Abstract
Background:
Epidural analgesia has been the reference standard for the provision of post-operative pain relief in patients recovering from major upper abdominal operations, including liver resections. However, a failure rate of 20–32% has been reported.
Aim:
The aim of the study was to analyse the success rates of epidural analgesia and the outcome in patients who underwent liver surgery.
Methods:
We collected data from a prospectively maintained database of 70 patients who underwent open liver surgery by a bilateral subcostal incision during a period of 20 months (February 2009 to September 2010). Anaesthetic consultants with expertise in anaesthesia for liver surgery performed the epidural catheter placement. A dedicated pain team assessed the post-operative pain scores on moving or coughing using the Verbal Descriptor Scale. The outcome was measured in terms of epidural success rates, pain scores, post-operative chest infection and length of hospital stay.
Results:
The study group included 43 males and 27 females. The indication for resection was liver secondaries (70%), primary tumours (19%) and benign disease (11%). While major (≥3 segments) and minor resections (≤ 2 segments) were performed in 44% and 47% respectively, 9% of patients were inoperable. Epidural analgesia was successful in 64 patients (91%). Bacterial colonisation of epidural tip was noticed in two patients. However, no neurological complications were encountered. Five patients (7%) had radiologically confirmed chest infection. Four patients (6%) developed wound infection. One patient died due to liver failure following extended right hepatectomy and cholecystectomy for gall bladder cancer. The median length of stay was 6 days (3–27 days). The extent of liver resection (p = 0.026) and post-operative chest infection (p = 0.012) had a significant influence on the length of stay.
Conclusion:
Our experience shows that epidural analgesia is safe and effective in providing adequate pain relief following open liver surgery.
Keywords: Epidural analgesia, liver surgery, acute pain, drug therapy, prevention and control
Introduction
Epidural analgesia provides regional pain control through neuraxial blockade. Epidural analgesia has been shown to provide better pain relief (especially during movement) in the initial post-operative period following open abdominal surgery when compared with patient-controlled analgesia (PCA).1,2 Furthermore, the duration of post-operative tracheal intubation and the occurrence of prolonged post-operative mechanical ventilation, myocardial infarction, gastric complications and renal complications have all been demonstrated to be considerably reduced by the use of epidural analgesia.2 There are several physiological effects that provide a rationale for expecting improved outcome with this technique.3 It is considered to provide adequate analgesia to support mobilisation and significant reduction in pulmonary and cardiovascular morbidity in the early post-operative period.4 Epidural analgesia is therefore widely considered as the reference standard for pain management in major abdominal surgery. It has gained importance particularly after the implementation of enhanced recovery programmes. However, several studies have demonstrated that a considerable proportion (20–32%) of epidural catheters either fail or perform suboptimally in the immediate post-operative period resulting in inadequate pain management.5,6
Liver resection is being increasingly performed for a wide variety of benign and malignant liver lesions. It has become the treatment of choice for colorectal liver metastases and is associated with an overall 5-year survival rate of 35–58%.7–21 While laparoscopic liver resections are being increasingly performed, open resection still remains the optimal method for large tumours and those located in the posterior segments within the liver. Pain can impair pulmonary function, especially with large incisions close to the diaphragm. The reduction in functional residual capacity results in atelectasis and subsequent chest infections. Studies have demonstrated improved functional residual capacity, vital capacity and forced expiratory volume or peak expiratory flow in patients after the effective administration of epidural infusion analgesia.22,23
Hepatectomy is often performed with low central venous pressure (CVP) to reduce the blood loss from hepatic venous injury during parenchymal transection.24 Intraoperative neuraxial blockade during liver surgery can be useful for the maintenance of low CVP during hepatic transaction and has been shown to reduce the blood loss and transfusion requirements.25 However, the epidural analgesia associated post-operative hypotension may lead to administration of excessive intravenous fluid.26
The bilateral subcostal approach, extended to the right as far as the midaxillary line, to the left as far as the lateral border of the rectus muscle and in the midline superiorly to the xiphoid process, is the classical approach for major hepatectomies and for liver transplants.27 The central area of the incision represents the weakest point of the abdominal wall and predisposes to post-operative wound dehiscence and incisional hernia.28,29 The post-operative analgesia is considered to become progressively less effective with incisions reaching a higher level on the abdominal wall. Modifications of this incision include the J incision (without left subcostal component) and bilateral subcostal incision (without the midline component) with the main advantage of reduced pain28,30–32 and subsequent respiratory complications.
While potential complications of epidural analgesia such as epidural haematoma or abscess formation and their associated neurological sequelae can be debilitating, they are fortunately extremely rare.33 As post-operative coagulopathy can be commonly seen following liver resection, the advantages have to be carefully balanced against the risks of epidural catheter placement and removal.34,35 The aim of our study was to analyse the success rates and complications of epidural analgesia in liver resections performed with a standardised bilateral subcostal incision without a midline component. We also aimed at identifying any correlation between epidural failure, duration of stay and complications (chest infection and wound infection).
Methods
Data were collected from a prospectively maintained database for 70 consecutive patients who underwent open liver surgery during the period between February 2009 and September 2010. All the patients in our study had successful insertion of epidural catheter. Data included patient characteristics, indication, extent of liver resection, epidural data, post-operative pain scores, complications and overall length of hospital stay. All patients in the study underwent a bilateral subcostal incision, extended to the right as far as the midaxillary line, to the left as far as the lateral end of the rectus muscle. None of the patients had a midline extension. Resection was defined as major or minor, with minor resections comprising one or two segments, and major resections comprising three or more segments. All patients who had a major resection were managed in a high dependency unit for the first 24 hours following the operation. Patients were classified into three categories based on the indication for liver resection – benign disease, primary malignancy and secondary malignancy.
Epidural catheter insertion and management
Anaesthetic consultants with expertise in anaesthesia for liver surgery performed the epidural catheter placement. A mid-thoracic epidural was inserted aimed at placement into the T7-8 interspace in awake patients. Bupivacaine (0.1%) with fentanyl (2 µg/mL) infusions was utilised. All patients had a patient-controlled epidural analgesia (PCEA) infusion and were able to administer themselves an extra 3-mL bolus (maximum 2 per hour). Additionally, they were prescribed a continuous infusion rate of 3–12 mL/hour. Determination of rate was dependent upon the clinical decision of the acute pain service. Pain scores were assessed and documented by a clinical nurse specialist at specified time intervals. If patient reported severe pain, the rescue protocol dictated that initially a 5-mL clinician bolus would be administered via the epidural pump (bupivacaine 0.1% + fentanyl 2 mcg/mL). This would be performed by the clinical nurse specialist after a full clinical assessment.
All patients received paracetamol (1 g 6 hourly) as the standard adjuvant analgesia. However, dose reduction or omission was performed based on the extent of liver resection and hepatic impairment.36 A dedicated pain team assessed the post-operative pain scores on moving and/or coughing using the verbal descriptor scale (0 – no pain, 1 – mild pain, 2 – moderate pain, 3 – severe pain). Epidural failure was defined as the abandonment of the epidural analgesia and use of an alternative analgesic regimen in the form of intravenous PCA with opioids. Epidural catheter was usually removed on the third or fourth post-operative day following a day of discontinuation of epidural administration and checking of the clotting parameters on the day of removal. Despite a normal preoperative profile, coagulation disorders may occur after liver resection because of transient hepatic insufficiency which can predispose to epidural haematoma on removal.34,37 An activated partial thromboplastin time (aPTT) ratio less than 1.4 and a platelet count greater than 100 were considered as safe prior to the removal of epidural catheter. If the epidural site displayed any signs of infection, the epidural catheter was removed, and the tip of the catheter was sent to the microbiology department to check for any bacterial growth.
The complications of liver failure, chest infection and wound infection were recorded. Chest infection was defined as the presence of clinical symptoms along with consolidation on chest X-ray. Wound infection was defined as the presence of clinical signs along with confirmation on a microbiology swab. The outcome was measured in terms of epidural success rates, rates of chest and wound infection and length of hospital stay. All data were collected on an Excel spreadsheet and transferred to SPSS for analysis. SPSS version 20 was used foranalysis. Independent samples Mann–Whitney U-test and Kruskal–Wallis test were used as appropriate.
Results
The study group included 43 males and 27 females. The mean age was 66 years (32–84 years, median = 67 years, standard deviation (SD) = 9.2 years). The indication for resection was liver metastases (69%, n = 48), primary tumours (20%, n = 14) and benign disease (11%, n = 8). Among the metastatic tumours of liver that were resected, 90% (n = 44) were colorectal in origin and the remaining were metastases from carcinoid (n = 2), breast cancer (n = 1), anal squamous cell carcinoma (n = 1) and gall bladder cancer (n = 1). The primary tumours of the liver included hepatocellular carcinoma (HCC; n = 7), cholangiocarcinoma (n = 3) and gall bladder cancer (n = 4). While major and minor resections were performed in 44% (n = 31) and 47% (n = 33), respectively, 9% (n = 6) of patients were inoperable. Patient characteristics and complications in the patients belonging to above three categories are compiled in Table 1. The various types of liver resection procedures carried out either alone or in combination are enumerated in Table 2. Three patients underwent second liver resection.
Table 1.
Patient characteristics and complications in the various categories of liver resection.
| Variable | Major resection (≥3 segments) | Minor resection (<3 segments) | Inoperable | |
|---|---|---|---|---|
| Number (%) | 31 (44%) | 33 (47%) | 6 (9%) | |
| Age (years) | Median | 67 | 69 | 59 |
| Minimum–Maximum | 51–81 | 32–84 | 46–77 | |
| Gender | Male | 19 | 22 | 2 |
| Female | 12 | 11 | 4 | |
| Indication | Benign | 3 | 4 | 1 |
| Malignant | 28 | 29 | 5 | |
| Epidural | Success | 29 | 29 | 6 |
| Failure | 2 | 4 | 0 | |
| Chest infection | 3 | 2 | 0 | |
| Wound infection | 3 | 1 | 0 | |
| Length of stay | Median | 7 | 6 | 5.5 |
| Minimum–Maximum | 5–27 | 4–20 | 3–9 | |
Table 2.
Types of liver resection performed in our series of patients either alone or in combination.
| Type of resection | Number |
|---|---|
| Cyst excision | 1 |
| Cyst enucleation | 1 |
| Metastasectomy | 11 |
| Monosegmentectomy | 10 |
| Caudate lobectomy | 5 |
| Bisegmentectomy | 8 |
| Left lateral sectionectomy | 6 |
| Polysegmentectomy | 1 |
| Central hepatectomy | 3 |
| Left hepatectomy | 9 |
| Right hepatectomy | 16 |
| Extended right hepatectomy | 4 |
Epidural analgesia was successful in 64 patients (91%). Failure of epidural analgesia (n = 6) was noted only in male patients, and the association was statistically significant (p = 0.042, chi-square) in comparison to females. One patient did not have adequate analgesia upon waking in recovery despite clinician boluses and so was deemed a failure. A morphine PCA was subsequently prescribed as an alternative. Failure of analgesia was due to unilateral block in two patients. There were three other patients who also had a unilateral block initially; however, their pain scores reduced to mild pain (pain score = 1) with adjustments of the epidural catheter (aseptic withdrawal by 1 cm) and clinician boluses performed by the pain management clinical nurse specialists. These were therefore not deemed as failures. One patient developed an inadequate epidural block on second post-operative day, and despite clinician boluses performed by the pain management clinical nurse specialists, an effective block could not be re-established. The remaining two patients had epidural catheters which became disconnected from the bacterial filter. Due to the risk of infection, these catheters were subsequently removed. All patients with failed epidural were set up on a PCA with morphine. Post-operative pain scores were assessed for each patient for each day while on epidural. In all, 22 patients did not experience any pain (worst pain score = 0) while on epidural infusion. The worst pain score was 1 (mild pain) in 33 patients, 2 (moderate pain) in 7 patients and 3 (severe pain) in 7 patients. The pain scores could not be assessed for one patient due to stay in the intensive treatment unit (ITU), but the epidural catheter was considered to function optimally. Among the seven patients with worst pain score of 3, six did not respond to simple analgesia and needed alternative analgesia in the form of PCA with morphine, and hence considered as failure of epidural analgesia. The frequency of the pain scores up to the third post-operative day in patients who had a successful epidural analgesia is illustrated in Figure 1. None of the patients developed epidural haematoma or abscess. Bacterial colonisation of epidural tip was noticed in one patient. However, no neurological complications were encountered. The median post-operative time to epidural catheter removal was 3 days (minimum = 2, maximum = 5, mean = 3.4, SD = 0.98).
Figure 1.
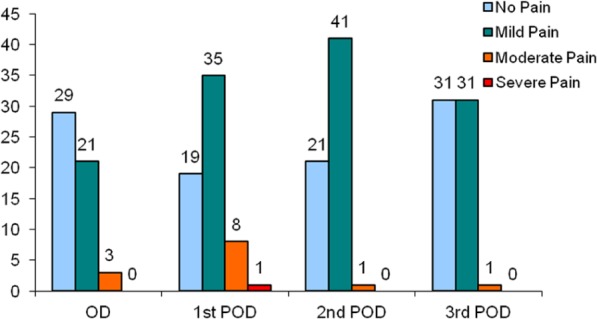
Clustered bar chart illustrating the frequency (number of patients) of pain scores in each of the post-operative days.
OD: operative day; POD: post-operative day.
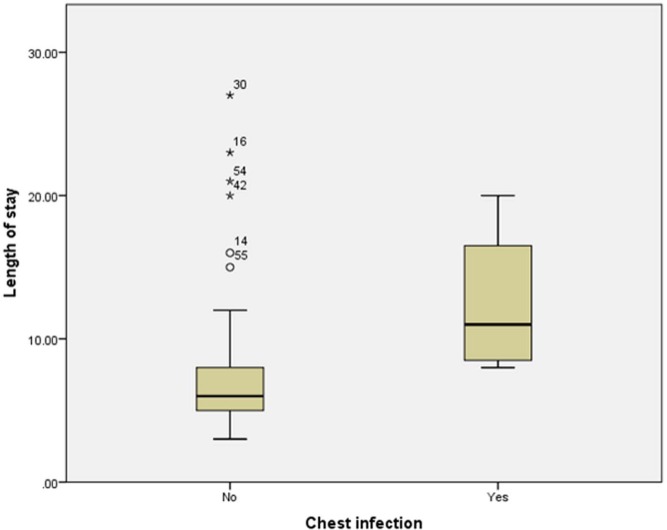
Five patients (7%) had radiologically confirmed chest infection. Four patients (6%) developed wound infection. One patient died due to liver failure following extended right hepatectomy for gall bladder cancer (1.4%). The median length of stay was 6 days (range = 3–27 days, SD = 4.6 days). There was no significant difference in the length of stay based on age, gender, indication for liver resection and the success of epidural analgesia. The extent of liver resection had a significant influence on the length of stay (p = 0.026, Kruskal–Wallis test; Figure 2), with patients undergoing major resections staying longer. Patients who developed post-operative chest infection also had significantly increased length of stay (p = 0.012, Mann–Whitney U-test; Figure 3).
Figure 2.
The length of stay was significantly higher in patients who had major liver resection (p = 0.026).
Figure 3.
The length of stay was significantly higher in patients who had a post-operative chest infection (p = 0.012).
Discussion
The majority of hepatic resections are still performed by open surgery with the help of large upper abdominal incisions. In this study, all the operations were performed or supervised by a single consultant with a standardised bilateral subcostal incision, as described in the methods section. Even though a mid-thoracic epidural is considered to provide a sensory block bilaterally across the upper abdomen, pain control and respiratory complications are considered to get worse as the level of incision is higher in the midline.32 The 50% diminished diaphragmatic function after major abdominal surgery triggers more post-operative muscular activity of the abdominal wall, which mainly affects the flat, transverse muscle.38,39 The deep fascial fibres cross the anterior abdominal wall in a transverse direction, like the Langer lines of cleavage, which run superficially. In the process of making a midline incision, both are divided, and the resulting higher distractive forces lead to greater impairment of the abdominal wall, which is responsible for a delayed post-operative pulmonary recovery. The same mechanism triggers an increase in perceived pain.32 Hence, this study analysed the effectiveness of epidural analgesia using a standardised incision.
In our study, epidural analgesia for post-operative pain relief was effective in 91% of the patients who had a successful insertion of epidural catheter. Suboptimal functioning of successfully inserted epidural catheters in a significant proportion of patients has been reported in the literature.5,40 In a recent study, Revie et al.41 had shown a 20% failure rate for epidural analgesia in liver surgery. Our study shows that if the epidural catheter placement is done by anaesthetists who perform the procedure on a regular basis and managed by a dedicated pain team, higher success rates can be achieved. While other forms of post-operative analgesia such as local wound infiltration along with PCA41 and intrathecal morphine42 have been suggested as alternative forms of analgesia with some advantages, epidural analgesia is still considered to be the reference standard for post-operative analgesia following upper abdominal surgery. This is mainly due to the fact that an optimally functioning epidural is considered to provide the best form of analgesia in the immediate post-operative period. While epidural analgesia is widely recommended as part of the enhanced recovery programmes, other forms of analgesia using opioids could be counterproductive. Even though, in our study, males were more likely to have a failure of epidural analgesia than females, this will need to be tested in studies of larger sample size. A similar finding has not been reported in the past.
While epidural-associated hypotension is commonly seen, this can be of advantage intra-operatively to maintain a low CVP and thereby reducing the blood loss from hepatic venous injury during parenchymal transection.24 However, anaesthetists did not routinely use the epidural to keep the CVP low in this series. Administration of excessive volumes of intravenous fluid due to the epidural-associated hypotension is well known.43 As the liver surgery is performed in specialised centres and with patients undergoing major liver resections being observed in a high dependency environment in the immediate post-operative period with specialised nursing staff, this problem should largely be overcome. Even though the rate of chest infection was low in our study (7%), it was still a significant factor in influencing the length of stay. As epidural analgesia reduces the systemic opioid requirements, it is associated with better post-operative bowel function, pulmonary function and earlier ambulation.44,45
While complications such as epidural haematoma and abscess formation along with their neurological sequelae need to be considered in the context of liver surgery, these remain extremely rare. Even though we had two patients with bacterial colonisation of the epidural tip, there was no evidence of epidural infection, and no neurological complications were encountered. The reduction in these serious complications is likely to be the improved expertise in the management of epidural catheters by dedicated pain team. The median length of stay in our study was 6 days, which was very similar to a recent study.41 However, the time required in fulfilling criteria for discharge rather than actual discharge was calculated in that study. While they had reported a shorter discharge time for wound infiltration along with PCA group, the quality of analgesia was reported to be superior with epidural catheter. Our observation that the length of stay was higher in patients undergoing major liver resection was on expected lines.
The strengths of this study are the standardisation of the incision and technique of liver surgery, and prospective data collection. The limitations are the small sample size and single-centre experience.
Conclusion
Our experience shows that epidural analgesia is successful in providing adequate pain relief following open liver surgery. We have shown a success rate of 91% with epidural analgesia in patients operated with a standardised bilateral subcostal incision without a midline component. This is much lower than has previously been reported within the literature and may be the result of having a small team of consultant anaesthetists who specialise in liver anaesthesia and from having an acute pain service who manage the epidural catheters more effectively. While the issue of neurological complications merits consideration, they are extremely rare and not reported in the recent studies. When the epidural catheter insertion is performed routinely by competent anaesthetists and managed on the ward by a dedicated pain team, it does seem to be effective.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Werawatganon T, Charuluxanun S. Patient controlled intravenous opioid analgesia versus continuous epidural analgesia for pain after intra-abdominal surgery. Cochrane Database Syst Rev 2005; 1: CD004088. [DOI] [PubMed] [Google Scholar]
- 2. Nishimori M, Ballantyne JC, Low JH. Epidural pain relief versus systemic opioid-based pain relief for abdominal aortic surgery. Cochrane Database Syst Rev 2006; 3: CD005059. [DOI] [PubMed] [Google Scholar]
- 3. Kehlet H. Modification of responses to surgery by neural blockade: clinical implications. In: Cousins M, Bridenbaugh P. (eds) Neural blockade in clinical anesthesia and management of pain, 2nd edn. Philadelphia, PA: J. B. Lippincott & Co, 1988, pp. 145–188. [Google Scholar]
- 4. Kehlet H, Holte K. Effect of postoperative analgesia on surgical outcome. Br J Anaesth 2001; 87(1): 62–72. [DOI] [PubMed] [Google Scholar]
- 5. McLeod G, Davies H, Munnoch N, et al. Postoperative pain relief using thoracic epidural analgesia: outstanding success and disappointing failures. Anaesthesia 2001; 56(1): 75–81. [DOI] [PubMed] [Google Scholar]
- 6. Ready LB. Acute pain: lessons learned from 25,000 patients. Reg Anesth Pain Med 1999; 24(6): 499–505. [DOI] [PubMed] [Google Scholar]
- 7. Hughes KS, Rosenstein RB, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases. A multi-institutional study of long-term survivors. Dis Colon Rectum 1988; 31(1): 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sasaki A, Iwashita Y, Shibata K, et al. Analysis of preoperative prognostic factors for long-term survival after hepatic resection of liver metastasis of colorectal carcinoma. J Gastrointest Surg 2005; 9(3): 374–380. [DOI] [PubMed] [Google Scholar]
- 9. Yasui K, Shimizu Y. Surgical treatment for metastatic malignancies. Anatomical resection of liver metastasis: indications and outcomes. Int J Clin Oncol 2005; 10(2): 86–96. [DOI] [PubMed] [Google Scholar]
- 10. Kanas GP, Taylor A, Primrose JN, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol 2012; 4: 283–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004; 239(6): 818–825, discussion 825–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scheele J, Stangl R, Altendorf-Hofmann A, et al. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery 1991; 110(1): 13–29. [PubMed] [Google Scholar]
- 13. Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002; 235(6): 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999; 230(3): 309–318, discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adson MA, van Heerden JA, Adson MH, et al. Resection of hepatic metastases from colorectal cancer. Arch Surg 1984; 119(6): 647–651. [DOI] [PubMed] [Google Scholar]
- 16. Gayowski TJ, Iwatsuki S, Madariaga JR, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery 1994; 116(4): 703–710, discussion 710–711. [PMC free article] [PubMed] [Google Scholar]
- 17. Jenkins LT, Millikan KW, Bines SD, et al. Hepatic resection for metastatic colorectal cancer. Am Surg 1997; 63(7): 605–610. [PubMed] [Google Scholar]
- 18. Jamison RL, Donohue JH, Nagorney DM, et al. Hepatic resection for metastatic colorectal cancer results in cure for some patients. Arch Surg 1997; 132(5): 505–510, discussion 511. [DOI] [PubMed] [Google Scholar]
- 19. Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 2005; 241(5): 715–722, discussion 722–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg 2009; 250(3): 440–448. [DOI] [PubMed] [Google Scholar]
- 21. Mayo SC, Pulitano C, Marques H, et al. Surgical management of patients with synchronous colorectal liver metastasis: a multicenter international analysis. J Am Coll Surg 2013; 216(4): 707–716, discussion 716–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bromage PR, Camporesi E, Chestnut D. Epidural narcotics for postoperative analgesia. Anesth Analg 1980; 59(7): 473–480. [PubMed] [Google Scholar]
- 23. Van der Auwera D, Verborgh C, Camu F. Analgesic and cardiorespiratory effects of epidural sufentanil and morphine in humans. Anesth Analg 1987; 66(10): 999–1003. [PubMed] [Google Scholar]
- 24. Melendez JA, Arslan V, Fischer ME, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg 1998; 187(6): 620–625. [DOI] [PubMed] [Google Scholar]
- 25. Jones RM, Moulton CE, Hardy KJ. Central venous pressure and its effect on blood loss during liver resection. Br J Surg 1998; 85(8): 1058–1060. [DOI] [PubMed] [Google Scholar]
- 26. Sakowska M, Docherty E, Linscott D, et al. A change in practice from epidural to intrathecal morphine analgesia for hepato-pancreato-biliary surgery. World J Surg 2009; 33(9): 1802–1808. [DOI] [PubMed] [Google Scholar]
- 27. Mazziotti A, Cavallari A. (eds). Techniques in liver surgery. London: Greenwich Medical Media, 1997. [Google Scholar]
- 28. D’Angelica M, Maddineni S, Fong Y, et al. Optimal abdominal incision for partial hepatectomy: increased late complications with Mercedes-type incisions compared to extended right subcostal incisions. World J Surg 2006; 30(3): 410–418. [DOI] [PubMed] [Google Scholar]
- 29. Heisterkamp J, Kazemier G. A J-shaped subcostal incision reduces the incidence of abdominal wall complications in liver transplantation. Liver Transpl 2009; 15(4): 453. [DOI] [PubMed] [Google Scholar]
- 30. Armstrong PJ, Burgess RW. Choice of incision and pain following gallbladder surgery. Br J Surg 1990; 77(7): 746–748. [DOI] [PubMed] [Google Scholar]
- 31. Halasz NA. Vertical vs horizontal laparotomies I. Early postoperative comparisons. Arch Surg 1964; 88: 911–914. [DOI] [PubMed] [Google Scholar]
- 32. Proske JM, Zieren J, Muller JM. Transverse versus midline incision for upper abdominal surgery. Surg Today 2005; 35(2): 117–121. [DOI] [PubMed] [Google Scholar]
- 33. Cook TM, Counsell D, Wildsmith JA. Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth 2009; 102(2): 179–190. [DOI] [PubMed] [Google Scholar]
- 34. Tsui SL, Yong BH, Ng KF, et al. Delayed epidural catheter removal: the impact of postoperative coagulopathy. Anaesth Intensive Care 2004; 32(5): 630–636. [DOI] [PubMed] [Google Scholar]
- 35. Matot I, Scheinin O, Eid A, et al. Epidural anesthesia and analgesia in liver resection. Anesth Analg 2002; 95(5): 1179–1181. [DOI] [PubMed] [Google Scholar]
- 36. Chandok N, Watt KD. Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc 2010; 85(5): 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Siniscalchi A, Begliomini B, De Pietri L, et al. Increased prothrombin time and platelet counts in living donor right hepatectomy: implications for epidural anesthesia. Liver Transpl 2004; 10(9): 1144–1149. [DOI] [PubMed] [Google Scholar]
- 38. Dureuil B, Cantineau JP, Desmonts JM. Effects of upper or lower abdominal surgery on diaphragmatic function. Br J Anaesth 1987; 59(10): 1230–1235. [DOI] [PubMed] [Google Scholar]
- 39. Cresswell AG, Grundstrom H, Thorstensson A. Observations on intra-abdominal pressure and patterns of abdominal intra-muscular activity in man. Acta Physiol Scand 1992; 144(4): 409–418. [DOI] [PubMed] [Google Scholar]
- 40. Burstal R, Wegener F, Hayes C, et al. Epidural analgesia: prospective audit of 1062 patients. Anaesth Intensive Care 1998; 26(2): 165–172. [DOI] [PubMed] [Google Scholar]
- 41. Revie EJ, McKeown DW, Wilson JA, et al. Randomized clinical trial of local infiltration plus patient-controlled opiate analgesia vs epidural analgesia following liver resection surgery. HPB (Oxford) 2012; 14(9): 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. De Pietri L, Siniscalchi A, Reggiani A, et al. The use of intrathecal morphine for postoperative pain relief after liver resection: a comparison with epidural analgesia. Anesth Analg 2006; 102(4): 1157–1163. [DOI] [PubMed] [Google Scholar]
- 43. Walsh SR, Walsh CJ. Intravenous fluid-associated morbidity in postoperative patients. Ann R Coll Surg Engl 2005; 87(2): 126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brimioulle S, Vachiery JL, Brichant JF, et al. Sympathetic modulation of hypoxic pulmonary vasoconstriction in intact dogs. Cardiovasc Res 1997; 34(2): 384–392. [DOI] [PubMed] [Google Scholar]
- 45. Warner DO, Warner MA, Ritman EL. Human chest wall function during epidural anesthesia. Anesthesiology 1996; 85(4): 761–773. [DOI] [PubMed] [Google Scholar]