Abstract
The present study aimed to explore how prescription of opioid medication for chronic non-malignant pain (CNMP) is managed in primary care. We used audit as a research tool, and one general practitioner (GP) practice in West London acted as an exemplar. Of the practice population with CNMP, 1% had repeat prescription of at least 12 months duration for opioid analgesics at the time of data collection. These 1% are on highly controlled opioids. Our study showed the following: (1) long-term opioid prescription appears to follow a fluctuating course as opposed to staying the same; (2) we found that medication reviews were done in most cases (85.7%), but the quality of the process is difficult to assess and ascertain; and (3) we identified two incidences where opioid contract was implemented. In both cases, contracts were used as a last chance warning for patients who were already problematic, suggesting that opioid contracts served as a disciplinary tool rather than a preventative measure. Our findings highlight a need for a more structured and specific review of analgesic medication, and a need for a simple and effective way to identify patients at high risk of developing problematic use, to ensure better monitoring and early presentations.
Keywords: Non-malignant pain, chronic therapy pain, opioid, primary health care, community health services
Introduction
The treatment of chronic non-malignant pain (CNMP) is a complex challenge facing primary care physicians.1,2 While studies have found opioids to provide moderately effective pain reduction in the short term, there is little to suggest that opioids have any long-term efficacy in treating various types of chronic pain disorders.3–9 On the other hand, there is well-established evidence demonstrating strong association of numerous adverse effects with long-term use of opioids.10 Moreover, prolonged use of opioids is also associated with poor employment status, negative quality of life indicators11,12 and addiction.13,14 Without specialist knowledge and tools, many primary care physicians report feeling ill trained when it comes to dealing with long-term opioid prescribing.15 In spite of this, in practice, the absence of other effective treatment means that opioids are often the ‘go-to’ medication for CNMP.16 A large proportion of patients continue on to long-term opioids treatment,5 with many ending up with lifelong usage.17 Prescriptions for strong opioids have increased markedly in the United Kingdom in recent times,18 and similar trends were also observed in other countries.19
Unsurprisingly, long-term use of opioids is often coupled with problematic prescription patterns and drug use behaviours. In a recent study, a group of Norwegian researchers reported high dose escalation and high discontinuation rates over a 5-year period.20 However, research suggests that a stable (non-escalation) dose of opioids is needed in CNMPs in order to achieve satisfactory analgesia with minimal risk of addition.21 The same Norwegian study also reported a link between high-dose opioids and co-medication with benzodiazepines, which can lead to increased health risks of addiction, overdose and death.20
In recognition of these difficulties, many national and international organisations have formulated guidelines aiming to prevent adverse events and to maximise effective pain control.22–24 A commonly recommended tool in these guidelines is the use of an ‘opioid contract’. The principle of the contract is to form a behaviour agreement between the physician and the patient, where a well-defined treatment goal is decided and the patient is adequately informed of the possible risks. Proponents of the contracts argue that using such a contract establishes a systematic approach to opioid administration and monitoring in primary care practices, thus ensuring safety and effectiveness.25 Although contracts are recommended in other countries, they are not standard practice in the United Kingdom. In reality, there are no specific tools in primary care to address the safety issues associated with opioid prescribing. The only regulatory framework in place at the moment is the practice of medication review for repeat prescriptions.
In the current study, we aim to examine the extent to which long-term strong opioid therapy is monitored in primary care. We investigate (1) whether opioid contracts are in place in primary care, using one general practitioner (GP) practice in West London as exemplar; and (2) to what extent medication reviews are carried out in a population of patients on long-term strong opioids in primary care.
Methods
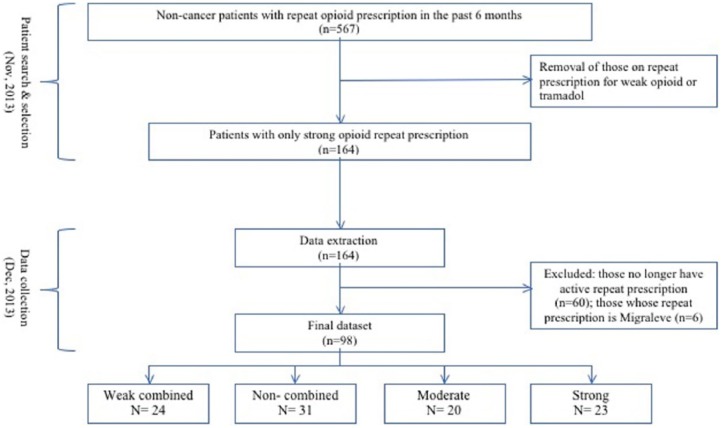
Patient search
All patient data were stored in the practice on the information technology (IT) program ‘SystmOne’.26 Tow GPs who were experienced with the system began patient search in November 2013. The first search was made by selecting ‘on opioids’ and ‘repeat templates’ under the rubric ‘reporting’. Only those who received repeat prescriptions in the past 6 months were selected. More refinements were made with a Venn diagram which represents ‘either/or’ or ‘and/or’ as Boolean operators. These allowed patients on weak combined opioids (co-codamol and co-dydramol) to be excluded. Of these patients, those with ‘cancer diagnoses’ on the quality and outcomes framework (QOF) register were excluded from the study. Finally, following the removal of patients who were on tramadol, the total number of patient subjects was reduced to 164 (1.6% of the practice population) (Figure 1). (Tramadol is classified as a weak opioid on the World Health Organization (WHO) pain ladder. Indeed, until 2011, tramadol was described as a weak opioid in the British National Formulary (BNF). However, tramadol has since been reclassified by the BNF. Presently, tramadol is considered to be a strong opioid by many authorities.27 To complicate the matter further, there exist some controversies as to whether tramadol should be considered as opiate; as an analogue of codeine, it has partial mu receptor agonist activity, but it also has central gamma aminobutyric acid (GABA), catecholamine and serotonergic effects, making its main mechanism of action more similar to tricyclic antidepressants (TCA) or selective serotonin reuptake inhibitors (SSRIs). It is therefore difficult to differentiate the potency of tramadol as an opioid to its analgesic potency in general. More importantly, despite the reclassification, in practice, GPs do not have the same psychological barriers to tramadol as they have to other controlled drugs; as a result, tramadol is as commonly and freely prescribed as codeine and other weaker opioid preparations. Due to these complexities, tramadol was excluded from the present study.)
Figure 1.
Flow chart illustrating the process of patient selection and data extraction. The intention during patient search was to select only those with strong opioid repeat prescriptions. As shown here, during data collection (1 month post patient search), we found that 60 patients no longer had active repeat prescription, and only a quarter of the selected patients still had strong opioid prescription. Errors during the search may account for some of the result found, but unlike to account for such a dramatic deviance to the original intention.
Data extraction
Data were collected 1 month after the patient search and collection in December 2013. Data were extracted from patients’ electronic medical records accessed in the practice via ‘SystmOne’. Only data on repeat medications were recorded, including the total number of medications, the number of analgesics overall (analgesics included all opioids, non-steroidal anti-inflammatory drugs (NSAIDs), paracetamol, antidepressants (SSRIs and TCAs) and anticonvulsants (pregabalin, gabapentin) that are established in the treatment of chronic pain.), the number of opioid medications and benzodiazepine co-prescription. Demographic information, including patient’s initials, age and gender, were also recorded. In addition, their mental health diagnosis status was recorded from the summary of diagnoses section of the electronic patient record.
Of the 164 patients, 60 patients were excluded as they had no active repeat prescription of strong opioids at the time of data collection. A further six patients were excluded as their only opioid prescriptions were of the anti-migraine drug Migraleve. Following these further exclusions, data collected from a total of 98 patients (ca. 1% of the patient population) were subjected to statistical analysis (Figure 1).
Patient categorisation
At the selection process, patients taking tramadol or codeine-based opioids (e.g. co-codamol) were excluded from the study, theoretically leaving only those on strong opioids. However, at the time of data collection, roughly 1 month later, the type of opioid uptake within the study cohort had changed considerably. With as many patients receiving only weak opioid repeat prescriptions as there were for strong opioids (Figure 1). For the purpose of statistical analysis, we divided the types of opioids into four commonly recognised categories based on strength.28 The categories were ranked from 1 to 4 according to their potency/strength (Table 1). Patients were assigned to one of the categories based on the most potent opioid repeat prescription they had at the time. We found that in general, there was minimal co-prescribing between each opioid category. The majority of patients received repeat prescriptions from only one of the categories, and none received repeat prescription for more than two categories of opioids.
Table 1.
Opioid categorisation, key demographic information and their average mean OMED.
| Categories | Criteria | Rank of strength | Examples | N | Gender (F:M) | Age (range), years | Mean OMED (SD), mg |
|---|---|---|---|---|---|---|---|
| Weak combined | Weak opioid combined with paracetamol | 1 | Co-codamol, co-dydramol | 24 | 20:4 | 66 (27–96) | 6.34 (8.686) |
| Weak non-combined | Weak opioids | 2 | Codeine | 31 | 20:11 | 58 (24–86) | 4.81 (3.421) |
| Moderate | Strong analgesics with relatively small proportion of opioid-mediated analgesic activity | 3 | Tramadol | 20 | 16:6 | 56 (31–86) | 35.13 (54.81) |
| Strong | Strong opioids (controlled drugs) | 4 | Morphine, oxycodone, methadone, fentanyl, buprenorphine, diamorphine | 23 | 12:11 | 63 (35–88) | 62.59 (67.61) |
OMED: oral morphine equivalent dose; SD: standard deviation.
Total oral morphine equivalent dose
The total oral morphine equivalent dose (OMED) of all opioids on repeat prescription was calculated for each patient as another measure of opioid intake. The calculation was done using an online opioid converter29 and verified with National Health Service (NHS) trust guidelines.30
Data analysis
Descriptive statistics were used to analyse the number of repeat prescriptions, OMED and medication reviews. Simple t-test was used to examine difference in OMED between patients co-prescribed with benzodiazepine and those without. Kruskal–Wallis test was used to compare the difference in number of repeat prescriptions and medication reviews between the different opioid potency categories.
Spearman’s ranking test was used to investigate relationships between opioid categories, numbers of medications, medication review and OMED. Simple logistic regression analysis was used to examine whether the presence of medication review can be predicted by any of the variables investigated (patient’s age, gender, number of total medication repeat prescriptions, number of total opioid repeat prescriptions, number of total analgesic repeat prescriptions, benzodiazepine co-prescription and mental health diagnosis).
Results
Patient demographics
Of the 98 patients, 66 (67%) were female and 32 (33%) were male. Patient’s age ranged from 24 to 96 years. The mean age was 61 years (standard deviation (SD) = 17.18 years). In all, 40 patients (40.8%) had a mental illness diagnosis, and 9 had a repeat prescription for benzodiazepine (9.2%).
Opioid contract
A behaviour contract in the past 12 months with regard to their opioid medication existed with two patients. Further reading of the medical record revealed that the reason for implementing the opioid contract was due to problematic opioid use (dose escalation, obtaining medication from various providers, lost prescriptions).
Repeat prescriptions
The mean total number of prescriptions was 10.90 (SD = 6.19). The mean total number of repeat prescriptions that were analgesics was 3.07 (SD = 1.69), and the mean total number of opioid repeat prescriptions was 1.29 (SD = .69). The majority of the patients had one opioid repeat prescription (80.6%). Broadly speaking, for the majority of the patients, analgesics accounted for half of the medications they received on repeat prescription (Mode = 50%, Median = 27.92%), indicating that pain is not their only medical problem. Opioids appear to be the mainstay of pain treatment for a large proportion of patients and commonly account for half of the analgesics on repeat prescription (Mode = 50%, Median = 50%).
Opioid potency categories
Kruskal–Wallis test was used to compare differences between the four opioid categories. There was no significant difference in age between the categories (p < .404). There was a significant difference in gender (p = .05) for most groups; there were more females than males with the exception of the strong opioid group. There was no difference in the number of mental health diagnoses between each opioid group (χ2(3, N = 98) = 3.437, p = .329). But a significant difference in number of benzodiazepine co-medication was found between each opioid group (χ2(3, N = 98) = 8.519, p = .0.36).
Prescription patterns
Significant differences were found between each of the opioid categories in the total number of opioid prescription (χ2(3, N = 98) = 20.938, p < .001), number of analgesic repeat prescriptions (χ2(3, N = 98) = 15.754, p = .001) and medication review (χ2(3, N = 98) = 11.512, p = .009) but not total number of repeat prescriptions (χ2(3, N = 98) = 7.223, p = .074). Spearman’s ranking test showed that stronger opioids were correlated with an increased number of analgesics (R(98) = .400, p < .001) and opioids (R(98) = .446, p < .001).
Morphine equivalent dose
The entire patient population investigated had a mean dose of 25 mg OMED (SD = 47.108 mg; range: 1–250 mg). The amount of OMED varied significantly between each opioid category (χ2(3, N = 98) = 58.568, p < .001). A strong positive relationship was found between morphine equivalent dosage and categories of opioid strength (R(98) = .712, p < .001).
Medication review
In the majority of cases, medication reviews were conducted within the past 12 months (85.7%); a total of 14 patients did not receive a medication review. No significant difference was observed between each opioid category and medication review (χ2(3, N = 98) = 7.213, p = .065).
A logistic regression analysis was conducted to predict medication review using all of the available variables. A test of the full model against a constant-only model was statistically significant, indicating that the predictors as a set reliably distinguished between receiving and not receiving a medication review within a 12-month period (χ2(9, N = 98) = 26.869, p = .001). Nagelkerke R2 of .428 indicates a moderately strong relationship between prediction and grouping. The Wald criterion demonstrated that only age made a significant contribution as a predictor (p = .005).
Using Spearman’s correlation, we find that age is positively associated with total number of repeat medication (r(1, 98) = .194, p = .006) and negatively associated with the number of analgesics (r(1, 98) = −.200, p = .007). In those without medication review, the mean OMED was 16.09 mg (SD = 26.80 mg), including one patient with an OMED of 100 mg. Two patients (20%) with benzodiazepine co-prescriptions did not receive a medication review.
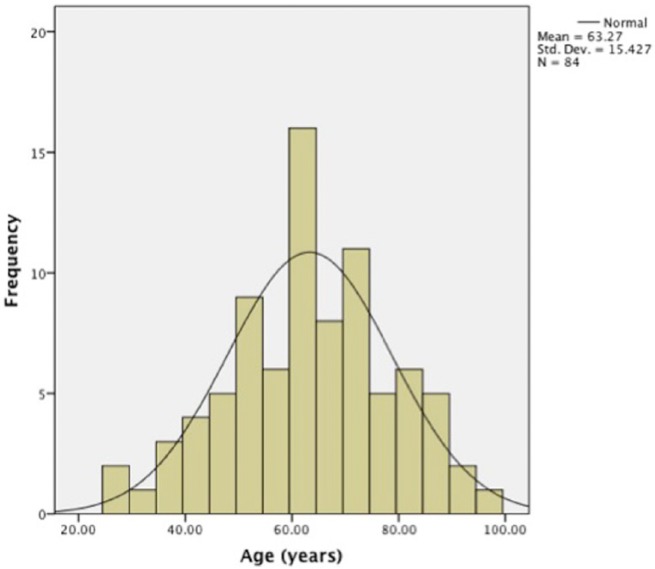
The mean age for those without medication review was 44 years (SD = 18.76 years) and 63.27 years (SD = 15.43 years) for those with medication review. The histograms show a normal distribution of age in patients who have received medication review, whereas in patients without medication review, the age distribution was skewed to the left (Figures 2 and 3).
Figure 2.
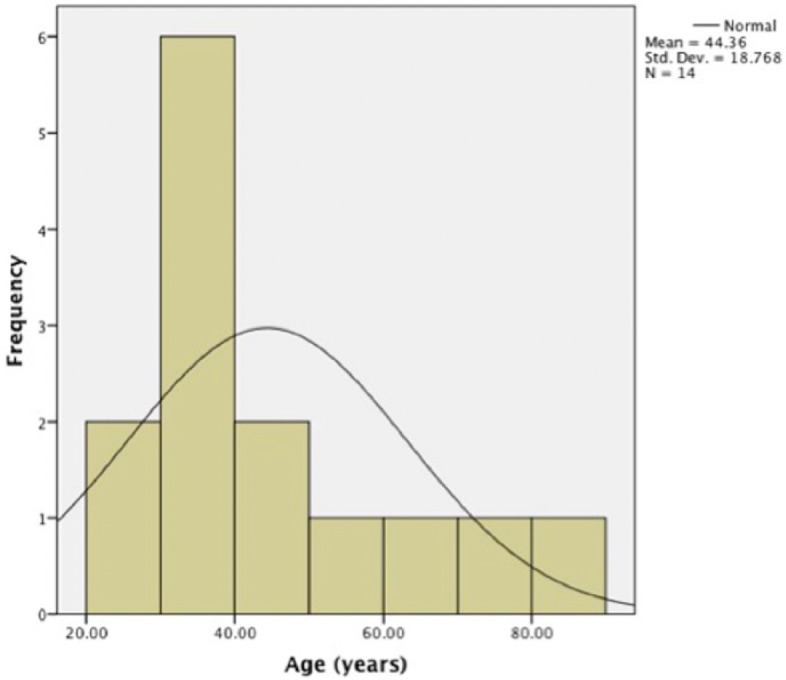
Histogram showing the age distribution in patients without medication review (N = 14).
Figure 3.
Histogram showing the age distribution in patients with medication review (N = 84).
Benzodiazepine co-medication
A significant correlation was found between benzodiazepine prescriptions and strong opioid prescriptions (r(98) = .289, p = .004) and more mental health diagnoses (r(98) = .311, p = .002). No other categories of opioids were significantly correlated with benzodiazepine prescriptions (weak combined: p = .711; weak: p = .619; tramadol: p = .091). There was no significant difference in OMED between those with benzodiazepine prescriptions and those without (t(96) = .644, p = .969).
Discussion
The present study aimed to examine the monitoring and management of opioid therapy in primary care by investigating the presence of an opioid contract and the practice of medication reviews. We observed that in the majority of cases, the opioid dosage was low (OMED average 25 mg/day),31 with the highest dosages found in those taking strong opioids. A significant proportion of patients suffered from mental illness (40.8%), and a notable number of patients had co-prescription of benzodiazepines and strong opioids (9.2%).
Opioid contract
Guidelines have suggested that opioid contracts would be a useful monitoring and potentially preventative tool for those who are at risk of problematic use.22–24 Our study found that opioid contracts were implemented only in cases when problematic use has already developed. The two patients observed in our study had exhibited disruptive behaviour and problematic opioid use (dose escalation, obtaining medication from various providers, lost prescriptions). The contract served as a last chance warning, whereby if the agreement were breached, the patient would be removed from the practice list. The finding suggests a need for more effective identification of high-risk patients so that opioid contracts can be implemented at an earlier stage to be used as a preventative rather than a disciplinary tool.
Medication review
Our study found a high prevalence of medication review, suggesting that, at the very least, the majority of patients on long-term opioids had their prescriptions re-evaluated by a physician at least once over a 12-month period. The high proportion of medication reviews indicated to some degree appropriate medical practice in this primary care centre, possibly supported by the automatic reminders generated by the IT system. However, we have found that the details of the review process were not recorded. It was therefore difficult to assess specific issues relating to long-term opioids.
Using statistical modelling, we found age as the only independent predictor for medication review. This finding may partly be explained by our observations that increased age is associated with a higher number of prescriptions. More prescriptions would lead to an increased number of automated reminders on the computer system, making medication review more likely to occur. In contrast, factors associated with increased risk of problematic opioid use, such as opioid strength, presence of benzodiazepine co-prescription, number of co-morbidities and mental health diagnosis, did not predict medication review. These findings highlight a need for a more specific and structured medication review system, aimed to assess harmful analgesic use and to identify high-risk cases.
Opioid prescription pattern
Our study was initially designed to be a cross-sectional analysis. To our surprise, 1 month post subject selection (a maximum of 7 months past the last issue of repeat prescription), the study cohort had changed considerably from what was originally intended at the selection process. Of the 164 patients, 60 no longer had any active repeat prescriptions, and only 23 patients were still receiving a repeat prescription for strong opioids. We found minimal overlapping/co-prescribing between the different strength categories, which suggests that the changes in prescriptions are mostly class switching rather an augmentation.
High discontinuation rates in long-term strong opioid therapy have previously been reported in a number of studies.3,20 Similarly, the practice of therapy augmentation, switching and discontinuation are well recognised in long-term opioid treatments.32 Our findings are consistent with these previously reported results. Unlike previous studies, which are longitudinal prospective studies where almost all of the patients received speciality input, our study was a cross-sectional analysis of patients in primary care. Despite these differences, it is interesting to find similar rates of discontinuation and augmentation rates. It is important to highlight that since our study was not intended to be a prospective study, these findings were incidental and interpretations will need to be taken with caution. However, we can say with some degree of certainty that our findings suggest long-term opioid prescription follows a fluctuating as opposed to a stable pattern.
It is difficult to determine what might be the cause for the fluctuating prescription pattern observed in our study. Poor efficacy, intolerance to side effects and fear of addiction have been reported previously as the most common causes of opioid therapy discontinuation.33 Other studies have shown that even among those who found opioids useful, a high percentage of patients still reported a desire to stop or cut down, and this ambivalence towards opioids was linked with depression.34 In addition, GPs’ own attitudes towards strong opioids have also been reported to influence opioid prescription. A large proportion of GPs have reported reluctance to prescribe strong opioids for CNMP.15 Moreover, many expressed concern about long-term commitment (managing dosing, repeat prescription, addiction and other adverse events). Finally, changes in therapy may be a reflection of the fluctuating pattern typically associated with the clinical presentation of CNMP. It has been suggested that to ensure effective pain relief and minimise harm, clinicians should expect and recognise treatment failure and be prepared for the option of stopping and switching.35
Limitations
The findings of our study must be considered in light of its limitations. As with all population-based studies, our patient selection protocol was not perfect, and the search terms used were not tested and tried. As a result, we cannot discount that errors might have been made during the selection. Such errors may have resulted in patients who were on strong opioids being missed in the search process, and those on tramadol and other weaker preparations being erroneously selected. An improvement to the current methodology would be to test out different search terms to ensure that the optimum selection protocol is used. Finally, it should be recognised that our study has limited external validity, as the population of our patients was from a single GP practice in West London. Therapeutic practices and patient population can differ not only across the country but also within London.36
Implications
Our study found that the generic medication review system implemented in the practice was lacking in specific structure and documentation for the review of analgesic medications. We also found that despite guideline recommendations, opioid contract is used as a last chance warning rather than a monitoring tool. The observation that there is considerable fluctuation in opioid prescription demonstrates that long-term analgesic prescribing is a dynamic process that needs constant monitoring and reassessment. These findings highlight the need for a simple and effective system to ensure that all analgesic medications are properly reviewed and high-risk patients are readily identified.
It has been suggested that ongoing assessment of the four As – analgesia, activities of daily living, adverse events, aberrant drug seeking behaviour – can provide structure in the assessment and monitoring of pain treatment.37 We propose that the practice of medication reviews in opioid prescription/pain prescribing could be helped by electronic reminders to physicians to assess and document the four As.
In our study, benzodiazepine co-prescriptions were found to be associated with strong opioids. Moreover, we observed a significant correlation between benzodiazepine prescription and mental health illness. Although there was no significant difference in OMED between benzodiazepine co-prescription, other studies have reported associations between benzodiazepine co-medication and high opioid dosage.20,38 Elsewhere, it has been shown that benzodiazepine use is associated with higher risk of developing problematic opioid use or addiction.39 Given these findings, we tentatively suggest that the presence of benzodiazepine co-prescription may serve as a practical point for selection of high-risk cases without the necessity for administering other more time-consuming screen tools such as Screener and Opioid Assessment for Patients with Pain (SOAPP).40
Conclusion
Opioid management in primary care is a complex and difficult task. We have found that medication reviews are being done in a majority of patients and opioid contracts are implemented when problems arise. Our findings highlight the need for a system in which opioid prescription can be easily and effectively assessed in primary care, and a way in which high-risk patients can be identified for better opioid monitoring to prevent problematic use. We suggest that the presence of benzodiazepine co-prescription and high opioid dosage could serve as a simple and effective way to identify high-risk patients. A structured medication review system to include the four As (analgesia, activities of daily living, adverse events, aberrant drug seeking behaviour) may be one way to achieve effective monitoring.
Acknowledgments
The authors would like to thank the office staff at Richford Gate Medical Practice for their support and assistance.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Jens Foell is funded by an NIHR clinical lecturer fellowship.
References
- 1. Breivik H, Collett B, Ventafridda V, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006; 10: 287–333. [DOI] [PubMed] [Google Scholar]
- 2. Gureje O, Simon GE, Von Korff M. A cross-national study of the course of persistent pain in primary care. Pain 2001; 92: 195–200. [DOI] [PubMed] [Google Scholar]
- 3. Kalso E, Edwards JE, Moore RA, et al. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain 2004; 112: 372–380. [DOI] [PubMed] [Google Scholar]
- 4. Furlan AD, Sandoval JA, Mailis-Gagnon A, et al. Opioids for chronic noncancer pain: a meta-analysis of effectiveness and side effects. CMAJ 2006; 174: 1589–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev 2010; 1: CD006605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noble M, Tregear SJ, Treadwell JR, et al. Long-term opioid therapy for chronic noncancer pain: a systematic review and meta-analysis of efficacy and safety. J Pain Symptom Manage 2008; 35: 214–228. [DOI] [PubMed] [Google Scholar]
- 7. Deshpande A, Furlan A, Mailis-Gagnon A, et al. Opioids for chronic low-back pain. Cochrane Database Syst Rev 2007; 3: CD004959. [DOI] [PubMed] [Google Scholar]
- 8. Russell IJ, Kamin M, Bennett RM, et al. Efficacy of tramadol in treatment of pain in fibromyalgia. J Clin Rheumatol 2000; 6: 250–257. [DOI] [PubMed] [Google Scholar]
- 9. Avouac J, Gossec L, Dougados M. Efficacy and safety of opioids for osteoarthritis: a meta-analysis of randomized controlled trials. Osteoarthritis Cartilage 2007; 15: 957–965. [DOI] [PubMed] [Google Scholar]
- 10. Baldini A, Von Korff M, Lin EHB. A review of potential adverse effects of long-term opioid therapy: a practitioner’s guide. Prim Care Companion CNS Disord 2012; 14 DOI: 10.4088/PCC.11m01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eriksen J, Sjøgren P, Bruera E, et al. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. Pain 2006; 125: 172–179. [DOI] [PubMed] [Google Scholar]
- 12. Jensen MK, Thomsen AB, Højsted J. 10-year follow-up of chronic non-malignant pain patients: opioid use, health related quality of life and health care utilization. Eur J Pain 2006; 10: 423–433. [DOI] [PubMed] [Google Scholar]
- 13. Jovey RD. Opioids, pain and addiction – practical strategies. Br J Pain 2012; 6: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kissin I. Long-term opioid treatment of chronic nonmalignant pain: unproven efficacy and neglected safety? J Pain Res 2013; 6: 513–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCracken LM, Velleman SC, Eccleston C. Patterns of prescription and concern about opioid analgesics for chronic non-malignant pain in general practice. Prim Health Care Res Dev 2008; 9: 146–156. [Google Scholar]
- 16. Collett B-J. Chronic opioid therapy for non-cancer pain. Br J Anaesth 2001; 87: 133–143. [DOI] [PubMed] [Google Scholar]
- 17. Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of the Interventional Pain Physicians’ (ASIPP) Guidelines. Pain Physician 2008; 11(Suppl. 2): S5–S62. [PubMed] [Google Scholar]
- 18. Zin CS, Chen L-C, Knaggs RD. Changes in trends and pattern of strong opioid prescribing in primary care. Eur J Pain 2014; 18: 1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hamunen K, Paakkari P, Kalso E. Trends in opioid consumption in the Nordic countries 2002–2006. Eur J Pain 2009; 13: 954–962. [DOI] [PubMed] [Google Scholar]
- 20. Fredheim OMS, Borchgrevink PC, Mahic M, et al. A pharmacoepidemiological cohort study of subjects starting strong opioids for nonmalignant pain: a study from the Norwegian Prescription Database. Pain 2013; 154: 2487–2493. [DOI] [PubMed] [Google Scholar]
- 21. Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med 2003; 349: 1943–1953. [DOI] [PubMed] [Google Scholar]
- 22. Opioids for persistent pain: summary of guidance on good practice from the British Pain Society. Br J Pain 2012; 6: 9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009; 10: 113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain. Part I – evidence assessment. Pain Physician 2012; 15(Suppl. 3): S1–S65. [PubMed] [Google Scholar]
- 25. Hariharan J, Lamb GC, Neuner JM. Long-term opioid contract use for chronic pain management in primary care practice. A five year experience. J Gen Intern Med 2007; 22: 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. SystmOne, http://www.tpp-uk.com/; Wikipedia, the free encyclopedia, http://en.wikipedia.org/wiki/SystmOne_http://www.tpp-uk.com/ (accessed 14 May 2014).
- 27. Letter to ACMD re: tramadol, https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/288017/TramadolLetterACMD.pdf (accessed 25 April 2014).
- 28. WHO. WHO’s cancer pain ladder for adults. WHO, http://www.who.int/cancer/palliative/painladder/en/ (accessed 25 April 2014).
- 29. Opioid converter, opioid conversions, pain management, http://www.globalrph.com/narcoticonv.htm
- 30. Microsoft word, http://www.wales.nhs.uk/sites3/documents/814/OpiateConversionDoses%5BFinal%5DNov2010.pdf
- 31. Von Korff M, Kolodny A, Deyo RA, et al. Long-term opioid therapy reconsidered. Ann Intern Med 2011; 155: 325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gore M, Sadosky AB, Leslie DL, et al. Therapy switching, augmentation, and discontinuation in patients with osteoarthritis and chronic low back pain. Pain Pract 2012; 12(6): 457–468. [DOI] [PubMed] [Google Scholar]
- 33. Maier C, Schaub C, Willweber-Strumpf A, et al. Long-term efficiency of opioid medication in patients with chronic non-cancer-associated pain. Results of a survey 5 years after onset of medical treatment. Schmerz 2005; 19: 410–417. [DOI] [PubMed] [Google Scholar]
- 34. Howe CQ, Sullivan MD, Saunders KW, et al. Depression and ambivalence toward chronic opioid therapy for chronic noncancer pain. Clin J Pain 2012; 28: 561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moore A, Derry S, Eccleston C, et al. Expect analgesic failure; pursue analgesic success. BMJ 2013; 346: f2690. [DOI] [PubMed] [Google Scholar]
- 36. NHS Business Services Authority NHS prescription services. Variation between strategic variation between strategic authorities in prescribing of opioid analgesics (Quarter to March 2013), 2013, http://www.nhsbsa.nhs.uk/PrescriptionServices.aspx (accessed 15 May 2014).
- 37. Passik SD. Issues in long-term opioid therapy: unmet needs, risks, and solutions. Mayo Clin Proc 2009; 84: 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mellbye A, Svendsen K, Borchgrevink PC, et al. Concomitant medication among persistent opioid users with chronic non-malignant pain. Acta Anaesthesiol Scand 2012; 56: 1267–1276. [DOI] [PubMed] [Google Scholar]
- 39. Morasco BJ, Duckart JP, Carr TP, et al. Clinical characteristics of veterans prescribed high doses of opioid medications for chronic non-cancer pain. Pain 2010; 151: 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. SOAP, http://nhms.org/sites/default/files/Pdfs/SOAPP-14.pdf