-nisin hybrid peptides which were used for the investigation of SpaK/NisK specificity. The N-terminal tryptophan is decisive for SpaK activation and further prevents NisK activation. Subtilin-nisin hybrids which were able to activate both the SpaK and the NisK histidine kinases could be generated.
MATERIALS AND METHODS
Bacterial and yeast strains.
The strains and plasmids used in the present study are listed in Table 1. The oligonucleotides used for construction of the plasmids are listed in Table 2.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or descriptiona | Source or referenceb |
|---|---|---|
| Strains | ||
| B. subtilis | ||
| B15029 | Wild type (Ent+) | DSM15029 |
| B6633 | Wild type (Sub+) | ATCC 6633 |
| B6633.MB1 | ΔspaS amyE::PspaS-lacZ (Specr Cmr Sub−) | 26 |
| B15029.TSp01 | ΔetnS (Specr Ent−) | This work |
| B15029.TSp03 | ΔetnS amyE::PspaS-spaS (Specr Neor) | This work |
| B15029.TSp05 | ΔetnS amyE::PspaS-spaS(W1I) (Specr Neor) | This work |
| B15029.TSp10 | ΔetnS amyE::PspaS-spaS(W1I/K2T) (Specr Neor) | This work |
| B15029.TSp11 | ΔetnS amyE::PspaS-spaS(W1I/K2T/E4I) (Specr Neor) | This work |
| B15029.TSp24 | ΔetnS amyE::PspaS-spaS(W1I/K2T/E4I/V12K) (Specr Neor) | This work |
| B15029.TSp32 | ΔetnS amyE::PspaS-spaS(W1I/K2T/E4I/V12K/Q17 M) (Specr Neor) | This work |
| B15029.TSp57 | ΔetnS amyE::PspaS-spaS(W1E) (Specr Neor) | This work |
| B15029.TSp58 | ΔetnS amyE::PspaS-spaS(W1Y) (Specr Neor) | This work |
| B15029.TSp59 | ΔetnS amyE::PspaS-spaS(W1F) (Specr Neor) | This work |
| B15029.TSp60 | ΔetnS amyE::PspaS-spaS(W1G) (Specr Neor) | This work |
| B15029.TSp66 | ΔetnS amyE::PspaS-spaS(W1A) (Specr Neor) | This work |
| B15029.TSp67 | ΔetnS amyE::PspaS-spaS(W1D) (Specr Neor) | This work |
| B15029.TSp68 | ΔetnS amyE::PspaS-spaS(K2T/E4I/V12K/Q17M) (Specr Neor) | This work |
| B15029.TSp69 | ΔetnS amyE::PspaS-spaS(W1H) (Specr Neor) | This work |
| B15029.TSp71 | ΔetnS amyE::PspaS-spaS(W1K) (Specr Neor) | This work |
| B15029.TSp72 | ΔetnS amyE::PspaS-spaS(W1R) (Specr Neor) | This work |
| B15029.TSp81 | ΔetnS amyE::PspaS-spaS(W1L) (Specr Neor) | This work |
| B15029.TSp82 | ΔetnS amyE::PspaS-spaS(W1V) (Specr Neor) | This work |
| B15029.TSp89 | amyE::PspaS-spaS(W1E) (Neor) | This work |
| B15029.TSp90 | amyE::PspaS-spaS(W1D) (Neor) | This work |
| B15029.TSp91 | amyE::PspaS-spaS(W1A) (Neor) | This work |
| B15029.TSp98 | amyE::PspaS-spaS(W1K) (Neor) | This work |
| L. lactis | ||
| NZ9800 | NZ9700 derivative ΔnisA | 39 |
| NZ9800.AUT1 | NZ9800 containing pNZ8148 PnisA-lacZ (Cmr) | This work |
| E. coli DH5α | recA1 endA1 gyrA96 thi hsdR17(rK− mK+) relA1 supE44 ϕ80ΔlacZΔM15 Δ(lacZYA-argF)U169 | Laboratory stock |
| Kocuria rhizophila K9341 | Test strain for MIC determination | ATCC 9341 |
| S. cerevisiae CEN.PK2 | MATa/α ura3-52/ura3-52 trp1-289/trp1-289 leu2-3,112/leu2-3,112 his3Δ1/his3Δ1 MAL2-8c/MAL2-8c SUC2/SUC2 | 34 |
| Plasmids | ||
| pRS416 | bla URA3 shuttle vector | 33 |
| ECE74 | pCm::Spc | 35 |
| pMLK83 | amyE integrative plasmid (BGSC accession no. ECE103) | 36 |
| pSD27 | pMLK83 removal of gusA by EcoRI | This work |
| pRS426 | bla URA3 shuttle vector | 37 |
| pTSp10 | bla etnS′ Specr ′etnS | This work |
| pTSp16 | bla amyE′ PspaS-spaS Neor ′amyE | This work |
| pTSp25 | bla amyE′ PspaS-spaS(W1I) Neor ′amyE | This work |
| pTSp33 | bla amyE′ PspaS-spaS(W1I/K2T) Neor ′amyE | This work |
| pTSp34 | bla amyE′ PspaS-spaS(W1I/K2T/E4I) Neor ′amyE | This work |
| pTSp45 | bla amyE′ PspaS-spaS(W1I/K2T/E4I/V12K) Neor ′amyE | This work |
| pTSp49 | bla amyE′ PspaS-spaS(W1I/K2T/E4I/V12K/Q17 M) Neor ′amyE | This work |
| pCG2 | bla amyE′ PspaS BamHI Neor ′amyE | This work |
| pTSp99 | bla amyE′ PspaS-spaS(W1E) Neor ′amyE | This work |
| pTSp100 | bla amyE′ PspaS-spaS(W1Y) Neor ′amyE | This work |
| pTSp101 | bla amyE′ PspaS-spaS(W1F) Neor ′amyE | This work |
| pTSp102 | bla amyE′ PspaS-spaS(W1G) Neor ′amyE | This work |
| pTSp108 | bla amyE′ PspaS-spaS(W1D) Neor ′amyE | This work |
| pTSp109 | bla amyE′ PspaS-spaS(W1A) Neor ′amyE | This work |
| pTSp110 | bla amyE′ PspaS-spaS(K2T/E4I/V12K/Q17M) Neor ′amyE | This work |
| pTSp112 | bla amyE′ PspaS-spaS(W1H) Neor ′amyE | This work |
| pTSp115 | bla amyE′ PspaS-spaS(W1K) Neor ′amyE | This work |
| pTSp116 | bla amyE′ PspaS-spaS(W1R) Neor ′amyE | This work |
| pTSp124 | bla amyE′ PspaS-spaS(W1L) Neor ′amyE | This work |
| pTSp125 | bla amyE′ PspaS-spaS(W1V) Neor ′amyE | This work |
| pNZ8148 | Vector carrying nisA promoter Cmr | 38 |
| pSB5 | bla amyE′ PspaS-lacZ Cmr ′amyE | 18 |
| pAUT3 | pNZ8148 PnisA-lacZ | This work |
Ent+, entianin producer; Ent−, entianin nonproducer; Sub+, subtilin producer; Sub−, subtilin nonproducer; Cmr, chloramphenicol resistant; Specr, spectinomycin resistant; Neor, neomycin resistant. Letters in parentheses indicate the amino acid residues exchanged (corresponding to the IUPAC abbreviation), and numbers in parentheses indicate the positions of the exchanged amino acids in the respective subtilin variants. BGSC, Bacillus Genetic Stock Center.
DSM, German Resource Centre for Biological Material; ATCC, American Type Culture Collection.
TABLE 2.
Oligonucleotides used in this study
| Use and oligonucleotide | Sequence | Descriptiona |
|---|---|---|
| Amplification | ||
| TSp26 | CATCGAACTTTGACATATTG | |
| TSp27 | GGTATCGATAAGCTTGATATCGAATTCCTGCAGCCCGGGGGAAGACATTACCGTAGATGG | |
| TSp28 | GTCTATTGCAAACTTGCTTC | |
| TSp29 | AGCTCCACCGCGGTGGCGGCCGCTCTAGAACTAGTGGATCCCATTGTTTCAGATGTATCG | |
| TSp30 | AGGTATTGAAAGGAGGTGACCAATATGTCAAAGTTCGATGGGATCGATCTGTATAATAAAG | |
| TSp31 | TTACAAGTGATTGTTTGAAGGAAGCAAGTTTGCAATAGACGTTTATAAGTGGGTAAACCGTG | |
| BS53 | GGCCAGTGAGCGCGCGTAATACGACTCACTATAGGGCGAACCATCATTGATGGTTTCTTTC | |
| BS54 | GATTACGCCAAGCGCGCAATTAACCCTCACTAAAGGGAACAAGCGGAAGAATGAAGTAAG | |
| TSp19 | TTCAGGTGCTTTTTTTATTTTATAAACTCATTCCCTGATCGCCTAAAATGTTAACACTTC | |
| TSp45 | TCATCATCGCTCATCCATGTCGACGGTATCGATAAGCTTG CATGGTTACAGCGGTATCGGTC | |
| CG1 | ATTGGTCACCTCCTTTCAATAC | |
| CG2 | GATATTTGTCTGTTACTATTTAGGTATTGAAAGGAGGTGACCAATGGATCCGTAAAACCATTAGCATCACCTTGC | |
| NN16 | TCAGCCATGGGATCCCCAGCTTGTTGATACACTA | |
| NN17 | TCAGAAGCTTGGCAGACATGGCCTGCCCG | |
| In vitro mutagenesis | ||
| TSp48 | CTCCGCAAATTAAAAGTGAATCAC | fw W1I |
| TSp49 | GTGATTCACTTTTAATTTGCGGAG | rv W1I |
| TSp73 | CGCAAATTACAAGTGAATCAC | fw W1I/K2T |
| TSp74 | GTGATTCACTTGTAATTTGCG | rv W1I/K2T |
| TSp75 | GCAAATTACAAGTATTTCACTTTGTACACC | fw W1I/K2T/E4I |
| TSp76 | GGTGTACAAAGTGAAATACTTGTAATTTGC | rv W1I/K2T/E4I |
| TSp76_1 | GGATGTAAAACTGGTGCATTGC | fw W1I/K2T/E4I/V12K |
| TSp76_i | GCAATGCACCAGTTTTACATCC | rv W1I/K2T/E4I/V12K |
| TSp69 | GGTGCATTGATGACTTGCTTCCTTC | fw Q17 M |
| TSp70 | GAAGGAAGCAAGTCATCAATGCACC | rv Q17 M |
| Gap repair | ||
| CG11 | ACTTGCTTCCTTCAAACACTAACTTGTAACTGCAAAATCTCTAAATAAGTAAAACCATTAGCATCACCTTGCTCTGACTCCTTGCACT | Universal oligonucleotide |
| CG12 | TTATTTAGAGATTTTGCAGTTACAAGTTAGTGTTTGAAGGAAGCAAGTTTGCAATGCACCAGTTACACATCCTGGTGTACAAAGTGATTCACTTTT | Universal oligonucleotide |
| TSp191 | TGTGAAAGTCTCTAAACAAGACTCAAAAATCACTCCGCAAGCAAAAAGTGAATCACTTTGTACACCAGGATGTGTAACTGGTGCATTGCAA | W1A |
| TSp192 | TGTGAAAGTCTCTAAACAAGACTCAAAAATCACTCCGCAATTCAAAAGTGAATCACTTTGTACACCAGGATGTGTAACTGGTGCATTGCAA | W1F |
| TSp193 | TGTGAAAGTCTCTAAACAAGACTCAAAAATCACTCCGCAATATAAAAGTGAATCACTTTGTACACCAGGATGTGTAACTGGTGCATTGCAA | W1Y |
| TSp194 | TGTGAAAGTCTCTAAACAAGACTCAAAAATCACTCCGCAAGATAAAAGTGAATCACTTTGTACACCAGGATGTGTAACTGGTGCATTGCAA | W1D |
| TSp195 | TGTGAAAGTCTCTAAACAAGACTCAAAAATCACTCCGCAAGAGAAAAGTGAATCACTTTGTACACCAGGATGTGTAACTGGTGCATTGCAA | W1E |
| TSp196 | TGTGAAAGTCTCTAAACAAGACTCAAAAATCACTCCGCAACATAAAAGTGAATCACTTTGTACACCAGGATGTGTAACTGGTGCATTGCAA | W1H |
| TSp197 | TGTGAAAGTCTCTAAACAAGACTCAAAAATCACTCCGCAAAAAAAAAGTGAATCACTTTGTACACCAGGATGTGTAACTGGTGCATTGCAA | W1K |
| TSp198 | TGTGAAAGTCTCTAAACAAGACTCAAAAATCACTCCGCAAAGAAAAAGTGAATCACTTTGTACACCAGGATGTGTAACTGGTGCATTGCAA | W1R |
| TSp208 | TGTGAAAGTCTCTAAACAAGACTCAAAAATCACTCCGCAATGGACAAGTATTTCACTTTGTACACCAGGATGTAAAACTGGTGCATTGATG | K2T/E4I/V12K/Q17 M |
| TSp209 | TTATTTAGAGATTTTGCAGTTACAAGTTAGTGTTTGAAGGAAGCAAGTCATCAATGCACCAGTTTTACATCCTGGTGTACAAAGTGAAATACTTGT | K2T/E4I/V12K/Q17 M |
| TSp225 | TGTGAAAGTCTCTAAACAAGACTCAAAAATCACTCCGCAACTTAAAAGTGAATCACTTTGTACACCAGGATGTGTAACTGGTGCATTGCAA | W1L |
| TSp226 | TGTGAAAGTCTCTAAACAAGACTCAAAAATCACTCCGCAAGTAAAAAGTGAATCACTTTGTACACCAGGATGTGTAACTGGTGCATTGCAA | W1V |
Letters indicate the amino acid residues exchanged (corresponding to the IUPAC abbreviation), and numbers indicate the positions of the exchanged amino acids in the respective subtilin variants. fw, forward; rv, reverse.
pTSp10 was constructed for the deletion of the etnS structural gene in B. subtilis B15029. Therefore, pRS416 (33) was linearized with BamHI and transformed into Saccharomyces cerevisiae CEN.PK2 (34) together with the PCR products TSp26/27 (upstream etnS region), TSp28/29 (downstream etnS region), and TSp30/31 (spectinomycin resistance cassette, amplified from ECE74 [35]). Plasmid pBS09 was constructed for the integration of mutated spaS genes into the amyE locus of B. subtilis. Therefore, gusA of plasmid pMLK83 (36) was removed by EcoRI restriction, resulting in plasmid pSD27. Plasmid pRS426 (37) was linearized with EcoRI and transformed into CEN.PK2 together with the PCR product BS53/54 (the amyE locus integration region with the neomycin resistance cassette, amplified from pSD27), resulting in plasmid pBS09. Plasmid pTSp16 was constructed by the transformation of linearized pBS09 (EcoRI) and the PCR product of TSp45/19 (PspaS-spaS fragment) into CEN.PK2. The mutations W1I, W1I/K2T, W1I/K2T/E4I, W1IK2T/E4I/V12K, and W1I/K2T/E4I/V12K/Q17M of spaS were introduced step by step by oligonucleotides via in vitro mutagenesis PCR (Table 2), resulting in plasmids pTSp25, pTSp33, pTSp34, pTSp45, and pTSp49, respectively. For pCG02 construction, pBS09 was linearized with EcoRI and transformed into CEN.PK2 together with the PCR products TSp45/CG1 (PspaS-BamHI) and CG02/TSp19 (the 3′ region downstream spaS). Plasmid pCG02 was henceforth used as the backbone for the integration of various mutated spaS genes. For example, pTSp99 was created by transformation of BamHI-linearized pCG02 together with the universal oligonucleotides CG11 and CG12 and the oligonucleotide TSp195 into CEN.PK2 (Table 1). Plasmids with mutated subtilin structural genes were transformed into B15029.TSp01. Plasmid pAUT3 was constructed after restriction of pNZ8148 (38) and the PCR product NN16/17 (lacZ, amplified from pSB5 [18]) with NcoI/HindIII and subsequent ligation into Escherichia coli. The plasmid pAUT3 was transformed into L. lactis NZ9800 (39), resulting in nisin reporter strain NZ9800.AUT1.
Transformation for gap repair in Saccharomyces cerevisiae strain CEN.PK2 was performed as described by Schiestl and Gietz (40). Isolation of recombinant plasmids from CEN.PK2 was performed as described by Robzyk and Kassir (41). Plasmids isolated from CEN.PK2 were transformed into E. coli DH5α for further amplification. Isolation of recombinant plasmids from E. coli was carried out by an alkaline extraction procedure (42). Transformation of B. subtilis was performed as described previously (25).
Growth conditions for bacterial and yeast strains.
B. subtilis strain B15029 derivatives were grown at 37°C in medium A (43, 44) for optimized production of subtilin and subtilin variants. The autoinduction reporter strain was grown in TY medium containing 0.3 M NaCl (26). For selection, the following antibiotics were used at the indicated concentrations: spectinomycin, 100 μg ml−1; neomycin, 15 μg ml−1; and chloramphenicol, 5 μg ml−1. When selecting with two or more antibiotics simultaneously, the concentrations were reduced to half. L. lactis strains were grown in M17 medium (Fluka) supplemented with 0.5% glucose (GM17) at 30°C. E. coli DH5α strains with recombinant plasmids were grown at 37°C in TB medium (25) containing 100 μg ml−1 ampicillin. S. cerevisiae was grown at 30°C in YPD medium (1% yeast extract, 2% peptone, 2% glucose) or in synthetic dropout medium (0.5% ammonium sulfate, 0.17% yeast nitrogen base, 2% glucose) without uracil for plasmid marker selection.
Autoinduction plate assay.
For the rapid detection of lantibiotic production and autoinduction capacity, a simple plate assay was established. In that assay, a fresh overnight culture of subtilin reporter B6633.MB1 was inoculated to an optical density at 600 nm (OD600) of 0.1 and incubated at 37°C until an OD600 of 1 was reached. Subsequently, 100 μl culture was used to inoculate 100 ml cooled down LB agar medium supplemented with 4 mg 5-bromo-4-chloro-3-indoxyl-β-d-galactopyranoside (X-Gal). Thereafter, 10 μl of a fresh overnight culture of the strain of interest was spotted onto the plate. The plate was incubated overnight at 37°C.
Purification of lantibiotics.
Culture supernatants of subtilin and subtilin variant producers were prepurified according to a modified protocol described previously (25). Subtilin and subtilin variants were separated on a semipreparative reverse-phase (RP) high-performance liquid chromatography (HPLC) column (5 μm, 110 Å, 250 by 10 mm; Gemini-NX C18 liquid chromatography [LC] column [Phenomenex, CA, USA]) using eluents A (20% acetonitrile [HPLC grade], 0.1% 2,2,2-trifluoroethanoic acid [TFA]) and B (99.9% acetonitrile [HPLC grade], 0.1% TFA). Subtilin and subtilin variants could be separated with a linear gradient of 22% to 26% eluent B within 40 min. Nisin purification was performed as described previously (25). The absorbance was monitored at 214 nm. The collected fractions were dried under vacuum and resuspended in 5% acetonitrile for in vivo tests or in 30% acetonitrile, 0.1% TFA for identification by mass spectrometry.
Quantification of subtilin, subtilin variants, and nisin.
Subtilin and subtilin variants were quantified as described previously (24).The absorption of various dilutions of a subtilin stock solution (purified and mass spectrophotometrically confirmed) was monitored at 280 nm. Molarities were calculated using the molar extinction coefficient of subtilin (ε280 = 5,750 liter mol−1 cm−1; ProtParam; ExPASy). Different dilutions were prepared from this stock solution and analyzed by use of an analytical RP-HPLC column (5 μm, 250 by 4.6 mm; Gemini-NX). The absorbances of the samples were recorded at 214 nm and 280 nm. The peak areas were recorded using an Agilent 1200 series ChemStation for LC three-dimensional systems offline program and used to form equations (x = peak area; y = micrograms of lantibiotic), resulting in y = 1,195.8x + 370.97 and y = 77.684x + 7.4 for 214 nm and 280 nm, respectively. Solutions of subtilin variants and nisin were analyzed by analytic RP-HPLC at 214 nm, the peak areas were recorded, and concentrations were determined by using the equation y = 1,195.8x + 370.97.
Measurement of induction capacity by β-galactosidase assay.
Samples for β-galactosidase assay were harvested as described previously (25). In short, a fresh overnight culture of subtilin reporter strain B6633.MB1 was inoculated to an OD600 of 0.1 in TY medium containing 0.3 M NaCl. The strain was grown to an optical density of approximately 1.0. Thereafter, 2 ml of the culture was transferred into small test tubes containing different concentrations of lantibiotics. After 1 h, samples were taken and cells were harvested by centrifugation and stored at −20°C for β-galactosidase assay. The β-galactosidase activity was measured as described previously and normalized to the cell density (45). The nisin reporter strain NZ9800.AUT1 was grown at 30°C in GM17 medium supplemented with 25 μg ml−1 chloramphenicol and handled as described above.
Antibiotic activities of lantibiotic variants and hybrids.
The lowest concentration that prevented the growth of cells of the lantibiotic-sensitive indicator strain Kocuria rhizophila (formerly called Micrococcus luteus) ATCC 9341 (46) was determined to be the MIC. K. rhizophila was cultivated at 37°C in LB medium. A fresh overnight culture was inoculated to an OD600 of 0.001. For MIC determination, 2-fold serial dilutions of the lantibiotics were made and added to 2 ml culture, resulting in end concentrations of between 0.6 nM and 19.2 nM. The results were evaluated after 12 h of incubation.
RESULTS AND DISCUSSION
Subtilin-nisin hybrids show reduced SpaK induction but trigger NisK induction.
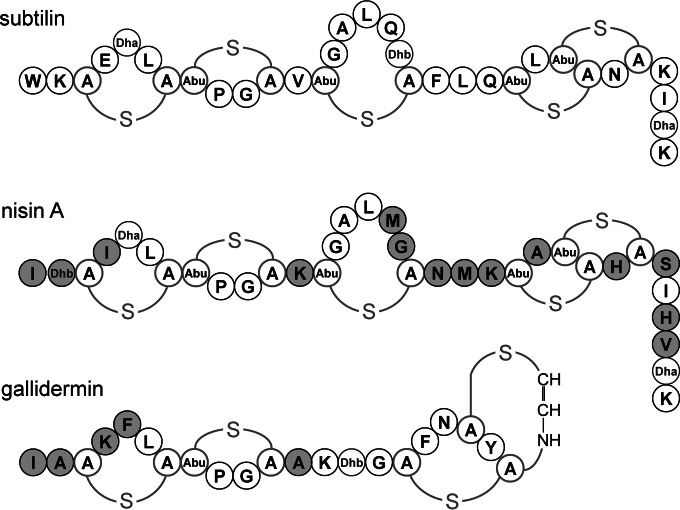
Class I lantibiotics like subtilin and nisin act as peptide pheromones and induce their biosynthesis in a quorum-sensing manner via the respective two-component system (TCS). Subtilin and nisin share a highly similar lanthionine ring structure, but their sequences differ at 14 amino acids (Fig. 1). The activation of the corresponding histidine kinase resulting in signal transduction is highly specific for its own cluster-derived lantibiotic (19, 25, 26).
FIG 1.
Structures of the lantibiotics subtilin, produced by B. subtilin ATCC 6633; nisin A, produced by several L. lactis strains; and gallidermin, produced by Staphylococcus gallinarum. The amino acids of nisin and the first 12 N-terminal amino acids of gallidermin that differ from those in subtilin are highlighted in gray. Abbreviations: A-S-A, meso-lanthionine; Abu-S-A, 3-methyl-lanthionine; Abu, α-aminobutyric acid; Dha, 2,3-didehydroalanine; Dhb, 2,3-didehydroburyrine.
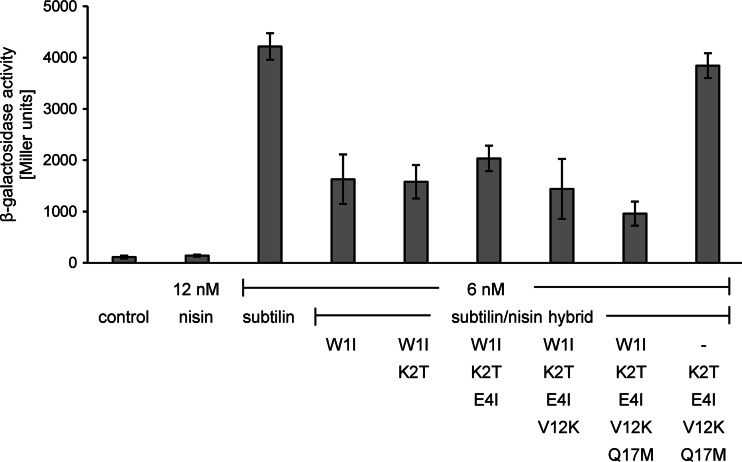
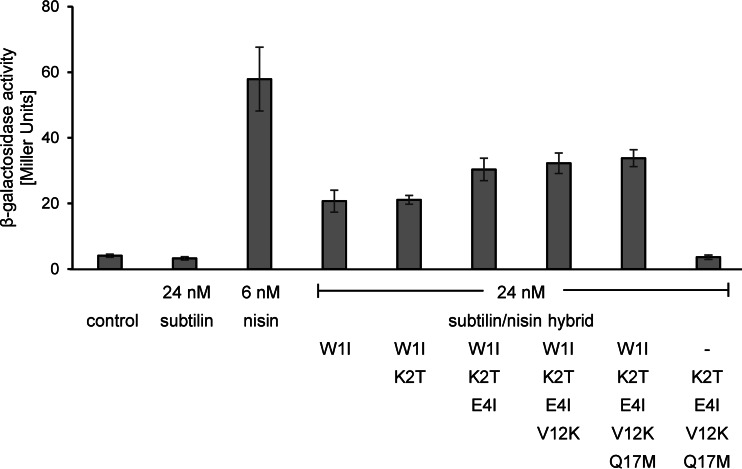
At nontoxic concentrations, nisin cannot activate SpaK, whereas at low concentrations, subtilin provides strong SpaK activation (Fig. 2). This is also the case vice versa, where low nisin concentrations provide a strong NisK response, whereas subtilin cannot activate NisK (Fig. 3).
FIG 2.
Induction response of the subtilin reporter B6633.MB1 upon addition of subtilin-nisin hybrid peptides with changes at the N termini. β-Galactosidase activities were measured after 60 min of induction. Error bars represent the standard deviations for samples from two separate cultures (n ≥ 3). Each measurement was carried out in duplicate. Control, no lantibiotic was added.
FIG 3.
Induction response of the nisin reporter NZ9800.AUT1 upon addition of subtilin-nisin hybrid peptides with changes at the N termini. β-Galactosidase activities were measured after 60 min of induction. Error bars represent the standard deviations for samples from two separate cultures (n = 2). Each measurement was carried out in duplicate. Control, no lantibiotic was added.
Knowledge of the structural features of the class I lantibiotics subtilin and nisin needed for autoinduction is an important prerequisite for genetic improvement of their antimicrobial activities.
To investigate the specificity of SpaK autoinduction, the N-terminal amino acids of subtilin were gradually adapted to those of nisin (Fig. 1). The resulting hybrid lantibiotics were expressed in B15029, where the etnS structural gene encoding entianin, a subtilin-like lantibiotic, was deleted. Entianin-producing strain B15029 was used instead of the subtilin producer B6633, as B15029 is easier to transform and to handle. The lantibiotic gene clusters in the two strains are nearly identical and behave absolutely similarly with respect to autoinduction, as shown previously (3, 25). After HPLC purification and quantification as previously described (24, 25), the autoinduction capacities of the hybrid lantibiotics were followed using subtilin reporter strain B6633.MB1 (Fig. 2).
Surprisingly, with subtilin W1I, the first amino acid exchange to isoleucine already resulted in a drastic 50% reduction of the induction capacity. Additional amino acid exchanges did not further significantly reduce the induction capacity. However, when the N-terminal tryptophan was recombined with other exchanges, e.g., hybrid peptide K2T/E4I/V12K/Q17M, the induction capacity was completely restored. These results clearly show the importance of the N-terminal tryptophan residue for the activation of SpaK.
In a corresponding experiment, NisK autoinduction was followed by the subtilin-nisin hybrid lantibiotics described above in the nisin reporter strain NZ9800.AUT1 (Fig. 3).
In contrast to the findings for subtilin, which mediated no NisK activation in nisin reporter strain NZ9800.AUT1, all hybrids where the N-terminal tryptophan of subtilin was replaced by isoleucine mediated moderate NisK activation. NisK activation was already detectable at concentrations of 6 nM (data not shown) and further increased at a higher concentration of 24 nM with respect to the level of activation by all other hybrid peptides (Fig. 3). Exchange of the first 4 amino acid residues of subtilin for those of nisin (subtilin W1I/K2T/E4I) slightly improved the level of autoinduction compared to that achieved with subtilin W1I, whereas a further adaptation of the N-terminal amino acids to residue 17 (subtilin W1IK2T/E4I/V12K/Q17M) did not result in an additional increase in β-galactosidase activity. This shows that the first 4 amino residues of nisin, which has 3 differences in amino acid composition compared to that of subtilin, are an important trigger for NisK activation. However, NisK activation by subtilin hybrid peptides W1I/K2T/E4I, W1IK2T/E4I/V12K, and W1IK2T/E4I/V12K/Q17M in NZ9800.AUT1 was still less efficient than that by nisin, and concentrations 4 times higher (24 nM) were needed to reach 70% of the entire level of autoinduction achieved with nisin. This shows that the C-terminal part of nisin with a further seven amino acid substitutions and the two additional amino acids, histidine and valine, compared to the sequence of subtilin also contributes to full nisin autoinduction.
To further prove the importance of isoleucine at position 1, the level of autoinduction achieved with the subtilin-nisin hybrid K2T/E4I/V12K/Q17M with tryptophan at the N terminus was measured. This hybrid peptide also failed to activate NisK in the reporter strain NZ9800.AUT1, which is in accordance with previous findings, in which replacement of the isoleucine at nisin position 1 by tryptophan abolished nisin autoinduction (19).
Importance of N-terminal amino acids for SpaK and NisK activation.
Our current results indicate that the tryptophan N terminus seems to be of major importance for SpaK activation. Therefore, the N-terminal tryptophan was replaced by small amino acids, aromatic amino acids, and charged amino acids. The mutated spaS genes were expressed in B15029 with an etnS deletion and tested in the autoinduction plate assay for color formation on X-Gal plates (see Fig. S1 in the supplemental material). Most subtilin peptides with N-terminal mutations showed autoinduction. Very weak color formation was observed when alanine and positively charged amino acids were at the N terminus of subtilin, whereas strains with peptides with glycine and negatively charged amino acids at the N termini completely failed to form color.
Obviously, peptides with glycine and negatively charged amino acids at the N termini either did not autoinduce or were not accessible for the lantibiotic biosynthesis machinery.
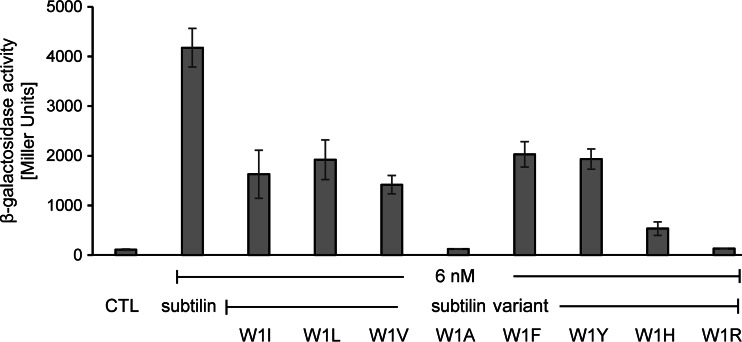
To compare the autoinduction capacities of the mutated lantibiotics, the respective peptides were HPLC purified to homogeneity and quantified, and their autoinduction was followed (Fig. 4).
FIG 4.
Induction response of the subtilin reporter B6633.MB1 upon addition of peptides with variations at subtilin position 1. β-Galactosidase activities were measured after 60 min of induction. Error bars represent the standard deviations for samples from two separate cultures (n = 2). Each measurement was carried out in duplicate. CTL, control to which no lantibiotic was added.
Similar to the activity obtained with hybrid peptide subtilin W1I, the peptides with leucine and valine showed reduced β-galactosidase activity that was approximately 50% of that of native subtilin. Replacement of tryptophan by other aromatic amino acids (F, Y) did not restore the full autoinduction of native subtilin, and histidine further reduced the level of autoinduction to 10% of that of native subtilin. The amount of peptide produced when the positively charged lysine was at the N terminus was too small for HPLC purification. The peptide with arginine at the N terminus could be purified, but nearly no autoinduction was measured. When these results are taken together with the failure to express SpaS mutations consisting of negatively charged amino acids at the N terminus, these data show that charged residues at the N terminus prevent autoinduction. This also coincides with the strong diminished autoinduction achieved with histidine (pKa of the side chain [pKR] = 6.0) in subtilin W1H, which also had a moderate charge under our experimental conditions.
Most remarkable was the very weak autoinduction obtained with subtilin W1A and the failure of subtilin W1G to activate SpaK, whereas the autoinduction capacity of peptides with valine, leucine, and isoleucine at position 1 was 50% of that of native subtilin. Obviously, the larger aliphatic side chain is advantageous for SpaK activation.
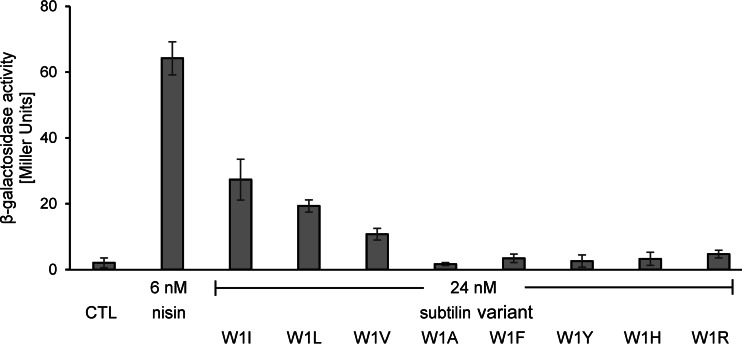
In a corresponding experiment, subtilin peptides with the N-terminal mutations were also tested with the nisin reporter NZ9800.AUT1 (Fig. 5), where 24 nM subtilin with the W1I mutation provided NisK activation that was 40% of that achieved with 6 nM nisin.
FIG 5.
Induction response of the nisin reporter NZ9800.AUT1 upon addition of peptides with variations at subtilin position 1. β-Galactosidase activities were measured after 60 min of induction. Error bars represent the standard deviations for samples from two separate cultures (n = 2). Each measurement was carried out in duplicate. CTL, control to which no lantibiotic was added.
As was also found for SpaK, the subtilin peptides with changes to leucine and valine activated NisK, whereas the peptide with alanine failed to significantly activate NisK. Interestingly, with a decreasing size of the aliphatic side chain, autoinduction becomes more diminished (the levels of autoinduction achieved with peptides with I, L, or V at the N terminus were 40%, 30%, and 20% of those achieved with the native peptide). As was also observed for SpaK, the positively charged arginine at the N terminus of subtilin did not provide NisK activation, and in contrast to the activation of SpaK by peptides with aromatic amino acids and histidine at the N terminus, all peptides with these amino acids at the N terminus failed to activate NisK.
Our results show that amino acid residues with larger aliphatic side chains can activate SpaK and NisK as well and that any charge at the N terminus of subtilin prevents the activation of both autoinduction systems. For SpaK, aromatic side chains and, preferably, tryptophan at the N terminus provide activation, whereas for NisK, aromatic side chains are not effective.
A functional analysis to support the findings presented above was performed by determination of the antimicrobial activity of some hybrids and variants against the lantibiotic-sensitive indicator strain Kocuria rhizophila ATCC 9341. All hybrids and variants showed MICs similar to those of subtilin and nisin for K. rhizophila (subtilin and nisin MICs, 4.8 nM), with the MICs of subtilin W1L, W1Y, and W1R, K2T/E4I/V12K/Q17M being 4.8 nM and those of subtilin W1I and W1I/K2T/E4I/V12K/Q17M being 9.6 nM.
Correlation of autoinduction and lantibiotic production.
Many subtilin variants and hybrid peptides were produced in reduced amounts. This raised the question of whether the biosynthetic machinery or a reduced autoinduction efficiency was responsible for the decreased levels of production. Therefore, we tested the correlation between lantibiotic production and autoinduction capacity. Using analytical HPLC, we determined the amount of lantibiotic in the culture supernatants of variant peptide producers in comparison to that in the culture supernatant of the wild-type subtilin producer (24). Thereafter, we compared the autoinduction capacities of the purified subtilin variants and subtilin. As aliphatic or aromatic amino acid residues at position 1 caused a reduction in autoinduction capacity to about 50% of that of native subtilin, the amounts of the variants with these changes at position 1 produced was reduced to about 70% of that of subtilin (data not shown). This shows that autoinduction and not the biosynthetic machinery is the strongest determinant of the amount of lantibiotic produced.
The amounts of peptides with positively charged amino acid residues and the small amino acid residue alanine at the N terminus produced were drastically reduced, and peptides with negatively charged residues at the N terminus were not produced at all. To test if a failure of autoinduction was responsible for the lack of peptide production, the respective subtilin variants were coexpressed in a wild-type subtilin-like lantibiotic producer. Hence, the genes coding for subtilin W1A, subtilin W1K, and subtilin W1E were introduced under the control of PspaS into the amyE locus of wild-type strain B15029.
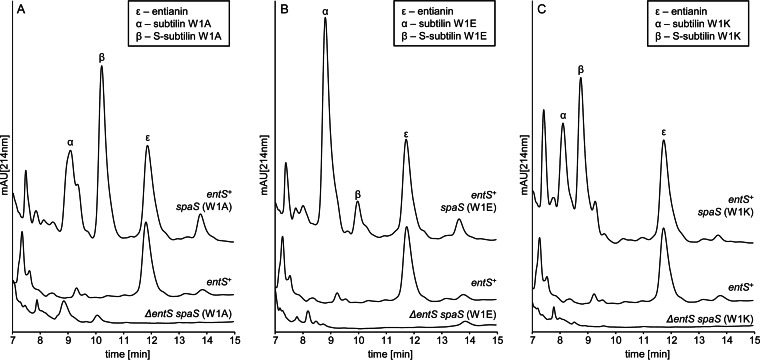
For all three strains, expression of the additionally introduced subtilin variant gene could be shown via analysis of the respective culture supernatants by HPLC (Fig. 6). This shows that the production of mutated subtilin can be restored by coexpression of entianin and excludes the possibility that the lanthionine synthetase complex is not able to interact with and modify the respective precursors. Moreover, the additional amount of the subtilin variants produced did not interfere with the total amount of entianin produced. In each case, the amount of entianin produced was not altered from the amount produced in wild-type strain B15029. Surprisingly, the amount of each subtilin variant produced was significantly higher than the amount of entianin produced, suggesting a higher rate of transcription of the mutated gene introduced ex loco. Thus, the turnover limit of the lanthionine synthetase complex was not reached, which is in accordance with previous findings, where an AbrB deletion strain exhibited a 6-fold-increased subtilin production rate (24).The higher ex loco transcription rate is also in accordance with the previously found discrepancy between autoinduction capacity and the amounts of variants with aliphatic or aromatic amino acid residues at position 1 produced.
FIG 6.
Coexpression of subtilin variants. Shown are comparative RP-HPLC chromatograms of subtilin variant producers and wild-type entianin producer B15029. The y axes of all chromatograms display the same intercepts. Greek letters indicate the corresponding lantibiotic, verified by mass spectrometry analysis. S-subtilin, succinylated lantibiotic molecules. (A) RP-HPLC chromatograms of B.15029.TSp66 ΔetnS spaS (W1A), B15029 entS+, and B15029.TSp91 etnS+ spaS (W1A). (B) RP-HPLC chromatograms of B15029.TSp57 ΔetnS spaS (W1E), B15029 etnS+, and B15029.TSp89 etnS+ spaS (W1E). (C) RP-HPLC chromatograms of B15029.TSp71 ΔetnS spaS (W1K), B15029 etnS+, and B15029.TSp98 etnS+ spaS (W1K). mAU, milli-absorbance units.
As reported previously, subtilin-like lantibiotics are succinylated at the N terminus (S-subtilin) to a certain extent. Succinylation depends on the medium composition and is also strain dependent (24). Remarkably, the amount of succinylation is different for the three subtilin variants (Fig. 6).
Gallidermin quenches entianin-mediated induction.
In addition to the importance of tryptophan at the N terminus, the N-terminal ring structure of subtilin also seems to be important for SpaK activation. As recently shown, the subtilin-like lantibiotics entianin and nisin and their corresponding N-terminal fragments compete at the sensory binding site of the histidine kinase SpaK, which suggests that the three N-terminal lanthionine rings are also involved in SpaK activation (25). Gallidermin and epidermin, which are the shortest class I lantibiotics (1, 47, 48), share an identical ring structure with respect to the first two N-terminal lanthionine rings. The third ring of gallidermin and epidermin, however, is very different from that of subtilin and nisin (Fig. 1) and also contains the C-terminal 2-aminovinyl ring structure (47, 49). Thus, gallidermin provided an excellent tool to verify the importance of the third ring for the assumed SpaK interaction.
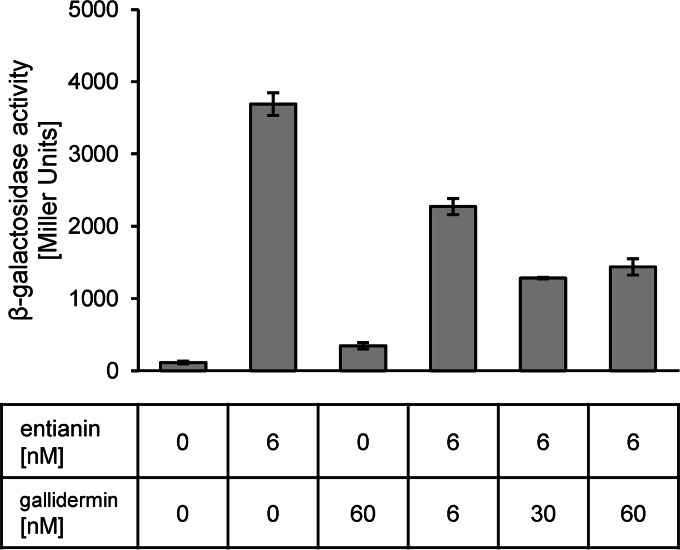
As shown in Fig. 7, similar to the results obtained with nisin, gallidermin quenches entianin autoinduction to 70% of the level of autoinduction mediated by entianin alone when the lantibiotics are used at equimolar concentrations (6 nM). If gallidermin was applied in 5-fold excess (30 nM), the entianin-mediated autoinduction was reduced to 30% of the level of autoinduction mediated by entianin (6 nM) alone; however, a higher concentration of gallidermin (60 nM) did not further reduce β-galactosidase activity. No SpaK activation was observed with the addition of 60 nM gallidermin alone.
FIG 7.
Influence of gallidermin on entianin-mediated induction of PspaS-lacZ. β-Galactosidase activities of PspaS-lacZ in reporter strain B6633.MB1 after 60 min of induction. Error bars represent the standard deviations for samples from two separate cultures (n = 2). Each measurement was carried out in duplicate.
Quenching of subtilin autoinduction by gallidermin clearly showed that the first two lanthionine rings of subtilin-like lantibiotics are sufficient for an interaction with the SpaK sensory binding site.
Taken together, our results show that subtilin-mediated autoinduction via SpaRK is significantly different from nisin-mediated autoinduction via NisRK. Surprisingly, a subtilin peptide with an N-terminal alanine failed to activate SpaK and NisK, whereas peptides with aliphatic side chains with increased sizes activated SpaK and NisK to certain extents. Whereas the size of the I, L, and V side chains had no effect on the activation of SpaK, the activation of NisK was more efficient with side chains of increasing sizes. These data show that hydrophobic interactions support SpaK and NisK activation. A major difference from those findings was observed for peptides with N termini with aromatic amino acids. Whereas tryptophan is needed for full SpaK activation, the affinity pocket of NisK is obviously not accessible to any aromatic side chain.
In summary, the structural constraints of tryptophan at the N terminus and the first two lanthionine rings should be considered for future genetic engineering of subtilin-like lantibiotics with respect to their efficient autoinduction and expression in B. subtilis. More than that, the successful coexpression of variants which do not autoinduce provides additional possibilities for the genetic engineering of subtilin-like lantibiotics and opens new possibilities to improve the antimicrobial activity of lantibiotics and their efficient production in B. subtilis.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by DFG (En 134/11-1).
We thank Stephanie Düsterhus for excellent technical support and F. Götz for providing the lantibiotic gallidermin. We also acknowledge Goethe University and the state of Hesse for providing laboratory space, basic support, and the necessary equipment, as well as M. Karas for his support during MS analysis.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02392-15.
REFERENCES
- 1.Schnell N, Entian KD, Schneider U, Götz F, Zähner H, Kellner R, Jung G. 1988. Prepeptide sequence of epidermin, a ribosomally synthesized antibiotic with four sulphide-rings. Nature 333:276–278. doi: 10.1038/333276a0. [DOI] [PubMed] [Google Scholar]
- 2.Arnison PG, Bibb MJ, Bierbaum G, Bowers AA, Bugni TS, Bulaj G, Camarero JA, Campopiano DJ, Challis GL, Clardy J, Cotter PD, Craik DJ, Dawson M, Dittmann E, Donadio S, Dorrestein PC, Entian KD, Fischbach MA, Garavelli JS, Göransson U, Gruber CW, Haft DH, Hemscheidt TK, Hertweck C, Hill C, Horswill AR, Jaspars M, Kelly WL, Klinman JP, Kuipers OP, Link AJ, Liu W, Marahiel MA, Mitchell DA, Moll GN, Moore BS, Müller R, Nair SK, Nes IF, Norris GE, Olivera BM, Onaka H, Patchett ML, Piel J, Reaney MJT, Rebuffat S, Ross RP, Sahl H, Schmidt EW, Selsted ME, et al. 2013. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat Prod Rep 30:108–160. doi: 10.1039/C2NP20085F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuchs SW, Jaskolla TW, Bochmann S, Kötter P, Wichelhaus T, Karas M, Stein T, Entian KD. 2011. Entianin, a novel subtilin-like lantibiotic from Bacillus subtilis subsp. spizizenii DSM 15029T with high antimicrobial activity. Appl Environ Microbiol 77:1698–1707. doi: 10.1128/AEM.01962-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattick ATR, Hirsch A. 1944. A powerful inhibitory substance produced by group N streptococci. Nature 154:551. doi: 10.1038/154551a0. [DOI] [Google Scholar]
- 5.Jansen EF, Hirschmann DJ. 1944. Subtilin, an antibacterial product of Bacillus subtilis: culturing conditions and properties. Arch Biochem 4:297–304. [Google Scholar]
- 6.Stein T, Borchert S, Conrad B, Feesche J, Hofemeister B, Hofemeister J, Entian KD. 2002. Two different lantibiotic-like peptides originate from the ericin gene cluster of Bacillus subtilis A1/3. J Bacteriol 184:1703–1711. doi: 10.1128/JB.184.6.1703-1711.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piper C, Draper LA, Cotter PD, Ross RP, Hill C. 2009. A comparison of the activities of lacticin 3147 and nisin against drug-resistant Staphylococcus aureus and Enterococcus species. J Antimicrob Chemother 64:546–551. doi: 10.1093/jac/dkp221. [DOI] [PubMed] [Google Scholar]
- 8.Siegers K, Heinzmann S, Entian KD. 1996. Biosynthesis of lantibiotic nisin. Posttranslational modification of its prepeptide occurs at a multimeric membrane-associated lanthionine synthetase complex. J Biol Chem 271:12294–12301. [DOI] [PubMed] [Google Scholar]
- 9.Kiesau P, Eikmanns U, Gutowski-Eckel Z, Weber S, Hammelmann M, Entian KD. 1997. Evidence for a multimeric subtilin synthetase complex. J Bacteriol 179:1475–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li B, Yu JP, Brunzelle JS, Moll GN, van der Donk WA, Nair SK. 2006. Structure and mechanism of the lantibiotic cyclase involved in nisin biosynthesis. Science 311:1464–1467. doi: 10.1126/science.1121422. [DOI] [PubMed] [Google Scholar]
- 11.Ortega MA, Hao Y, Zhang Q, Walker MC, van der Donk WA, Nair SK. 2014. Structure and mechanism of the tRNA-dependent lantibiotic dehydratase NisB. Nature 517:509–512. doi: 10.1038/nature13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelke G, Gutowski-Eckel Z, Kiesau P, Siegers K, Hammelmann M, Entian KD. 1994. Regulation of nisin biosynthesis and immunity in Lactococcus lactis 6F3. Appl Environ Microbiol 60:814–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein T, Heinzmann S, Solovieva I, Entian KD. 2003. Function of Lactococcus lactis nisin immunity genes nisI and nisFEG after coordinated expression in the surrogate host Bacillus subtilis. J Biol Chem 278:89–94. [DOI] [PubMed] [Google Scholar]
- 14.Klein C, Entian KD. 1994. Genes involved in self-protection against the lantibiotic subtilin produced by Bacillus subtilis ATCC 6633. Appl Environ Microbiol 60:2793–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuipers OP, Beerthuyzen MM, Siezen RJ, de Vos WM. 1993. Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis. Requirement of expression of the nisA and nisI genes for development of immunity. Eur J Biochem 216:281–291. [DOI] [PubMed] [Google Scholar]
- 16.Klein C, Kaletta C, Entian KD. 1993. Biosynthesis of the lantibiotic subtilin is regulated by a histidine kinase/response regulator system. Appl Environ Microbiol 59:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleerebezem M. 2004. Quorum sensing control of lantibiotic production; nisin and subtilin autoregulate their own biosynthesis. Peptides 25:1405–1414. doi: 10.1016/j.peptides.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Stein T, Borchert S, Kiesau P, Heinzmann S, Kloss S, Klein C, Helfrich M, Entian KD. 2002. Dual control of subtilin biosynthesis and immunity in Bacillus subtilis. Mol Microbiol 44:403–416. doi: 10.1046/j.1365-2958.2002.02869.x. [DOI] [PubMed] [Google Scholar]
- 19.Kuipers OP, Beerthuyzen MM, de Ruyter PG, Luesink EJ, de Vos WM. 1995. Autoregulation of nisin biosynthesis in Lactococcus lactis by signal transduction. J Biol Chem 270:27299–27304. doi: 10.1074/jbc.270.45.27299. [DOI] [PubMed] [Google Scholar]
- 20.de Ruyter PG, Kuipers OP, Beerthuyzen MM, van Alen-Boerrigter I, de Vos WM. 1996. Functional analysis of promoters in the nisin gene cluster of Lactococcus lactis. J Bacteriol 178:3434–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutowski-Eckel Z, Klein C, Siegers K, Böhm K, Hammelmann M, Entian KD. 1994. Growth phase-dependent regulation and membrane localization of SpaB, a protein involved in biosynthesis of the lantibiotic subtilin. Appl Environ Microbiol 60:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubnau E, Weir J, Nair G, Carter L, Moran C, Smith I. 1988. Bacillus sporulation gene spo0H codes for sigma 30 (sigma H). J Bacteriol 170:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weir J, Predich M, Dubnau E, Nair G, Smith I. 1991. Regulation of spo0H, a gene coding for the Bacillus subtilis sigma H factor. J Bacteriol 173:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bochmann SM, Spieß T, Kötter P, Entian KD. 2015. Synthesis and succinylation of subtilin-like lantibiotics are strongly influenced by glucose and transition state regulator AbrB. Appl Environ Microbiol 81:614–622. doi: 10.1128/AEM.02579-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spieß T, Korn SM, Kötter P, Entian KD. 2015. Activation of histidine kinase SpaK is mediated by the N-terminal part of subtilin-like lantibiotics and is independent of lipid II. Appl Environ Microbiol 81:5335–5343. doi: 10.1128/AEM.01368-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burkard M, Entian K, Stein T. 2007. Development and application of a microtiter plate-based autoinduction bioassay for detection of the lantibiotic subtilin. J Microbiol Methods 70:179–185. doi: 10.1016/j.mimet.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Field D, Begley M, O'Connor PM, Daly KM, Hugenholtz F, Cotter PD, Hill C, Ross RP. 2012. Bioengineered nisin A derivatives with enhanced activity against both Gram positive and Gram negative pathogens. PLoS One 7:e46884. doi: 10.1371/journal.pone.0046884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Healy B, Field D, O'Connor PM, Hill C, Cotter PD, Ross RP. 2013. Intensive mutagenesis of the nisin hinge leads to the rational design of enhanced derivatives. PLoS One 8:e79563. doi: 10.1371/journal.pone.0079563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Kraaij C, Breukink E, Rollema HS, Siezen RJ, Demel RA, de Kruijff B, Kuipers OP. 1997. Influence of charge differences in the C-terminal part of nisin on antimicrobial activity and signaling capacity. Eur J Biochem 247:114–120. doi: 10.1111/j.1432-1033.1997.00114.x. [DOI] [PubMed] [Google Scholar]
- 30.Field D, Quigley L, O'Connor PM, Rea MC, Daly K, Cotter PD, Hill C, Ross RP. 2010. Studies with bioengineered nisin peptides highlight the broad-spectrum potency of nisin V. Microb Biotechnol 3:473–486. doi: 10.1111/j.1751-7915.2010.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu W, Hansen JN. 1992. Enhancement of the chemical and antimicrobial properties of subtilin by site-directed mutagenesis. J Biol Chem 267:25078–25085. [PubMed] [Google Scholar]
- 32.Field D, O'Connor PM, Cotter PD, Hill C, Ross RP. 2008. The generation of nisin variants with enhanced activity against specific gram-positive pathogens. Mol Microbiol 69:218–230. doi: 10.1111/j.1365-2958.2008.06279.x. [DOI] [PubMed] [Google Scholar]
- 33.Sikorski RS, Hieter P. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Entian KD, Kötter P. 2007. Yeast genetic strain and plasmid collections. Methods Microbiol 36:629–666. [Google Scholar]
- 35.Steinmetz M, Richter R. 1994. Plasmids designed to alter the antibiotic resistance expressed by insertion mutations in Bacillus subtilis, through in vivo recombination. Gene 142:79–83. doi: 10.1016/0378-1119(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 36.Karow ML, Piggot PJ. 1995. Construction of gusA transcriptional fusion vectors for Bacillus subtilis and their utilization for studies of spore formation. Gene 163:69–74. doi: 10.1016/0378-1119(95)00402-R. [DOI] [PubMed] [Google Scholar]
- 37.Christianson TW, Sikorski RS, Dante M, Shero JH, Hieter P. 1992. Multifunctional yeast high-copy-number shuttle vectors. Gene 110:119–122. doi: 10.1016/0378-1119(92)90454-W. [DOI] [PubMed] [Google Scholar]
- 38.Kuipers OP, de Ruyter PGGA, Kleerebezem M, de Vos WM. 1998. Quorum sensing-controlled gene expression in lactic acid bacteria. J Biotechnol 64:15–21. doi: 10.1016/S0168-1656(98)00100-X. [DOI] [Google Scholar]
- 39.de Ruyter PG, Kuipers OP, de Vos WM. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl Environ Microbiol 62:3662–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schiestl RH, Gietz RD. 1989. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet 16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- 41.Robzyk K, Kassir Y. 1992. A simple and highly efficient procedure for rescuing autonomous plasmids from yeast. Nucleic Acids Res 20:3790. doi: 10.1093/nar/20.14.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Birnboim HC, Doly J. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banerjee S, Hansen JN. 1988. Structure and expression of a gene encoding the precursor of subtilin, a small protein antibiotic. J Biol Chem 263:9508–9514. [PubMed] [Google Scholar]
- 44.Feeney R, Garibaldi J, Humphreys EM. 1948. Nutritional studies on subtilin formation by Bacillus subtilis. Arch Biochem Biophys 17:435–445. [PubMed] [Google Scholar]
- 45.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 46.Tang JS, Gillevet PM. 2003. Reclassification of ATCC 9341 from Micrococcus luteus to Kocuria rhizophila. Int J Syst Evol Microbiol 53:995–997. doi: 10.1099/ijs.0.02372-0. [DOI] [PubMed] [Google Scholar]
- 47.Kellner R, Jung G, Hörner T, Zähner H, Schnell N, Entian KD, Götz F. 1988. Gallidermin: a new lanthionine-containing polypeptide antibiotic. Eur J Biochem 177:53–59. doi: 10.1111/j.1432-1033.1988.tb14344.x. [DOI] [PubMed] [Google Scholar]
- 48.Schnell N, Entian KD, Götz F, Hörner T, Kellner R, Jung G. 1989. Structural gene isolation and prepeptide sequence of gallidermin, a new lanthionine containing antibiotic. FEMS Microbiol Lett 49:263–267. [DOI] [PubMed] [Google Scholar]
- 49.Allgaier H, Jung G, Werner RG, Schneider U, Zähner H. 1986. Epidermin: sequencing of a heterodetic tetracyclic 21-peptide amide antibiotic. Eur J Biochem 160:9–22. doi: 10.1111/j.1432-1033.1986.tb09933.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.