Abstract
Objectives:
To examine whether the prevalence of regional and chronic widespread pain (CWP) varies with rurality and to determine the characteristics of persons in rural locations in whom pain is found to be in excess.
Methods:
Participants, aged ≥55 years, from participating general practices in seven different geographical locations in Scotland were sent a postal questionnaire. The 1-month prevalence of 10 regional pain conditions plus CWP was identified using body manikins. Differences in the prevalence of pain with differing rurality were examined using Chi2 test for trend. Thereafter, among the rural population, the relationships between pain and putative risk factors were examined using Poisson regression. Thus, results are described as risk ratios.
Results:
There was some evidence to suggest that the prevalence of CWP increased with increasing rurality, although the magnitude of this was slight. No large or significant differences were observed with any regional pain conditions. Factors associated with the reporting of CWP included poor general health, feeling downhearted most of the time and selected measures of social contact. Factors independently associated with CWP included female gender (risk ratio: 1.24; 95% confidence interval (CI): 0.997–1.55), poor self-rated health (risk ratio: 3.50; 95% CI: 1.92–6.39) and low mood (risk ratio: 1.54; 95% CI: 1.07–2.20). Also, having fewer than 10 people to turn to in a crisis was associated with a decrease in the risk of CWP – risk ratio: 0.68 (95% CI: 0.50–0.93) and 0.78 (95% CI: 0.60–1.02) for those with 5–10 and <5 people, respectively.
Conclusions:
This study provides no evidence that the prevalence of regional musculoskeletal pain is increased in rural settings, although there is some evidence of a modest increase in CWP. Risk factors for CWP are similar to those seen in the urban setting, including markers of general health, mental health and also aspects of social contact. It may be, however, that social networks are more difficult to maintain in rural settings, and clinicians should be aware of the negative effect of perceived social isolation on pain in rural areas.
Keywords: Pain, rural, urban, chronic widespread pain, epidemiology, statistics and numerical data
Key points.
Some evidence to suggest that the prevalence of chronic widespread pain (CWP) increased with increasing rurality;
Risk factors for CWP are similar to those seen in the urban setting, including markers of general health, mental health and also aspects of social contact;
In rural areas, individuals who know few of their neighbours, or who rarely see friends/family/neighbours, are at increased risk of CWP.
Introduction
Musculoskeletal pain is common, particularly in the low back, hip, knee and shoulder,1 and it is associated with considerable disability, health care and societal costs.2 Estimates vary, but for low back pain, the most common regional pain condition, 1-year prevalence, has been estimated to be approximately 30–40%, while lifetime prevalence is 65–70%.3 Furthermore, population studies consistently show the prevalence of chronic widespread pain (CWP; the cardinal feature of fibromyalgia) to be around 12%.1
The majority of epidemiological studies of pain have considered urban or sub-urban populations with few studies in communities that are rural (small population size) or remote (distant from large towns).4 Although definitions vary, the Scottish Government defines ‘accessible rural’ areas as those with fewer than 3000 people and within 30 minutes drive of a settlement of 10,000 or more and ‘remote rural’ areas as settlements of <3000 people and with a drive time of >30 minutes to a settlement of 10,000 or more.5 Some authors have described elevated levels of chronic pain in a rural Swedish population6 and in a rural area of the United States, compared to an urban area.4 However, there were no significant differences in prevalence between individual pain sites. Others have demonstrated a high pain prevalence in a Canadian sample that included a disproportionately high number of rural participants.7 However, work in this area is limited: sample sizes and response rates are low,4,7 and although samples are described as rural, it is not always clear how this is actually defined.6
The essential difference between rural and urban populations, in terms of health care, is the relative accessibility of services, but there may also be differences with regard to aetiology. Risk factors for pain in the general population include female gender, poor psychological well-being, lower social class and occupational and psychosocial factors.1–3,8,9 While there are no real reasons to believe that a different set of risk factors will be important in rural areas, the relative importance of risk factors may differ. For example, studies have found that in those with chronic pain, higher quality of life is related to lower social constraints, suggesting benefits of strong social support networks,4 and it may be that these are easier to maintain in urban rather than rural communities. Also, generally speaking, rural populations are older, have higher levels of manual labour (and individuals remain in physical occupations later in life10) and higher levels of social isolation, with the dispersal of social groupings leading to distinct social interaction effects.10 Furthermore, some authors have suggested that individuals living in socially isolated environments are more likely to focus attention inward and are at increased risk of reporting physical symptoms.11
The aim of this study was to examine the epidemiology of regional and widespread pain in rural versus urban settings. In particular, we aimed first to compare prevalence of regional and widespread pain in areas of different rurality, and second, to determine the characteristics of persons in rural locations in whom pain is found to be in excess.
Methods
This study took advantage of two population surveys ongoing at a similar time, in different geographical areas. All persons aged ≥55 years on the registers of nine participating general practices were sent a questionnaire by post to collect data on pain, general health and well-being. Over 96% of persons resident in the United Kingdom are registered with a General Practice; therefore, this represents a suitable population sampling frame for epidemiological studies. Non-respondents were sent a further questionnaire after 2 weeks.
Rural sample
The rural sample came from practices participating in the Older People for Older People (O4O) study.12 Funded by the European Union (EU) Northern Periphery Programme (2007–2010), the O4O study aimed to improve services delivered to the population living in remote and rural areas, working with communities in Scotland, Finland, Sweden, Greenland and Northern Ireland (www.o4os.eu). Only practices from Scotland were selected for this study, and the sample comprised six rural communities within the Scottish Highlands – an area of low population density, with fewer than 100 people per square kilometre. These rural areas were also fairly remote, with an average drive time to a large community (>10,000 individuals) of >90 minutes.
Urban sample
The urban sample came from three practices participating in the MUSICIAN study. The MUSICIAN study was a 2 × 2 factorial randomised controlled trial investigating the management of CWP.13,14 However, for this study, participants comprised the respondents to a large-scale postal survey that was used to identify persons eligible for the trial. Only practices in Aberdeen were selected for this study. The city of Aberdeen in the north-east coast of Scotland has a population of 250,000 and is a relatively affluent city with high employment, particularly in the fields of oil and higher education.
Questionnaires
Study questionnaires gathered information on demographics (age, gender, location and employment), and pain was assessed by asking the participants, ‘Thinking back over the past month, have you had any aches or pains that have lasted for one day or longer?’ Participants answering positively were asked to shade the location(s) of their pain on a four-view body manikin; this was coded into regional areas as per Figure 1 allowing the identification of the following regional pain conditions: shoulder pain, elbow pain, forearm pain, hand pain, low back pain, hip pain, knee pain, foot pain and headache. In addition, CWP was also identified, defined according to the American College of Rheumatology 1990 criteria for fibromyalgia, that is, pain lasting more than 3 months, on both sides of the body, above and below the waist, and axial pain.15
Figure 1.
Manikin indicating regional pain areas.
Rural study participants were also asked about a number of putative risk markers for pain, including self-rated health and information on psychosocial (feeling calm and feeling downhearted) and social factors (knowing or trusting neighbours; attendance/participation at community projects and local groups; recently speaking to or seeing friends, neighbours and family; people to turn to in a crisis). A composite index of social contact was created using the number of times participants saw their friends, neighbours and relatives, and a separate index was created using the number of times participants spoke to friends, neighbours and relatives. Each was then divided into quartiles for analysis.
Analysis
Differences in the prevalence of pain across differing levels of rurality (i.e. areas of decreasing population size), compared to the urban population, were examined using the Chi2 test for trend. Thereafter, for pain conditions shown to be in excess in rural areas, the relationship between pain and potential risk markers was examined using Poisson regression. Thus, results are presented as risk ratios with 95% confidence intervals (CIs), the latter being derived using robust estimates of standard error.16 Estimates from univariate analyses were initially adjusted for age, sex and geographical location and then used to build a multivariable model in which variables were offered to the model if the adjusted risk ratio was ≥1.25 (or its reciprocal, ≤0.8) or if it was significant at p ≤ 0.2. These criteria were applied for dichotomous variables, or for any category of categorical variables, and ensured that all potential confounding factors of even marginal significance were at least considered for the final model. The final multivariable model used forward Poisson regression, with variables included at p ≤ 0.10 and eliminated at p ≥ 0.15. Factors which were likely to be consequences of pain as opposed to potential risk markers (e.g. pain interference with social life) were not considered for multivariable analysis. All analyses were conducted using Stata v12.1 (StataCorp LP, College Station, TX, USA).
Results
Demographic characteristics of the study sample
In total, questionnaires were sent to 12,831 people. From rural areas, 1374/2462 responded and provided complete data on pain (56%) and 4639/10,369 from urban areas (45%). The characteristics of both study populations are detailed in Table 1.
Table 1.
Characteristics of study populations.
| Rural, n (%)a | Urban, n (%)a | ||
|---|---|---|---|
| Age | 55–64 years | 620 (45.5%) | 1890 (40.7%) |
| 65–74 years | 408 (29.9%) | 1479 (31.9%) | |
| 75–84 years | 270 (19.8%) | 1019 (22.0%) | |
| >84 years | 66 (4.8%) | 251 (5.4%) | |
| Sex | Male | 630 (46.3%) | 2073 (44.7%) |
| Female | 731 (53.7%) | 2566 (55.3%) | |
| Employment | Full-time | 264 (19.6%) | 895 (19.7%) |
| Part-time | 153 (11.3%) | 471 (10.4%) | |
| Retired | 900 (66.7%) | 2704 (59.5%) | |
| Unemployed | 32 (2.4%) | 11 (0.2%) | |
| Unable to workb | – | 193 (4.2%) | |
| Otherb | – | 273 (6.0%) | |
| Locationc | Urban | – | 4639 (100%) |
| Ardersier (1000; 23 minutes) | 560 (40.8%) | – | |
| Tongue (1000; 123 minutes) | 130 (9.5%) | – | |
| Lochcarron (950; 87 minutes) | 260 (18.9%) | – | |
| Lochinver (600; 122 minutes) | 230 (16.7%) | – | |
| Torridon (400; 88 minutes) | 131 (9.5%) | – | |
| Applecross (250; 118 minutes) | 63 (4.6%) |
Numbers vary due to missing data.
Not recorded in the rural study.
Numbers in parentheses denote (1) approximate population size (an indication of the extent to which the community is rural) and (2) approximate drive time to a community of >10,000 (an indication of the extent to which the community is remote).
Prevalence of regional and widespread pain
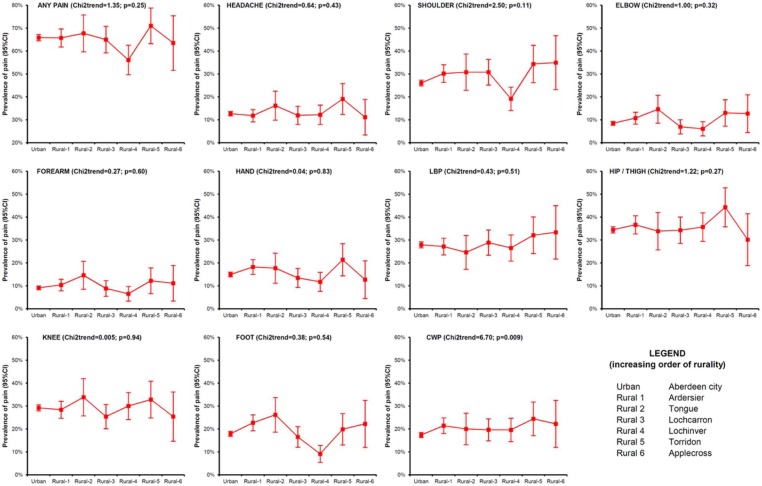
The prevalence of pain in rural and urban areas was 64.6% and 65.8%, respectively. There was no difference in the overall pain prevalence (‘any pain’) with increasing rurality across the seven sites (: 1.35; p = 0.25). The most common regional pain conditions in both populations were hip pain (35%), knee pain (29%), low back pain (28%) and shoulder pain (27%). Figure 2 shows the prevalence of all pain conditions by increasing rurality and, further, provides a Chi2 test for trend to investigate significant differences in pain prevalence. Only for CWP there was a significant trend in the prevalence of pain with increasing rurality (: 6.70; p = 0.009). However, the magnitude of the difference in prevalence across categories was relatively small (17.4% in the urban sample versus 22.2% in the most rural sample).
Figure 2.
Prevalence of regional and widespread pain conditions with increasing rurality.
CWP: chronic widespread pain; CI: confidence interval; LBP: low back pain.
Risk markers for CWP in the rural population
The prevalence of CWP in the rural population as a whole was 21.0%. Women were significantly more likely to report CWP than men (risk ratio: 1.30; 95% CI: 1.05–1.60). Persons who were retired were more likely to report CWP (risk ratio: 1.54; 95% CI: 1.12–2.11) although there was no clear association with age (Table 2).
Table 2.
Association between chronic widespread pain and demographic factors, in rural population.
| Chronic widespread paina |
Risk ratio |
|||
|---|---|---|---|---|
| Yes | No | (95% CI) | ||
| Age | 55–64 years | 130 (21.0%) | 490 | 1.00 |
| 65–74 years | 86 (21.1%) | 322 | 1.01 (0.79–1.28) | |
| 75–84 years | 48 (17.8%) | 222 | 0.85 (0.63–1.14) | |
| >84 years | 20 (30.3%) | 46 | 1.45 (0.97–2.15) | |
| Sex | Male | 113 (17.9%) | 517 | 1.00 |
| Female | 170 (23.3%) | 561 | 1.30 (1.05–1.60) | |
| Employment | Full-time | 38 (14.4%) | 226 | 1.00 |
| Part-time | 33 (21.6%) | 120 | 1.50 (0.98–2.28) | |
| Retired | 199 (22.1%) | 701 | 1.54 (1.12–2.11) | |
| Unemployed | 7 (21.9%) | 25 | 1.52 (0.74–3.12) | |
| Educationb | H-grade or above | 143 (19.6%) | 585 | 1.00 |
| S-grade or below | 134 (22.8%) | 455 | 1.16 (0.94–1.43) | |
CI: confidence interval.
Numbers vary due to missing data.
H-grade: Scottish ‘Higher’ grade exams, typically taken at an age of 17–18 years; S-grade: Scottish ‘Standard’ grade exams, typically taken at an age of 15–16 years.
A dose–risk relationship was found between self-rated health and CWP (Table 3); those reporting poor self-rated health were five times more likely also to report CWP (risk ratio: 4.99; 95% CI: 2.83–8.81) compared to those in excellent health. Participants who knew (risk ratio: 1.50; 95% CI: 1.15–1.95) or trusted (risk ratio: 1.40; 95% CI: 1.08–1.81) few or none of their neighbours were more likely to report CWP. There was also some evidence that those who rarely saw friends, family or neighbours were at increased risk (risk ratio: 1.33; 95% CI: 0.98–1.81). Interestingly, however, individuals living in two-person (risk ratio: 0.60; 95% CI: 0.45–0.80) or single person households (risk ratio: 0.78; 95% CI: 0.56–1.08) were less likely to report CWP than those living in households of more than two people.
Table 3.
Association between chronic widespread pain and health, social and psychosocial factors, in rural population.
| Chronic widespread paina |
Risk ratiob |
|||
|---|---|---|---|---|
| Yes | No | (95% CI) | ||
| Self-rated health | Excellent | 16 (9.7%) | 149 | 1.00 |
| Very good | 57 (12.3%) | 405 | 1.32 (0.76–2.28) | |
| Good | 98 (21.4%) | 361 | 2.26 (1.34–3.80) | |
| Fair | 83 (38.6%) | 132 | 4.23 (2.5–7.16) | |
| Poor | 34 (48.6%) | 36 | 4.99 (2.83–8.81) | |
| Number of people in household | >2 people | 46 (28.6%) | 115 | 1.00 |
| 2 people | 146 (18.1%) | 672 | 0.60 (0.45–0.80) | |
| 1 person | 89 (23.5%) | 289 | 0.78 (0.56–1.08) | |
| Know neighbours | Most | 107 (17.9%) | 490 | 1.00 |
| Many | 98 (21.1%) | 367 | 1.20 (0.93–1.54) | |
| A few/none | 82 (26.7%) | 225 | 1.50 (1.15–1.95) | |
| Trust neighbours | Most | 149 (19.0%) | 635 | 1.00 |
| Many | 66 (20.4%) | 258 | 1.12 (0.86–1.47) | |
| A few/none | 72 (27.9%) | 186 | 1.40 (1.08–1.81) | |
| Recent social group attendance | Yes | 166 (19.6%) | 683 | 1.00 |
| No | 120 (23.4%) | 393 | 1.12 (0.89–1.40) | |
| See friends/family/neighbours | Regularly | 71 (18.9%) | 305 | 1.00 |
| Often | 61 (19.4%) | 253 | 1.05 (0.77–1.45) | |
| Sometimes | 62 (20.1%) | 247 | 1.07 (0.78–1.48) | |
| Rarely | 66 (24.4%) | 205 | 1.33 (0.98–1.81) | |
| Speak to friends/family/neighbours | Regularly | 87 (23.8%) | 278 | 1.00 |
| Often | 63 (17.6%) | 294 | 0.76 (0.56–1.03) | |
| Sometimes | 65 (20.9%) | 246 | 0.91 (0.68–1.23) | |
| Rarely | 53 (21.3%) | 196 | 0.98 (0.71–1.34) | |
| Number of people to turn to in a crisis | >10 | 54 (24.3%) | 168 | 1.00 |
| 5–10 | 68 (17.7%) | 317 | 0.72 (0.52–0.99) | |
| <5 | 151 (23.1%) | 503 | 0.89 (0.67–1.18) | |
| Feeling calm | All of the time | 27 (11.5%) | 207 | 1.00 |
| Most of the time | 167 (19.7%) | 679 | 1.74 (1.18–2.56) | |
| Little or none of the time | 88 (33.2%) | 177 | 2.98 (1.99–4.45) | |
| Feeling downhearted | None of the time | 84 (13.4%) | 541 | 1.00 |
| A little of the time | 158 (26.1%) | 447 | 1.88 (1.47–2.40) | |
| Most or all of the time | 41 (33.3%) | 82 | 2.27 (1.61–3.20) | |
CI: confidence interval.
Numbers vary due to missing data.
Adjusted for age, sex, employment status, educational qualifications and geographical location.
Those reporting low mood – as indicated by feeling downhearted – experienced a significantly elevated risk of CWP (risk ratio: 2.27; 95% CI: 1.61–3.20). The same was true of those who reported that they rarely felt calm (risk ratio: 2.98; 95% CI: 1.98–4.45).
Contrary to what one might expect, compared to participants who reported that they have more than 10 people they could turn to in a crisis, those with fewer confidants reported a reduction in the risk of CWP (risk ratio: 0.72; 95% CI: 0.52–0.99 and risk ratio: 0.89; 95% CI: 0.67–1.18 for those with 5–10 and <5 people, respectively).
Multivariable analysis
Three factors emerged as independent risk markers for CWP: poor self-rated health, low mood and the number of people one is able to turn to in a crisis (Table 4). Although forced into the model (and therefore not subject to the stepwise variable selection criteria), female gender was also significantly associated with the reporting of CWP.
Table 4.
Factors independently associated with chronic widespread pain in rural population (multivariable model).
| Risk ratio (95% CI)a | ||
|---|---|---|
| Sex | Male | 1.00 |
| Female | 1.24 (0.997–1.55) | |
| Self-rated health | Excellent | 1.00 |
| Very good | 1.13 (0.65–1.95) | |
| Good | 1.91 (1.13–3.23) | |
| Fair | 3.33 (1.93–5.73) | |
| Poor | 3.50 (1.92–6.39) | |
| Feeling downhearted | None of the time | 1.00 |
| A little of the time | 1.50 (1.15–1.94) | |
| Most or all of the time | 1.54 (1.07–2.20) | |
| Number of people to turn to in a crisis | >10 | 1.00 |
| 5–10 | 0.68 (0.50–0.93) | |
| <5 | 0.78 (0.60–1.02) |
CI: confidence interval.
Adjusted for age, employment status, educational qualifications and geographical location which were forced into model.
Discussion
We have demonstrated that, for the main part, the prevalence of pain is similar in urban/rural communities. However, we provide some evidence to suggest that CWP occurs in excess in rural populations. Furthermore, we have shown that, in a rural population, individuals with poor self-rated health, low mood and who know few of their neighbours or who rarely see friends/family/neighbours are at increased risk of CWP.
A number of methodological issues must be considered when interpreting these findings. First, the response rate for the rural and urban populations was 56% and 45%, respectively, and non-response bias is a potential concern. Age and sex are known markers of participation, with non-responders more likely to be male and younger. No data are available on non-responders in the rural sample due to restrictions on access to non-respondent data, although in this study, the distribution of gender (45.1% male) was exactly what would be expected in a Scottish sample of this age group (45.3% male),17 suggesting no differential response by gender. Also, because the whole sample was ≥55 years, any effect across age will be greatly reduced, although pain is known to increase with age1 and this may have influenced prevalence estimates. However, in the event that non-response bias was responsible for the observed increase in the prevalence of CWP with increasing rurality, one would also expect an increased prevalence of all pain(s), and this was not observed.
Second, there is the possibility of duplicates in the rural dataset. Due to requirements of the ethical approval for the O4O study, questionnaire respondents were not identifiable, and thus, reminders were sent to all sampled persons, rather than solely to non-responders. This ensured participation remained anonymous although, technically, it was possible for some people to respond twice (although they were asked not to). Potential duplicates were identified on SPSS (PASW Statistics Release Version 18.0.0) by comparing variables unlikely to change between mailings, such as age, gender, employment status, qualifications, income and number of people in household. This identified 69 potential duplicates, which were removed from further analyses. It is possible, therefore, that some participants may have erroneously been excluded and/or some individuals included twice. Two sensitivity analyses were concluded: first, pain prevalence was estimated in the entire dataset (i.e. potential duplicates included), and second, we estimated the prevalence of all pain conditions using bootstrap methodology. A total of 1000 estimates of prevalence were computed (for each pain condition) each time removing a random sample of 69 individuals in order to match the original ‘duplicate’ numbers. Both approaches resulted in estimates of pain prevalence almost identical to those of the main study. We believe, therefore, that our findings have not been biased to any great extent by the restrictions placed on the administration of the study.
Third, the cross-sectional nature of the analysis prevents us from establishing temporality, and in drawing conclusions, one must be wary of reverse causality. It is unlikely that CWP leads to rurality. However, stronger effects are observed with the perception of social contact and community rather than any objective markers of this, and it may be that pain influences one’s perception of these relationships and that these are in fact consequences of pain, rather than antecedents of it. This study is unable to tease apart these issues, and it is crucial, therefore, that future work should examine these relationships longitudinally.
Additionally, we made 11 comparisons of pain prevalence by rurality (any pain, nine regional pains and CWP), and only in the latter, a significant association was observed. We cannot rule out the possibility that the single association with CWP may have arisen by chance. However, although the magnitude of effect was not large, the relationship between rurality and CWP was far from statistically borderline, at p = 0.009. In other words, the probability of observing the trend we see in the data if, in reality, the null hypothesis is true (i.e. that there is no association) is less than 1%. A final point to be considered is the fact that our population sample was limited to those >55 years; therefore, while we can make conclusions based on that cohort, it is possible that results may differ in younger age groups. We do not have the data to assess these potential differences and therefore recommend future research with a younger population sample.
Previous works examining rural populations have reported low response rates (25%) and, often, no definition of rurality is given.4,6,11 This study is the first, to our knowledge, to examine the epidemiology of pain across several rural communities while also directly comparing pain prevalence to a contemporary urban population. The prevalence of rural regional pain reported here is consistent with urban populations,17 and the finding that low back, knee, shoulder and hip pain are the most common regional pains across both populations is in line with the literature in urban populations.1,4,18 Historically, urban population estimates of CWP have been fairly consistent ranging between 11% and 14%.1 However, a more recent study in the United Kingdom reported prevalence of 23%19 in an urban sample. This recent evidence and the current CWP prevalence reported for the urban and rural samples (17% and 21%, respectively) may reflect a shift over time with increasing CWP or it may be that previous literature has underestimated its burden.
This study only investigated the aetiology of those pain conditions which were found to increase significantly with increasing rurality. The approach was chosen to guard against the effects of multiple testing. However, for comparison, additional analysis (not shown) was conducted to determine the factors associated with regional pain conditions, and risk markers were broadly similar to those identified for CWP. In general, the risk factors for CWP in this study were very similar to those previously reported for CWP in urban populations.1 Furthermore, the current findings suggest that, in a rural population, individuals who know few of their neighbours or who rarely see friends/family/neighbours are at increased risk of CWP. This, again, is consistent with other studies that have shown that a better sense of neighbourhood is associated with better physical and mental health, lower stress, better social support and being physically active.20
We have previously shown in older adults living in an urban setting that perceived loneliness is a risk factor for musculoskeletal pain.21 Our findings also provide support for Pennebaker who claimed that those who live in socially isolated environments may be at greater risk of reporting physical symptoms due to a lack of external distractions,11 and it is certainly conceivable that the benefits of social networks may be harder to realise in remote and rural communities. In addition, geographical isolation may also limit access to treatments, and without support from neighbours, older rural residents may have greater difficulty reaching health/social care. While our results suggest that individuals are at increased risk of CWP if they are isolated, we must consider that these self-reported issues are difficult to separate from symptoms such as anxiety and social phobia, which are collectively known to influence self-reported chronic pain. Therefore, we must consider that it may not necessarily be rurality or feeling alone which impacts on CWP, but predisposing feelings of anxiety.
An intriguing observation was that participants who reported fewer than 10 people to whom they could turn to in a crisis experienced a decrease in the likelihood of CWP. Initially counter-intuitive, one can only speculate on the mechanism underpinning this association. However, it may be that these relationships are bidirectional and the more people you have to turn to, the more that may turn to you, and that this is in some way detrimental. Or, it may be that it is the perception of the quality of these relationships that is important rather than the quantity.
Conclusion
Hitherto, there has been little research looking at the epidemiology of pain in rural communities, and there are no previous direct comparisons of pain prevalence in rural versus urban populations. We have shown that while the prevalence of regional pain conditions is similar, there is some evidence that CWP occurs more commonly in increasingly rural areas, although the magnitude of this increase it not large. Our findings need to be corroborated with longitudinal investigations; we have demonstrated that while a number of aspects of the aetiology of CWP in rural populations are similar to those reported in urban settings, not all objective measures of social contact are associated with pain prevalence. However, there is a negative effect of perceived social isolation. Those who know/trust their neighbours are less likely to report CWP, not only providing evidence for the benefit of a strong neighbourhood community but also stressing the importance of the perceived quality of these relationships. While this is important for the maintenance of musculoskeletal health generally, it is of particular importance in rural areas where individuals are more likely to be physically and socially isolated.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article. However, the MUSICIAN study was funded by Arthritis Research UK. The O4O study was funded through the EU Northern Periphery Programme, Scottish Government, Highland Council and Highlands and Islands Enterprise. Funding was matched by contributions to the project in each region. In addition, O4O received support from organisations including the Scottish Government, Highlands & Islands Enterprise and the Highland Council.
References
- 1. Macfarlane GJ, Jones GT, McBeth J. Epidemiology of Pain. In McMahon S, Koltzenburg M, Tracey I, Turk DC. (eds): Wall and Melzack’s Textbook of Pain; 6th edition Seattle: Elsevier Ltd, 2013. [Google Scholar]
- 2. Van Tulder MW, Koes B, Bombardier C. Low back pain. Best Pract Res Clin Rheumatol 2002; 16: 761–775. [DOI] [PubMed] [Google Scholar]
- 3. Papageorgiou AC, Croft PR, Ferry S, et al. Estimating the prevalence of low back pain in the general population. Evidence from the South Manchester Back Pain Survey. Spine 1995; 20: 1889–1894. [DOI] [PubMed] [Google Scholar]
- 4. Hoffman PK, Meier BP, Council JR. A comparison of chronic pain between an urban and rural population. J Community Health Nurs 2002; 19: 213–224. [DOI] [PubMed] [Google Scholar]
- 5. Scottish Government. Effective interventions unit – rural and remote areas, http://www.scotland.gov.uk/Publications/2005/06/28112330/23387 (2005, accessed 13 February 2014).
- 6. Andersson HI. The epidemiology of chronic pain in a Swedish rural area. Qual Life Res 1994; 3(Suppl. 1): S19–S26. [DOI] [PubMed] [Google Scholar]
- 7. Tripp DA, VanDenKerkhof EG, McAlister M. Prevalence and determinants of pain and pain-related disability in urban and rural settings in south-eastern Ontario. Pain Res Manag 2006; 11: 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walsh K, Cruddas M, Coggon D. Low back pain in eight areas of Britain. J Epidemiol Community Health 1992; 46: 227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andersson GB. Epidemiological features of chronic low-back pain. Lancet 1999; 354: 581–585. [DOI] [PubMed] [Google Scholar]
- 10. Joshi VL, Chopra A. Is there an urban-rural divide? Population surveys of rheumatic musculoskeletal disorders in the Pune region of India using the COPCORD Bhigwan model. J Rheumatol 2009; 36: 614–622. [DOI] [PubMed] [Google Scholar]
- 11. Pennebaker JW. Psychological factors influencing the reporting of physical symptoms. In: Stone AA, Turkkan JS, Bachrach CA, et al. (eds) The science of self-report: implications for research and practice. Mahwah, NJ: Erlbaum Publishers, 1999, pp. 299–316. [Google Scholar]
- 12. Farmer J, Bradley S. Measuring the value of social organisations as service providers. In: Farmer J, Hill C, Munoz S-A. (eds) Community co-production: social enterprise in remote and rural communities. Northampton, MA: Edward Elgar Publishing, Inc, 2012, pp. 133–158. [Google Scholar]
- 13. McBeth J, Prescott G, Scotland G, et al. Cognitive behavior therapy, exercise, or both for treating chronic widespread pain. Arch Intern Med 2012; 172: 48–57. [DOI] [PubMed] [Google Scholar]
- 14. Macfarlane GJ, Beasley M, Jones EA, et al. The prevalence and management of low back pain across adulthood: results from a population-based cross-sectional study (the MUSICIAN study). Pain 2012; 153: 27–32. [DOI] [PubMed] [Google Scholar]
- 15. Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum 1990; 33: 160–172. [DOI] [PubMed] [Google Scholar]
- 16. Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol 2004; 160: 301–305. [DOI] [PubMed] [Google Scholar]
- 17. Office of the Chief Statistician, Scottish Government. Mid-2010 population estimates Scotland: population estimates by sex, age and administrative area. Available at: http://www.gro-scotland.gov.uk/files2/stats/population-estimates/mid-2010/mid-year-pop-est-2010.pdf (2011).
- 18. Hunt IM, Silman AJ, Benjamin S, et al. The prevalence and associated features of chronic widespread pain in the community using the ‘Manchester’ definition of chronic widespread pain. Rheumatology (Oxford) 1999; 38: 275–279. [DOI] [PubMed] [Google Scholar]
- 19. Nicholl BI, Macfarlane GJ, Davies KA, et al. Premorbid psychosocial factors are associated with poor health-related quality of life in subjects with new onset of chronic widespread pain – results from the EPIFUND study. Pain 2009; 141: 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Young AF, Russell A, Powers JR. The sense of belonging to a neighbourhood: can it be measured and is it related to health and well being in older women? Soc Sci Med 2004; 59: 2627–2637. [DOI] [PubMed] [Google Scholar]
- 21. Docking RE, Fleming J, Brayne C, et al. Epidemiology of back pain in older adults: prevalence and risk factors for back pain onset. Rheumatology (Oxford) 2011; 50: 1645–1653. [DOI] [PubMed] [Google Scholar]