Abstract
Chronic non-cancer pain (CNCP) is significant global health issue, accounting for a substantial increase in prescription analgesics worldwide, in recent decades. This clinical burden is evident in the UK prison population, where the prevalence of CNCP has never previously been determined. This study, conducted in June/July 2013, used prescribing data and a systematic review of clinical records from two UK prison establishments to derive a figure for point-prevalence of CNCP. Results showed that 20% of the total aggregated prisoner rolls (N = 1944) described CNCP and had been in receipt of treatment with daily analgesia, for a period of at least 3 months prior to observation date. This prevalence of CNCP was related to increasing age group (Spearman’s rank correlation 0.94). Of those on continuous analgesic therapy (CAT), 44% were taking continuous opioid therapy (COT) of any sort. Prisoners with a diagnosis of opioid-type drug dependence (OTDD) were more than twice as likely to complain of CNCP and be on continuous medication for it (odds ratio 2.3). The issues relating to CNCP in prisons are discussed. Further research is recommended, identifying factors influencing CNCP prevalence in prisons, and enabling comparisons to CNCP prevalence in the UK general population.
Keywords: Chronic pain, prison, prisoners, persistent pain, offender health, statistics and numerical data
Introduction
Chronic non-cancer pain (CNCP) has been defined as a painful condition lasting at least 3 months, including musculoskeletal pain, neuropathic pain, fibromyalgia, osteoarthritis and rheumatoid arthritis, but excluding headache, migraine, angina pectoris, cancer pain and pain associated with specific disease conditions (e.g. multiple sclerosis).1 Its prevalence in a general population has been estimated by a number of researchers: 19% in representative samples across eight European countries2 and 19% for chronic spinal pain in the United States,3 both based on self-reported symptoms described in cross-sectional surveys; 25% for chronic somatic pain reported in primary care records in the United States,4 while Gallagher in 2008, quoted the National Center for Health Statistics (United States) that ‘… 25% of Americans report chronic pain’.5
There is a substantial gap, within the international literature, of research into chronic non-cancer pain in prisons, and the actual prevalence of CNCP in prisons is hitherto unknown.6,7 This is a significant deficiency, given that states have a particular responsibility for the welfare of prisoners in their care, and that the diversion and abuse of medications prescribed to treat chronic pain are acknowledged as serious problems within the UK prison estate.7–9
CNCP is widely recognised as being associated with psychiatric co-morbidity, including depressive illness and/or anxiety.1,3,5,10–13,14–16 In 2005, Sullivan reported increased rates of dysthymia, panic disorder, depression and anxiety among patients on opioid drugs for CNCP.17 CNCP also has an association with insomnia.13,14 These associations are particularly marked where chronic physical co-morbidities are also present, such as diabetes mellitus or obesity, or in the presence of functional disability.18 Hennings et al. in 2012 identified lack of exercise as being an important adjunct in patients with CNCP and depressive illness.19 The frequent co-existence of CNCP and chronic anxiety or depression implies a likelihood that one begets the other, and that unsatisfactory treatment of one exacerbates both.10 There is also much evidence for a substantial impact of CNCP on general quality of life, with negative effects on individuals’ emotional well-being, economic independence, perception of general health, everyday functional ability and quality of relationships with others.1,3,5,15,20,21
Prisons in the United Kingdom can be regarded as pockets of deprivation where pain might assume a disproportionate magnitude. There is a relatively high prevalence of mental ill-health22–26 and chronic physical co-morbidity27–29 among prisoners, especially elderly prisoners, so the risk factors for CNCP described above would appear to be increased. As clinicians in the (male) UK prison estate, our personal impression is that chronic pain is very prevalent, with a substantial number of young, middle-aged and elderly prisoners prescribed continuous analgesic therapy (CAT) on account of chronic pain or acute-on-chronic pain, often of uncertain origin. This is mostly musculoskeletal, in which case it is usually spinal/truncal; it is sometimes peripheral, in which case it may be described in terms which suggest neuropathy. It is occasionally visceral. It is usually associated with functional disability (real or perceived), may be of spontaneous origin, but is often described as the legacy of alleged trauma (injuries sustained in industrial accidents, road traffic collisions, as a result of assault, or during arrest/legal control and restraint episodes or in the course of abortive suicide attempts). It may be associated with diagnosable disease, but often occurs in the absence of any obvious physical pathology. Usually diagnostic imaging has revealed evidence of degenerative disease, which is then held to be the origin of the pain, despite, in many cases, a poor anatomical correlation.
In sum, the evidence supports the assertion that CNCP, as a public health issue, has a significant prevalence, is burdensome to those individuals who suffer from it and is closely related to a lack of mental and physical well-being. It also provokes an increased rate of consumption of analgesic agents: over the last two decades, both in the United Kingdom and the United States, there have been significant increases in the number of opioid prescriptions,30–32 and these have been attributed to the more widespread use of COT (continuous opioid therapy) in CNCP.33 The UK prison estate is having to grapple with the challenge of meeting the health needs of an ageing prisoner population,34 and must also address the security implications of increased prescribing of psychoactive and habit-forming drugs. Research is therefore needed to get a clearer picture of the current problem of CNCP in the prison population.
Study objective
The purpose of the study was to provide basic prevalence figures for CNCP treated with CAT/COT (prescribed both for CNCP and opioid-type drug dependence) in a UK prison population. This would inform UK prison healthcare needs assessments and provide the foundation of further and more elaborate studies, designed to promote good practice in the management of CNCP in the prison environment.
Research questions
What is the prevalence of CNCP treated with CAT among prisoners in a two-prison population in the UK prison estate?
What proportion of these prisoners with CNCP is treated with COT?
What is the prevalence of CNCP among those prisoners with a historical or current diagnosis of opioid-type drug dependence (OTDD), who are currently being treated with COT?
Glossary
Chronic non-cancer pain (CNCP) is defined in this article as a painful condition lasting at least 3 months, including musculoskeletal pain, neuropathic pain, fibromyalgia, osteoarthritis and rheumatoid arthritis, but excluding headache, migraine, angina pectoris, cancer pain and pain associated with specific disease conditions (e.g. multiple sclerosis),1 but also for which a current prescription of continuous analgesic therapy (CAT) has been in force for that period of time. For the purposes of this study, we excluded pain of visceral or uro-genital origin.
Continuous analgesic therapy (CAT) is defined as prescribed pain-killing medication prescribed continuously for a period of at least 3 months. This comprises paracetamol, non-steroidal anti-inflammatory drug (NSAID) therapy, nefopam, lignocaine patches, amitriptyline, notriptyline, carbamazepine, pregabalin and gabapentin (where these agents are prescribed for a chronic pain condition, as they are also licensed for non-pain conditions) and continuous opioid therapy (COT).
Continuous opioid therapy (COT) is defined as prescribed opioid medication issued for a period of at least 3 months, either on account of a chronic painful condition, or opioid dependency.35 For the purposes of this study, we are also including codeine and all codeine/paracetamol combinations within this definition.
Methodology
We audited the management of prisoners in receipt of CAT, and identified that fraction of prisoners who have been in receipt of CAT for at least 3 months, in respect of a painful condition which meets the definition proposed by Reid et al.,1 but excluding pain of visceral or uro-genital origin. This was done by reviewing the computerised clinical record of each prisoner at one of two prisons in that part of the UK prison healthcare estate managed by Care UK, (an independent provider of prison healthcare), who were either in receipt of CAT, and establishing that the prisoner meet these criteria for CAT/CNCP; or were prescribed opioid substitution therapy on account of OTDD. Further details were then recorded, including age, prison number, description of CAT and description of COT. We wished to identify a prevalence of CNCP, significant enough from the patients’ perspective, to result in a demand for analgesic therapy which was then dispensed in sufficient quantity to allow consumption of this on a daily basis, for a period of at least 3 months. This would, therefore, exclude those prisoners whose pain was only acute or short-lived, and exclude those prisoners whose chronic pain, though noticeable, was not significant enough to motivate them to seek a regular and ongoing daily prescription remedy.
Comprehensive lists of analgesic drugs issued at the two prisons under study were therefore obtained from the prison pharmacies: these provided medication reports in the SystmOne primary care computer programmes utilised at both establishments. These reports were configured to generate two lists of prisoners, from each of the two prison sites, who had been issued with a prescription for one of these drugs, between 3 and 6 months prior to the dates of recruitment. The case records of these prisoners were then reviewed in order to identify the precise names of the drug(s); whether their prescription was still current; the symptom for which the prescription was issued; any excluding conditions as defined above; and co-existing diagnosed OTDD. The review process was validated to exclude observer bias: methodology was replicated by another observer (Rachel Mayhew) and the results concurred.
These lists were coded to ensure retrievability, and were then anonymised. The data were grouped according to age intervals, and compared with the total roll of prisoners in the two prisons, on the dates of recruitment. The data were then aggregated between the two prisons, and percentages calculated of prisoners in each age group, fulfilling the criteria for CAT for CNCP; COT for CNCP; non-COT for CNCP and COT for CNCP after OTDD prisoners had been excluded. The proportion of prisoners on CAT who were taking COT was also studied, as was the age-related prevalence of prisoners on COT who had an established diagnosis of OTDD. Spearman’s rank correlation coefficient was used to test any correlation with age, and a standard odds ratio calculation made to compare the differences in prevalence of CNCP between a general prisoner population and one of known OTDD prisoners. Boxes 1 and 2 describe the characteristics of the two establishments studied.
Box 1. Characteristics of Prison 1.
Located in the south of England, Category B training establishment holding an adult male population, comprising vulnerable prisoners/sex offenders and ordinary prisoners, including some serving Indeterminate for Public Protection (IPP) and Life sentences. A few prisoners held on remand awaiting trial at local courts.
Box 2. Characteristics of Prison 2.
Located in the north of England, a Category A and B establishment holding an adult male population, including those serving 4 years and over, and IPP or Life sentences plus High and Standard Risk Category A remands.
Results
Figure 1 and Box 3 show the prevalence of CNCP among the prisoners studied. Table 1 shows CAT for all excluded conditions.
Figure 1.

Breakdown of total prisoner roll showing identification of CNCP cases.
CAT: continuous analgesic therapy; CNCP: chronic non-cancer pain; OTDD: opioid-type drug dependence.
Table 1.
CAT for all excluded conditions.
| Aggregated data: exclusions | Total |
|---|---|
| Headache including migraine | 22 |
| Cancer pain | 5 |
| Abdominal pain including IBS, Crohn’s disease | 5 |
| Sickle cell disease | 1 |
| Testicular pain | 2 |
| Pain from urolithiasis | 1 |
| Toothache | 2 |
| CAT for all excluded conditions | 38 |
CAT: continuous analgesic therapy; IBS: inflammatory bowel syndrome.
Box 3. The Prevalence of CNCP amongst patients with diagnosed opioid-type drug dependence.
Of a total of 46 prisoners diagnosed with opioid-type drug dependence, 26 had no evident complaint of CNCP.
The other 20 opioid-type drug dependent prisoners described CNCP and were taking continuous medication for it: 43% of the total. This compares with the 20% figure for the general prison population studied (odds ratio 2.23).
What is the prevalence of CNCP treated with CAT in a two-prison population in the UK prison estate?
The prevalence was 18.3% for Prison 1 and 22.7% for Prison 2, giving an aggregated prevalence of 20%. Charts 1 and 2 demonstrate that the age profile for the total roll in both establishments to be broadly similar, with a corresponding similarity in age profiles for prisoners on CAT and COT for CNCP, respectively.
Chart 1.

Histogram showing numbers of patients (y-axis) in each age range (x-axis), for Prison 1.
CNCP: chronic non-cancer pain.
Blue = total prisoners in roll (N = 1154).
Red = patients on continuous analgesic therapy for CNCP (n = 211).
Orange = patients on continuous opioid therapy for CNCP (n = 89).
Chart 2.
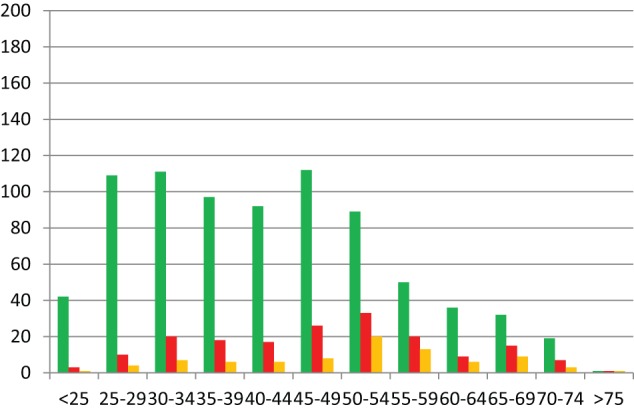
Histogram showing numbers of patients (y-axis) in each age range (x-axis), for Prison 2.
CNCP: chronic non-cancer pain.
Green = total prisoners in roll (N = 790).
Red = patients on continuous analgesic therapy for CNCP (n = 179).
Orange = patients on continuous opioid therapy for CNCP (n = 84).
Aggregation of these results, and their expression as a percentage of the total roll in each age group, demonstrated that the prevalence of CNCP, treated with CAT of any sort, appeared to correlate with increasing patient age group (Chart 3, Spearman’s rank correlation 0.94).
Chart 3.

Histogram showing percentage of prisoners on continuous analgesic therapy for CNCP (y-axis) in each age range (x-axis), for aggregated populations from both prisons. Trend line included (n = 390, N = 1944). Spearman’s rank correlation 0.94.
CNCP: chronic non-cancer pain.
This correlation with increasing age group was also evident for the prevalence of CNCP treated with opioid drugs (Chart 4, Spearman’s rank correlation 0.95).
Chart 4.
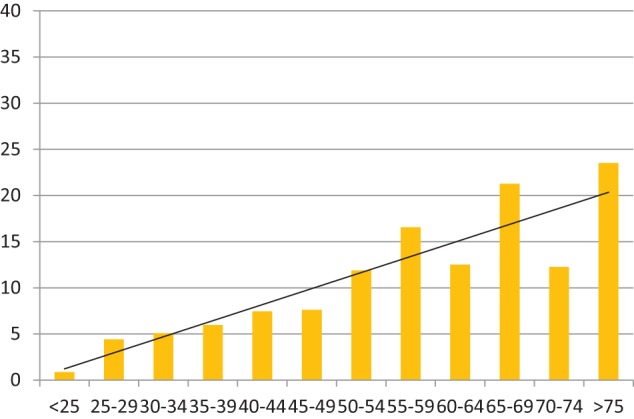
Histogram showing percentage of prisoners on continuous opioid therapy for CNCP (y-axis) in each age range (x-axis), for aggregated populations from both prisons. Trend line included (n = 173, N = 1944). Spearman’s rank correlation 0.95.
CNCP: chronic non-cancer pain.
This correlation with increasing age group was also evident for the prevalence of CNCP treated with simple analgesia and NSAIDs, which are neither habit-forming or prized in the prison shadow economy (Chart 5, Spearman’s rank correlation 0.76).
Chart 5.
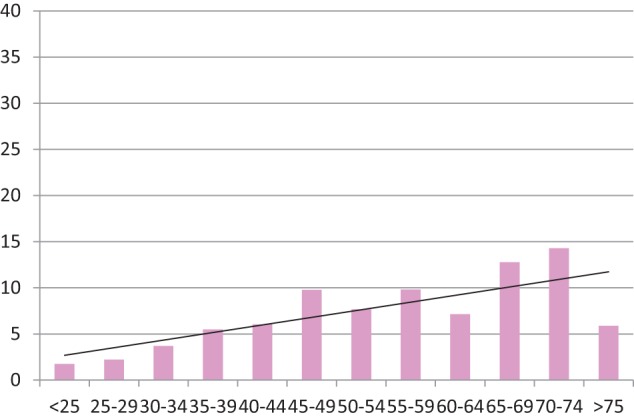
Histogram showing percentage of prisoners on simple analgesia and NSAIDs only for CNCP (y-axis) in each age range (x-axis), for aggregated populations from both prisons. Trend line included (n = 130, N = 1944). Spearman’s rank correlation 0.76.
CNCP: chronic non-cancer pain; NSAID: non-steroidal anti-inflammatory drug.
This correlation with increasing age group was also evident for the prevalence of CNCP treated with opioid drugs, after those with diagnosed opioid-style drug dependence had been excluded (Chart 6, Spearman’s rank correlation 0.96).
Chart 6.

Histogram showing percentage of prisoners on COT for CNCP, excluding those with diagnosed OTDD (y-axis) in each age range (x-axis), for aggregated populations from both prisons. Trend line included (n = 153, N = 1944) Spearman’s rank correlation 0.96.
CNCP: chronic non-cancer pain; COT: continuous opioid therapy; OTDD: opioid-type drug dependence.
What proportion of prisoners with CNCP treated with CAT, is treated with COT?
The proportion of prisoners with CNCP treated with CAT receiving COT (continuous treatment with opioid-containing drugs of any sort) was 44% (89/211 for Prison 1 (42%), and 84/179 for Prison 2 (47%): again, broadly similar). Aggregated figures demonstrated that a correlation with age was still evident, though weaker, with a greater proportion of older prisoners receiving opiate-based analgesia for their CNCP (Chart 7).
Chart 7.
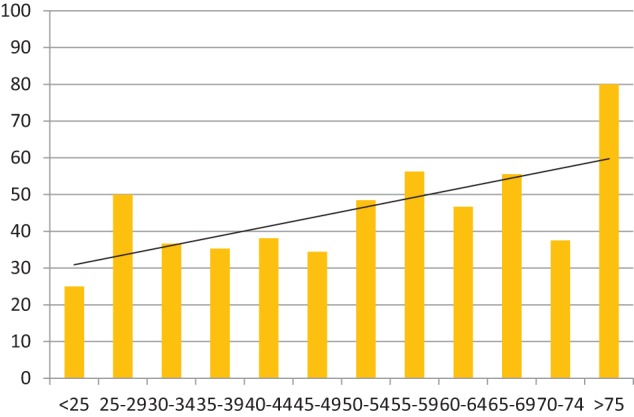
Histogram showing percentage of prisoners on CAT for CNCP, who are in receipt of continuous opioid therapy (y-axis) in each age range (x-axis), for aggregated populations from both prisons. Trend line included (n = 173, N = 390). Spearman’s rank correlation 0.59.
CAT: continuous analgesic therapy; CNCP: chronic non-cancer pain.
What is the prevalence of CNCP among those prisoners with a historical or current diagnosis of opioid-type drug dependence, who are currently being treated with COT?
The prevalence of CNCP among these prisoners was 43% (20/46 in aggregated data from both establishments). Compared to the general prisoner prevalence of CNCP of 20%, this gave an odds ratio of 2.3, showing prisoners with diagnosed OTDD as more than twice as likely to describe CNCP as those without.
Prescribing characteristics at the two prisons
Prescribing practice for CNCP at both establishments was broadly similar, as evidenced by proportions of prescriptions for each class of drug, when expressed in terms of the World Health Organization (WHO) ladder for analgesic use (Chart 8 and Table 2).
Chart 8.

WHO treatment ladder for analgesia in prisons. Cumulative percentage of patients treated with each class of drug, at each establishment.
Blue line = Prison 1 (n = 211).
Green line = Prison 2 (n = 179).
WHO: World Health Organization.
Table 2.
Percentage data and absolute numbers from which Chart 8 is derived.
| P1 | P2 | P1 | P2 | |
|---|---|---|---|---|
| Simple analgesia only | 15.2% | 21.2% | 32 | 38 |
| Stronger NSAIDs | 31.6% | 35.2% | 67 | 63 |
| TCA + carbamazepine | 37.4% | 39.1% | 79 | 70 |
| Nefopam | 46.9% | 42.5% | 99 | 76 |
| Codeine/co-codamol | 58.8% | 65.4% | 124 | 117 |
| Stronger opioids/GABA analogues | 100% | 100% | 211 | 179 |
P1 = prison 1 P2 = prison 2.
GABA: gamma-aminobutyric acid; NSAID: non-steroidal anti-inflammatory drug; TCA: tricyclic antidepressant.
The prevalence of diagnosed OTDD (and therefore prescribing rates for methadone and buprenorphine treatment) were, however, significantly different in the two establishments: 58/1154 in Prison 1 and 7/790 in Prison 2. This perhaps reflects Prison 2’s status as a high-security establishment, with a larger proportion of prisoners serving very long sentences.
Detailed data of results are given in Tables 3–6.
Table 3.
Results from Prison 1.
| Prison 1 on 2 June 2013 | Total | <25 | 25–29 | 30–34 | 35–39 | 40–44 | 45–49 | 50–54 | 55–59 | 60–64 | 65–69 | 70–74 | >75 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prisoners in roll | 1154 | 72 | 117 | 105 | 104 | 123 | 164 | 172 | 113 | 76 | 62 | 30 | 16 |
| Analgesics commenced 3–6 months ago | 562 | 25 | 41 | 41 | 56 | 65 | 91 | 83 | 64 | 39 | 34 | 15 | 8 |
| Acute/lapsed only | 302 | 23 | 28 | 23 | 33 | 30 | 51 | 47 | 34 | 13 | 11 | 5 | 4 |
| Chronic excluded | 26 | 1 | 0 | 2 | 2 | 3 | 3 | 5 | 2 | 5 | 2 | 1 | 0 |
| CAT for CNCP | 211 | 1 | 10 | 10 | 16 | 25 | 35 | 31 | 28 | 21 | 21 | 9 | 4 |
| As percentage of prisoners in roll | 18.3 | 1.4 | 8.5 | 9.5 | 15.4 | 20.3 | 21.3 | 18 | 24.8 | 27.6 | 33.9 | 30 | 25 |
| CAT for CNCP no COT | 122 | 1 | 4 | 6 | 10 | 15 | 22 | 20 | 14 | 13 | 10 | 6 | 1 |
| As percentage of prisoners on CAT for CNCP | 58.8 | 100 | 40 | 60 | 62.5 | 60 | 62.9 | 64.5 | 50 | 61.9 | 47.6 | 66.7 | 75 |
| COT for CNCP | 89 | 0 | 6 | 4 | 6 | 10 | 13 | 11 | 14 | 8 | 11 | 3 | 3 |
| As percentage of prisoners on CAT for CNCP | 41.2 | 0 | 60 | 40 | 37.5 | 40 | 37.1 | 35.5 | 50 | 38.1 | 52.4 | 33.3 | 25 |
| COT | 110 | 0 | 9 | 10 | 11 | 17 | 15 | 11 | 14 | 8 | 11 | 3 | 3 |
| As percentage of prisoners in roll | 9.5 | 0 | 7.7 | 9.5 | 10.6 | 13.8 | 9.1 | 6.4 | 12.4 | 10.5 | 17.7 | 10 | 6.3 |
| No h/o diagnosed OTDD with COT for CNCP | 71 | 0 | 0 | 3 | 4 | 6 | 10 | 10 | 13 | 8 | 11 | 3 | 1 |
| As percentage of prisoners on COT | 62.7 | 0 | 30 | 36.4 | 35.2 | 66.7 | 90.9 | 92.5 | 100 | 100 | 100 | 100 | |
| h/o diagnosed OTDD with COT for CNCP | 18 | 0 | 6 | 1 | 2 | 4 | 3 | 1 | 1 | 0 | 0 | 0 | 0 |
| As percentage of prisoners h/o diagnosed OTDD on COT | 43.9 | 66.7 | 14.3 | 28.6 | 36.3 | 60 | 100 | 100 | |||||
| h/o diagnosed OTDD | 58 | 2 | 11 | 8 | 14 | 14 | 6 | 2 | 1 | 0 | 0 | 0 | 0 |
| As percentage of prisoners in roll | 5 | 2.8 | 9.4 | 7.6 | 13.4 | 11.4 | 3.7 | 1.2 | 0.8 | 0 | 0 | 0 | 0 |
| h/o diagnosed OTDD on COT | 41 | 0 | 9 | 7 | 7 | 11 | 5 | 1 | 1 | 0 | 0 | 0 | 0 |
| As percentage of prisoners on COT | 37.3 | 100 | 70 | 63.6 | 64.7 | 33.3 | 9.1 | 7.1 | 0 | 0 | 0 | 0 | |
| h/o diagnosed OTDD not on COT | 17 | 2 | 2 | 1 | 7 | 3 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| h/o diagnosed OTDD on COT no CNCP | 23 | 0 | 3 | 6 | 5 | 7 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| As percentage of prisoners h/o diagnosed OTDD on COT | 56.1 | 33.3 | 85.7 | 71.4 | 63.6 | 40 | 0 | 0 |
CAT: continuous analgesic therapy; CNCP: chronic non-cancer pain; COT: continuous opioid therapy; OTDD: opioid-type drug dependence.
Table 4.
Results from Prison 2.
| Prison 2 on 19 July 2013 | Total | <25 | 25–29 | 30–34 | 35–39 | 40–44 | 45–49 | 50–54 | 55–59 | 60–64 | 65–69 | 70–74 | >75 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prisoners in roll | 790 | 42 | 109 | 111 | 97 | 92 | 112 | 89 | 50 | 36 | 32 | 19 | 1 |
| Analgesics commenced 3–6 months ago | 348 | 10 | 37 | 43 | 42 | 36 | 56 | 47 | 32 | 16 | 20 | 8 | 1 |
| Acute / lapsed only | 153 | 6 | 25 | 23 | 21 | 18 | 24 | 13 | 11 | 7 | 4 | 1 | 0 |
| Chronic excluded | 12 | 1 | 1 | 0 | 3 | 0 | 5 | 1 | 1 | 0 | 0 | 0 | 0 |
| CAT for CNCP | 179 | 3 | 10 | 20 | 18 | 17 | 26 | 33 | 20 | 9 | 15 | 7 | 1 |
| As percentage of prisoners in roll | 22.7 | 7.1 | 9.2 | 18 | 18.6 | 18.5 | 23.2 | 37.1 | 40 | 25 | 46.9 | 36.8 | 100 |
| CAT for CNCP no COT | 94 | 2 | 6 | 13 | 12 | 11 | 18 | 13 | 7 | 3 | 5 | 4 | 0 |
| As percentage of prisoners on CAT for CNCP | 52.5 | 66.7 | 60 | 65 | 66.7 | 64.7 | 69.2 | 39.4 | 35 | 33.3 | 33.3 | 57.1 | 0 |
| COT for CNCP | 84 | 1 | 4 | 7 | 6 | 6 | 8 | 20 | 13 | 6 | 9 | 3 | 1 |
| As percentage of prisoners on CAT for CNCP | 47.5 | 33.3 | 40 | 35 | 33.3 | 35.3 | 30.8 | 60.6 | 65 | 66.7 | 66.7 | 42.9 | 100 |
| COT | 95 | 2 | 6 | 13 | 12 | 11 | 18 | 13 | 7 | 3 | 6 | 4 | 0 |
| As percentage of prisoners in roll | 11.1 | 2.4 | 4.6 | 6.3 | 6.2 | 7.6 | 8 | 22.5 | 26 | 16.7 | 31.3 | 15.8 | 100 |
| No h/o diagnosed OTDD with COT for CNCP | 83 | 1 | 4 | 7 | 5 | 6 | 8 | 20 | 13 | 5 | 10 | 3 | 1 |
| As percentage of prisoners on COT | 94.3 | 100 | 80 | 100 | 83.3 | 85.7 | 88.9 | 100 | 100 | 83.3 | 100 | 100 | 100 |
| h/o diagnosed OTDD with COT for CNCP | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| As percentage of prisoners h/o diagnosed OTDD on COT | 40 | 0 | 100 | 0 | 0 | 100 | |||||||
| h/o diagnosed OTDD | 7 | 0 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| As percentage of prisoners in roll | 0.9 | 0 | 1.8 | 0.9 | 1 | 1 | 0.9 | 0 | 0 | 2.8 | 0 | 0 | 0 |
| h/o diagnosed OTDD on COT | 5 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| As percentage of prisoners on COT | 5.7 | 0 | 20 | 0 | 16.7 | 14.3 | 11.1 | 0 | 0 | 16.7 | 0 | 0 | 0 |
| h/o diagnosed OTDD not on COT | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| h/o diagnosed OTDD on COT no CNCP | 3 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| As percentage of prisoners h/o diagnosed OTDD on COT | 60 | 100 | 0 | 100 | 100 | 0 |
CAT: continuous analgesic therapy; CNCP: chronic non-cancer pain; COT: continuous opioid therapy; OTDD: opioid-type drug dependence.
Table 5.
Prescribing details from Prison 1.
| Prison 1 on 2 June 2013 | Total | <25 | 25-29 | 30-34 | 35-39 | 40-44 | 45-49 | 50-54 | 55-59 | 60-64 | 65-69 | 70-74 | >75 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prisoners in roll | 1154 | 72 | 117 | 105 | 104 | 123 | 164 | 172 | 113 | 76 | 62 | 30 | 16 |
| No more than simple analgesia for CNCP | 32 | 0 | 0 | 0 | 2 | 2 | 6 | 5 | 5 | 4 | 4 | 3 | 1 |
| No more than stronger NSAIDs (cum) for CNCP | 67 | 0 | 2 | 1 | 5 | 5 | 14 | 11 | 9 | 6 | 9 | 4 | 1 |
| No more than stronger NSAIDs + TCA + carbamazepine (cum) for CNCP | 79 | 0 | 2 | 3 | 6 | 6 | 16 | 14 | 12 | 6 | 9 | 4 | 1 |
| No more than nefopam (cum) for CNCP | 99 | 0 | 3 | 4 | 8 | 10 | 18 | 18 | 13 | 10 | 10 | 4 | 1 |
| No more than codeine / co-codamol (cum) for CNCP | 124 | 0 | 3 | 6 | 9 | 13 | 21 | 19 | 17 | 14 | 15 | 4 | 3 |
| Treatment with GABA analogues / stronger opioids (cum) for CNCP | 211 | 1 | 10 | 10 | 16 | 25 | 35 | 31 | 28 | 21 | 21 | 9 | 4 |
| Paracetamol prescriptions for CNCP | 62 | 0 | 0 | 1 | 1 | 6 | 12 | 7 | 9 | 10 | 9 | 4 | 3 |
| Ibuprofen prescriptions for CNCP | 13 | 0 | 1 | 0 | 1 | 1 | 1 | 4 | 3 | 0 | 1 | 1 | 0 |
| Stronger NSAID prescriptions for CNCP | 74 | 0 | 5 | 3 | 5 | 7 | 13 | 12 | 15 | 3 | 9 | 1 | 1 |
| Nefopam prescriptions for CNCP | 31 | 0 | 3 | 1 | 2 | 4 | 4 | 5 | 4 | 5 | 2 | 1 | 0 |
| TCA prescriptions for CNCP | 22 | 0 | 2 | 2 | 2 | 2 | 2 | 6 | 5 | 1 | 0 | 0 | 0 |
| Carbamazepine prescriptions for CNCP | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Co-codamol 8/500 prescriptions for CNCP | 16 | 0 | 0 | 2 | 0 | 1 | 2 | 1 | 2 | 2 | 5 | 0 | 1 |
| Co-codamol 30/500 prescriptions for CNCP | 8 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 2 | 2 | 0 | 0 | 0 |
| Codeine prescriptions for CNCP | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Tramadol prescriptions for CNCP | 36 | 0 | 0 | 2 | 2 | 3 | 5 | 7 | 9 | 4 | 4 | 0 | 0 |
| Buprenorphine prescriptions for CNCP | 7 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 2 | 1 |
| Methadone prescriptions for CNCP | 10 | 0 | 5 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Morphine prescriptions for CNCP | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Fentanyl prescriptions for CNCP | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 |
| DHC prescriptions for CNCP | 5 | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 1 | 0 |
| Gabapentin prescriptions for CNCP | 35 | 0 | 1 | 2 | 3 | 8 | 5 | 7 | 3 | 4 | 1 | 1 | 0 |
| Pregabalin prescriptions for CNCP | 15 | 1 | 1 | 1 | 1 | 2 | 4 | 1 | 1 | 1 | 0 | 1 | 1 |
| Stronger opiates and GABA analogue prescriptions | 112 | 1 | 8 | 5 | 9 | 16 | 19 | 18 | 13 | 9 | 7 | 5 | 2 |
| Stronger opiates and GABA analogues prescriptions minus LSD | 95 | 1 | 2 | 5 | 7 | 13 | 17 | 17 | 13 | 9 | 7 | 3 | 1 |
CAT: continuous analgesic therapy; CNCP: chronic non-cancer pain; COT: continuous opioid therapy; cum = cumulative total according to World Health Organization ladder; DHC: dihydrocodeine; LSD: licenced substitute drugs; OTDD: opioid-type drug dependence.
Table 6.
Prescribing details from Prison 2.
| Prison 2 on 19 July 2013 | Total | <25 | 25–29 | 30–34 | 35–39 | 40–44 | 45–49 | 50–54 | 55–59 | 60–64 | 65–69 | 70–74 | >75 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prisoners in roll | 790 | 42 | 109 | 111 | 97 | 92 | 112 | 89 | 50 | 36 | 32 | 19 | 1 |
| No more than simple analgesia for CNCP | 38 | 0 | 0 | 5 | 2 | 7 | 8 | 6 | 3 | 1 | 3 | 3 | 0 |
| No more than stronger NSAIDs (cum) for CNCP | 63 | 2 | 3 | 7 | 6 | 8 | 13 | 9 | 7 | 2 | 3 | 3 | 0 |
| No more than stronger NSAIDs + TCA + carbamazepine (cum) for CNCP | 70 | 2 | 3 | 8 | 7 | 9 | 14 | 10 | 7 | 3 | 4 | 3 | 0 |
| No more than nefopam (cum) for CNCP | 76 | 2 | 4 | 9 | 9 | 9 | 15 | 10 | 7 | 3 | 5 | 3 | 0 |
| No more than codeine / co-codamol (cum) for CNCP | 117 | 3 | 7 | 14 | 10 | 11 | 16 | 20 | 12 | 5 | 12 | 6 | 1 |
| treatment with GABA analogues / stronger opioids (cum) for CNCP | 179 | 3 | 10 | 20 | 18 | 17 | 26 | 33 | 20 | 9 | 15 | 7 | 1 |
| Paracetamol prescriptions for CNCP | 83 | 1 | 4 | 7 | 9 | 10 | 13 | 12 | 14 | 4 | 6 | 3 | 0 |
| Ibuprofen prescriptions for CNCP | 14 | 0 | 0 | 3 | 0 | 2 | 4 | 5 | 0 | 0 | 0 | 0 | 0 |
| Stronger NSAID prescriptions for CNCP | 45 | 2 | 6 | 3 | 5 | 3 | 6 | 9 | 9 | 2 | 0 | 0 | 0 |
| Nefopam prescriptions for CNCP | 8 | 0 | 1 | 2 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 |
| TCA prescriptions for CNCP | 10 | 0 | 0 | 2 | 2 | 1 | 2 | 2 | 0 | 0 | 1 | 0 | 0 |
| Carbamazepine prescriptions for CNCP | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Co-codamol 8/500 prescriptions for CNCP | 6 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 |
| Co-codamol 15/500 prescriptions for CNCP | 5 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 |
| Co-codamol 30/500 prescriptions for CNCP | 6 | 1 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 0 |
| Codeine prescriptions for CNCP | 23 | 0 | 0 | 4 | 0 | 1 | 1 | 6 | 4 | 2 | 5 | 0 | 0 |
| Tramadol prescriptions for CNCP | 40 | 0 | 2 | 2 | 4 | 3 | 7 | 9 | 7 | 3 | 2 | 1 | 0 |
| Buprenorphine prescriptions for CNCP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Methadone prescriptions for CNCP | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Morphine prescriptions for CNCP | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Fentanyl prescriptions for CNCP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DHC prescriptions for CNCP | 4 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Gabapentin prescriptions for CNCP | 18 | 0 | 2 | 2 | 1 | 1 | 5 | 3 | 1 | 1 | 2 | 0 | 0 |
| Pregabalin prescriptions for CNCP | 14 | 0 | 0 | 3 | 3 | 2 | 1 | 3 | 0 | 1 | 1 | 0 | 0 |
| Stronger opiates and GABA analogues | 79 | 0 | 4 | 8 | 10 | 7 | 13 | 16 | 9 | 6 | 5 | 1 | 0 |
| Stronger opiates and GABA analogues minus LSD | 78 | 0 | 4 | 8 | 9 | 7 | 13 | 16 | 9 | 6 | 5 | 1 | 0 |
CNCP: chronic non-cancer pain; cum = cumulative total according to World Health Organization ladder; DHC: dihydrocodeine; GABA: gamma-aminobutyric acid; LSD: licensed substitute drugs; NSAID: non-steroidal anti-inflammatory drug; TCA: tricyclic antidepressant.
Discussion
We therefore demonstrated a consistent finding at two prisons, situated in different regions of the United Kingdom, that approximately 20% of all prisoners on their rolls were in receipt of CAT for CNCP. This rate appears to correlate closely to age, with older prisoners more likely to report CNCP. Forty-four percent of prisoners on continuous medication for CNCP are prescribed opioid drugs of any sort. Those prisoners on opioid drugs, who have been diagnosed with OTDD, are more than twice as likely to report CNCP as those who have not acquired this diagnosis.
Validity and sources of error
In our study, the total number of prisoner patients was smaller in the age groups above 55 years, and there were very few who are older than 75 years. Numbers are also small in the opioid-dependent sub-group. Chance therefore threatens the results relating to these groups, and a larger application of this study would be needed to strengthen the validity of the observations.
Data collection involved a careful review of medical records, but a subjective call was sometimes required on the question of the inclusion criteria. In practice, excluding criteria were not hard to identify: continuous medication was usually just that, and there did seem to be a substantial gap between those patients who were taking medicines for pain on an intermittent basis, and those who were picking them up regularly, every week and every month, with enough supply to ensure medication was available for daily consumption. All patients were recruited on a single day for each establishment. Records were preserved and were analysed retrospectively, and all patients were accounted for at the end of the study: attrition bias can therefore be discounted.
Both these prisons are gaols accommodating longer-sentenced prisoners. The characteristics of these prisoners (including health) may have been significantly different from those in other establishments in the UK prison estate, especially local prisons, which have a higher turnover and which accommodate more remand prisoners. Female prisoners are not studied here. Caution should therefore be exercised concerning the external validity of this study, until later analyses, using the same tool, are applied to a broader array of prison types. The two establishments studied are at either end of the country, but demonstrate strikingly similar patient characteristics and prescribing profiles: such similarities would need to be confirmed or refuted by recruitment of other gaols and thus greater numbers of subjects.
The problem of prison pain
CNCP in a prison setting would appear to be a significant problem, with one in five of all prisoners in these two prisons consuming analgesic medication of some sort, on a daily basis, on account of it. Rates vary from less that 4% in the under-25 years age group, to 36% in the age group 65–69 years. Our results indicate that the prevalence of prison CNCP requiring daily analgesia, as defined in our study, accounted for 90% of prescribing for persistently painful conditions: chronic pain in these (male) prisons is mostly musculoskeletal in origin. Concern may be expressed that complaints of CNCP may cloak an underlying OTDD, or as a means to acquire drugs for sale; but the similarity in age-related correlation between consumption for CNCP of CAT of all types, of COT, and of simple analgesia or strong NSAIDs (which are neither prized as currency in prison, or habit-forming) suggest that most of CNCP in prisons is real pain (Charts 3–5). Analysis of the sub-group of patients with diagnosed OTDD reveals that, for them, CNCP seems even more of a problem, with a prevalence double that of the ordinary prison population. The total number of OTDD patients was small, however, and it would require a specialist study, looking at CNCP prevalence rates among a larger population of OTDD patients across a greater number of establishments, to determine what patterns might be evident, both in terms of age and in relation to co-morbidity, or use of particular drugs for CNCP. In respect of this, the data from this study draw on too small a sample for any conclusions to be drawn, beyond the odds ratio for CNCP of 0.23, as quoted above. The prevalence of diagnosed OTDD is heavily skewed towards the younger age group: this may reflect the increased mortality of OTDD, so that substantial numbers of these patients never reach old age; or may imply that OTDD in the older age group may remain undiagnosed due to the stigma; or that the disease itself is a recent product of a young modern society and has not yet made such inroads among older people.
It is unsurprising that this study, which looked in detail at the burden of CNCP in a prison population, should demonstrate that older patients consume more medication, presumably suffering from more CNCP. The prevalence of traumatic, inflammatory and degenerative musculoskeletal disease increases with age, as does the prevalence of diabetes mellitus and atheromatous cardiovascular disease, both of which ramp up an individual’s burden of chronic musculoskeletal pain as a result of neuropathy, ischaemia and increased vulnerability to infection. Depressive illness, with its close relationship to CNCP, is another important factor, relevant to all age groups. Personality characteristics, operating through a tendency to catastrophise pain,36–38 will also assume relevance here. It seems very likely that it is the effects of each of these, alone or in combination, which drive the demand for daily analgesia in prisons.
Further research
This basic prevalence study should be built upon, to develop an evidence base assisting in the effective management of CNCP in prisons, as described above. Are there prison-based prescribing frameworks for the use of COT for CNCP, and if so, are these utilised? Are there variations in prison prescribing practices, and, if so, what patterns do they follow? Do they correlate with particular beliefs and attitudes held by individual prescribers? Are prescribing decisions made by prison doctors exclusively, or on the recommendation of hospital-based specialists? What are the real-time trends in prescribing of CAT/COT for CNCP? And, crucially, when using the same research tool, is CNCP more prevalent, or less, in comparison to outside UK general practice, and if so, what are the likely factors which might account for these differences?
Acknowledgments
Thanks are due to Dr Sarah Bromley (National Medical Director for Offender Health, Care UK) Helen Parker, Joanne McCormick, and Joanne Thurston (North East Division Offender Health, Care UK) and Mark Landridge (Superintendent Pharmacist Care UK) for facilitating access to databases, and to Dr David Howard, of Lincoln University, for helpful advice and comments.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: This study is based on an anonymised retrospective audit of prescribing figures in two prisons in the UK prison estate. There was no assignment of subjects. Ethical approval therefore was not required, but institutional permission was sought and obtained from the National Offender Management Service to publish these findings in anonymous format.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was partially funded by educational grants from Lighthouse Educational Foundation and Care UK.
References
- 1. Reid KJ, Harker J, Bala MM, et al. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin 2011; 27(2): 449–462. [DOI] [PubMed] [Google Scholar]
- 2. Alonso J, Ferrer M, Gandek B, et al. Health-related quality of life associated with chronic conditions in eight countries: results from the International Quality of Life Assessment (IQOLA) Project. Qual Life Res 2004; 13(2): 283–298. [DOI] [PubMed] [Google Scholar]
- 3. Von Korff M, Crane P, Lane M, et al. Chronic spinal pain and physical-mental comorbidity in the United States: results from the national comorbidity survey replication. Pain 2005; 113(3): 331–339. [DOI] [PubMed] [Google Scholar]
- 4. Khan AA, Khan A, Harezlak J, et al. Somatic symptoms in primary care: etiology and outcome. Psychosomatics 2003; 44(6): 471–478. [DOI] [PubMed] [Google Scholar]
- 5. Gallagher RM, Rosenthal LJ. Chronic pain and opiates: balancing pain control and risks in long-term opioid treatment. Arch Phys Med Rehabil 2008; 89(3): S77–S82. [DOI] [PubMed] [Google Scholar]
- 6. RCGP Consensus Group. Management of persistent pain in secure environments (Draft), http://www.rcn.org.uk/__data/assets/pdf_file/0010/465841/Final_draft_-_Management_of_persistent_pain_in_secure_environments_01.07.2012.pdf (2012, accessed 12 August 2013).
- 7. Public Health England. Managing persistent pain in secure settings, http://www.nta.nhs.uk/uploads/persistentpain.pdf (2013, accessed 31 August 2013).
- 8. Royal College of General Practitioners & Royal Pharmaceutical Society. Safer prescribing in prisons: guidance for clinicians. RCGP Secure Environments Group, http://www.rcgp.org.uk/Clinical-and-research/Clinical-resources/Resources-for-GPs-working-in-secure-environments.aspx (2011, accessed 12 August 2013).
- 9. Levy M. Safer prescribing for prisoners. BMJ 2012; 344: e447. [DOI] [PubMed] [Google Scholar]
- 10. Fishbain DA, Cutler R, Rosomoff HL, et al. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clin J Pain 1997; 13(2): 116–137. [DOI] [PubMed] [Google Scholar]
- 11. Kroenke K. Patients presenting with somatic complaints: epidemiology, psychiatric co-morbidity and management. Int J Methods Psychiatr Res 2003; 12(1): 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ohayon MM, Schatzberg AF. Using chronic pain to predict depressive morbidity in the general population. Arch Gen Psychiatry 2003; 2003, 60(1): 39–47. [DOI] [PubMed] [Google Scholar]
- 13. Ohayon MM. Specific characteristics of the pain/depression association in the general population. J Clin Psychiatry 2004; 65: 5–9. [PubMed] [Google Scholar]
- 14. Ohayon MM. Relationship between chronic painful physical condition and insomnia. J Psychiatr Res 2005; 39(2): 151–159. [DOI] [PubMed] [Google Scholar]
- 15. Demyttenaere K, Bonnewyn A, Bruffaerts R, et al. Comorbid painful physical symptoms and depression: prevalence, work loss, and help seeking. J Affect Disord 2006; 92: 185–193. [DOI] [PubMed] [Google Scholar]
- 16. Demyttenaere K, Bruffaerts R, Lee S, et al. Mental disorders among persons with chronic back or neck pain: results from the World Mental Health Surveys. Pain 2007; 129: 332–342. [DOI] [PubMed] [Google Scholar]
- 17. Sullivan MD, Edlund MJ, Steffick D, et al. Regular use of prescribed opioids: association with common psychiatric disorders. Pain 2005; 119(1–3): 95–103. [DOI] [PubMed] [Google Scholar]
- 18. Sacco W, Bykowski C, Mayhew L. Pain and functional impairment as mediators of the link between medical symptoms and depression in type 2 diabetes. Int J Behav Med 2013; 20(1): 22–29. [DOI] [PubMed] [Google Scholar]
- 19. Hennings A, Schwarz MJ, Riemer S, et al. The influence of physical activity on pain thresholds in patients with depression and multiple somatoform symptoms. Clin J Pain 2012; 28(9): 782–789. [DOI] [PubMed] [Google Scholar]
- 20. McDermott AM, Toelle TR, Rowbotham, et al. The burden of neuropathic pain: results from a cross-sectional survey. Eur J Pain 2006; 10: 127–135. [DOI] [PubMed] [Google Scholar]
- 21. Breivik H, Collett B, Ventafridda V, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006; 10: 287–333. [DOI] [PubMed] [Google Scholar]
- 22. Singleton N., et al. Psychiatric morbidity among prisoners in England and Wales. London: Office for National Statistics, 1998. http://webarchive.nationalarchives.gov.uk//www.dh.gov.uk/en/Publicationsandstatistics/Publications/Publicationsstatistics/DH_4007132 [Google Scholar]
- 23. Ministry of Justice. Gender differences in substance misuse and mental health amongst prisoners. London: Ministry of Justice, 2013. [Google Scholar]
- 24. Wiles N, Zammit S, Bebbington P, et al. Self-reported psychotic symptoms in the general population. Br J Psychiatry 2006; 188: 519–526. [DOI] [PubMed] [Google Scholar]
- 25. Department of Health. Conference report, sharing good practice in prison health, 2007, http://webarchive.nationalarchives.gov.uk/20080910135103/dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_078070
- 26. Fazel S, Hope T, O’Donnell I, et al. Hidden psychiatric morbidity in elderly prisoners. Br J Psychiatry 2001; 179: 535–539. [DOI] [PubMed] [Google Scholar]
- 27. Fazel S, Hope T, O’Donnell I, et al. Health of elderly male prisoners: worse than the general population, worse than younger prisoners. Age Ageing 2001; 30: 403–407. [DOI] [PubMed] [Google Scholar]
- 28. Ginn S. Prison environment and health. BMJ; 2012: 345, http://dx.doi.org/10.1136/bmj.e5921 [DOI] [PubMed] [Google Scholar]
- 29. Ginn S. Promoting health in prison. BMJ; 2013: 346, http://dx.doi.org/10.1136/bmj.f2216 [DOI] [PubMed] [Google Scholar]
- 30. NHS National Treatment Agency for Substance Misuse. Addiction to medicine: an investigation into the configuration and commissioning of treatment services to support those who develop problems with prescription-only or over-the-counter medicine, http://www.nta.nhs.uk/uploads/addictiontomedicinesmay2011a.pdf (2011. accessed 12 August 2013).
- 31. Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician 2008; 11(2): S63–S88. [PubMed] [Google Scholar]
- 32. Centers for Disease Control and Prevention, Public Health Service, US Department Health and, Human Services. Opioid overdoses in the United States. J Pain Palliat Care Pharmacother 2012; 26(1): 44–47.22448941 [Google Scholar]
- 33. Manchikanti L, Fellows B, Ailinani H, et al. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain Physician 2010; 13(5): 401–435. [PubMed] [Google Scholar]
- 34. Ginn S. Elderly prisoners. BMJ; 2012: 345, http://dx.doi.org/10.1136/bmj.e6263 [DOI] [PubMed] [Google Scholar]
- 35. Washington State Agency Medical Directors Group. Interagency guideline on opioid dosing for chronic non-cancer pain (CNCP), http://www.agencymeddirectors.wa.gov/Files/OpioidGdline.pdf (2010, accessed 12 August 2013).
- 36. Granot M, Ferber SG. The roles of pain catastrophizing and anxiety in the prediction of postoperative pain intensity: a prospective study. Clin J Pain 2005; 21(5): 439–445. [DOI] [PubMed] [Google Scholar]
- 37. Drahovzal DN, Stewart SH, Sullivan MJL. Tendency to catastrophize somatic sensations: pain catastrophizing and anxiety sensitivity in predicting headache. Cogn Behav Ther 2006; 35(4): 226–235. [DOI] [PubMed] [Google Scholar]
- 38. Börsbo B, Peolsson M, Gerdle B. Catastrophizing, depression, and pain: correlation with and influence on quality of life and health – a study of chronic whiplash-associated disorders. J Rehabil Med 2008; 40(7): 562–569. [DOI] [PubMed] [Google Scholar]


