Abstract
Background:
Human basic fibroblast growth factor (hBFGF) is a heparin-binding growth factor and stimulates the proliferation of a wide variety of cells and tissues causing survival properties and its stability and biological activity improvements have received much attention.
Materials and Methods:
In the present work, hBFGF produced by engineered Escherichia coli and purified by cation exchange and heparin affinity chromatography, was PEGylated under appropriate condition employing 10 kD polyethylene glycol. The PEGylated form was separated by size exclusion chromatography. Structural, biological activity, and stability evaluations were performed using Fourier transform infrared (FITR) spectroscopy, 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) assay and effect denaturing agent, respectively.
Results:
FITR spectroscopy revealed that both PEGylated and native forms had the same structures. MTT assay showed that PEGyalated form had a 30% reduced biological activity. Fluorescence spectrophotometry indicated that the PEGylated form denatured at higher concentrations of guanidine HCl (1.2 M) compared with native, which denatured at 0.8 M guanidine HCl.
Conclusions:
PEGylation of hBFGF makes it more stable against denaturing agent but reduces its bioactivity up to 30%.
Keywords: 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide assay; Fourier transform infrared spectroscopy; human basic fibroblast growth factor; polyethylene glycol methyl ether maleimide; stability
INTRODUCTION
Biotherapeutic agents are successfully employed in the treatments of different pathophysiological disorders ever since the first approval of recombinant insulin in 1982.[1] Hundreds of recombinant drugs have found places in the market after their approval by respective authorities. Such products are termed as first generation of recombinant protein/peptides as they show an identical amino acid sequence to a native human protein and replacing or increasing levels of that protein in vivo.[1] Though the first generation of biopharmaceuticals have been approved and improved; But they possess limitations such as relatively short in vivo circulation time, poor solubility, physicochemical and proteolytic instability and immunogenicity.[2] Furthermore, second generation of biotherapeutic agents was engineered by different approaches like, amino acid manipulation to reduce immunogenicity and proteolytic digestion.[3] Slow release and protection through drug delivery system,[2,4] higher circulation (half-life) and stability[5,6] and efficiency,[5,7,8,9,10,11,12,13,14,15] tissue permeability[6,16] and lower immunogenicity[17,18,19] can be achieved as postproduction modifications by conjugating the target protein to some synthetic or natural polymers such as polyethyleneglycol, thereby the patients get benefited as their administration frequency can be reduced to large extent.[2] human basic fibroblast growth factor (hBFGF) is one of the multifunctional biopharmaceutics with growing therapeutic potential in cardiovascular disease, cancer and other disorders.[20] hBFGF is a heparin binding growth factor containing 146 amino acids polypeptide with a high affinity to its trans membrane receptors[21,22,23,24] and stimulates the proliferation of a wide variety of cells and tissues causing survival properties.[25] Mitogenic and angiogenic properties,[26,27,28,29,30] effectiveness in burn treatment,[31,32,33,34] tissue repair after myocardial infarction[35,36,37,38] and improvement in spinal injuries[39,40,41,42,43,44] are the most important roles of hBFGF.
In this article, with the aim of protection against proteolysis which cause reduced stability, short half-life and immunities;[6,45] our attempts were made to PEGylate recombinant hBFGF and study its biological activity and stability by comparison such properties with that of non-PEGylated form.
MATERIALS AND METHODS
For BFGF production, Escherichia coli BL21 (DE3) containing plasmid expressing hBFGF was cultivated in Luria-Bertani (LB) broth (Sigma-Aldrich). The production of target protein was induced by 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG: Thermo Scientific: Fermentas) at 30°C for 4 h. The cells were harvested by centrifugation and were slurried in an appropriate buffer and then disrupted by high pressure homogenizer. It was then centrifuged at 4°C and 12000 rpm for 30 min. The supernatant was subjected to cation exchange membrane Sartobind S (Sartorius Co.) and heparin affinity (heparin affinity (HiTrap: GE Health Care Life Sciences), both at a flow rate of 1 ml/min using fast purification liquid chromatography (Bio-Rad). Vivaspin 10 kD was used to exchange the buffer in order to reduce the salt concentration. In the final step, anion exchange membrane chromatography Sartobind Q (Sartorius Co.) at a flow rate of 1 ml/min was used to minimize presence of endotoxin to accepted value. This step was performed to avoid interference of endotoxin in 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Western blot and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) 12% was carried out to confirm the presence of BFGF.
PEGylation of the above mentioned protein was performed in presence of nitrogen gas at dark employing 10 kD maleimide-polyethyleneglycol (Jenken). The PEGylated form was separated from unPEGyalted hBFGF by size exclusion chromatography using a Hiload 16/600 Superde × 75 prep grade column (GE Health care life sciences) at a flow rate of 1 ml/min. Both the forms were treated with different concentrations of guanidine hydrochloride from 0.1 mol/ml up to 4 mol/ml as denaturing agent at 37°C. The effects of guanidine hydrochloride on both PEGylated and unPEGyalted BFGF were studied by fluorescence spectrophotometry (Luminescence spectrometer PERKIN ELMER LS 50 B). This assay use for BFGF as a unique test to separation stable and unstable BFGF molecule from each other.[46]
3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide assay was used to evaluate the biological activity of the samples. The balb/c 3T3 cells from mouse embryo tissue were cultivated in Dulbecco's modified Eagle's medium (DMEM: Gibco) containing 10% fetal bovine serum (FBS) (Gibco). 10,000 cells/ml per well added to plastic 96 well plates, were cultured at 37°C for 2 h in a humidified 5% CO2-95% air atmosphere. The cells then were treated with different concentration of hBFGF. The assay was going on by using the MTT exclusion dye. The range of sample concentration was from 15 pg/ml up to 2000 pg/ml. The MTT assay was used for PEGylated form too.
Fourier transform infrared spectroscopy tests of PEGylated and native forms were performed to study the effect of PEGylation on protein secondary structure. Samples were mixed with potassium bromide in 1/200 ratio and then were ground to form a very fine powder. This powder was then compressed into a thin pellet at a pressure of 8 tons. The changes of structure before and after PEGylation were analyzed at the scan range of 4000–400/cm.
RESULTS
Escherichia coli BL21 (DE3) containing plasmid was grown in LB broth. The expression of BFGF was induced by 1 mM IPTG when the growth reached an OD of 1 at λ 600 nm. The cells were harvested by centrifugation after 4 h of induction and expression of BFGF was confirmed by SDS-PAGE 12% and Western blot. Purification steps were started by passing the cells’ slurry through high pressure homogenizer. The cell debris were separated by centrifugation at 20,000 rpm, 4°C for 30 min. Thus the supernatant containing hBFGF was subjected to two steps purification processes including cation exchange and heparin affinity chromatography techniques as mentioned in methods. Figure 1 depicts the purified BFGF.
Figure 1.
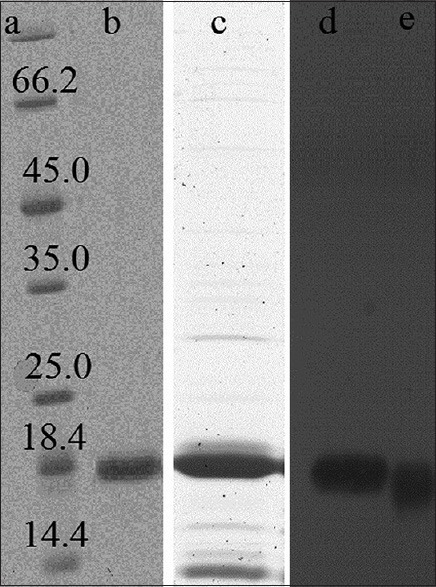
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis results of purified human basic fibroblast growth factor (hBFGF). Lane A: Ladder, Lane B: Heparin affinity eluate, Lane C: Cation exchange eluate, Lane D: Western of standard hBFGF, Lane E: Western of purified hBFGF
The purified hBFGF then was PEGylated and purified by size exclusion chromatography. Good separation of PEGylated hBFGF from reaction mixture, was achieved by using sufficient tall column (60 cm), low sample apply volume (2% column packed bed volume) and low flow rate (1 ml/min). Figure 2 shows the SDS-PAGE results of PEGylated recombinant BFGF separated from non-PEGylated form. The PEGylated form of the recombinant protein was separated.
Figure 2.
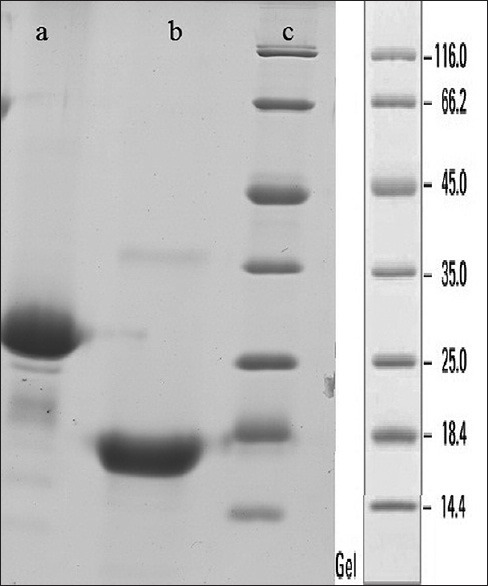
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis results of two fraction separated by size exclusion chromatography. Lane A: PEGylated form in first fraction, Lane B: Non-PEGyalated form in second fraction, Lane C: Ladder
Further, the purified human recombinant BFGF and its PEGylated form were subjected to MTT assay in order to evaluate the biological activity. In this assay 10,000 3T3 clone A31 cell line/well was cultured in RPMI1640 containing 10% FBS at 5% CO2 atmosphere in the presence of 50–200 ng of PEGylated and native recombinant BFGF and 68 and 98% of proliferation rate were observed respectively [Figure 3].
Figure 3.
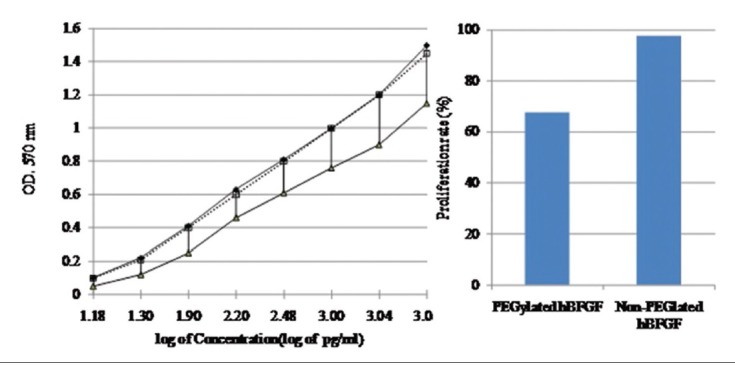
Biological activity analysis. Left: Proliferation assay for standard human basic fibroblast growth factor (hBFGF) (-♦-), PEGylated hBFGF (▪▪▫▪) and non-PEGylated form (-▴-). Right: Comparison of proliferation rate of PEGylated non-PEGylated hBFGF
Free BFGF and BFGF-polyethylene glycol (PEG) were treated with guanidine hydrochloride in order to study their stability by fluorescence spectroscopy. It was observed that BFGF was totally denatured by exposure to guanidine hydrochloride (37°C, 24 h) at 0.8 M concentration whereas BFGF-PEG was denatured by 1.2 M concentration of the same denaturing agent. Figure 4 shows the effect of denaturation on the fluorescence spectrophotometry results.
Figure 4.
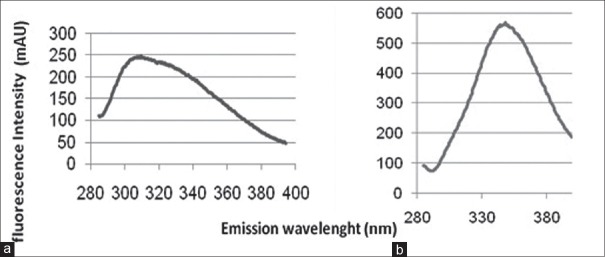
Fluorescence spectrophotometry. (a) intact PEGylated basic fibroblast growth factor (BFGF), (b) denatured form of PEGylated BFGF
Figure 5 depicts FTIR of free BFGF-PEG, BFGF and PEG where there is no structural change occurring in BFGF after PEGylation.
Figure 5.
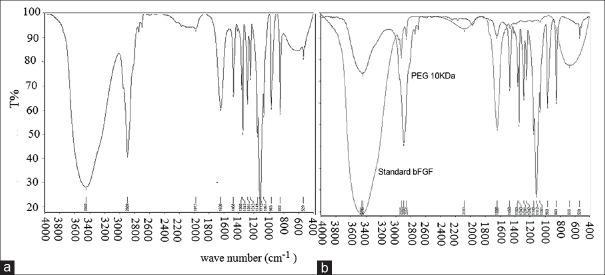
Fourier transform infrared (FITR) spectroscopy of free basic fibroblast growth factor-polyethylene glycol (BFGF-PEG), BFGF and PEG. (a) FTIR of PEGylated form, (b) FTIR of standard BFGF and PEG 10 kDa
DISCUSSION
PEGylation of proteins is a method through which the stability of protein can be improved. Random and nonselective PEGylation occurs at N-termini or lysine residues causing heterogeneous with low biological activity products while selective PEGylation reduces the above disadvantages brought about by nonselective PEGylation. Stability of PEGylated form is measure with different methods like heat stability or exposure to denaturing reagent. In this study, we use exposing to denaturing reagent like guanidine hydrochloride and determine by using fluorescence spectrophotometry as a unique assay for BFGF that recognize the stable, semi stable and un stable form of molecule.[46] We showed that the PEGylated form of hBFGF is resistant to denaturing agent like guanidine HCl though its biological activity to some extent is approximately 30% reduced. FTIR assay confirmed that PEGylation did not effect on the protein structure and masking the active site of protein partially is the reason of decreased bioactivity of PEGylated hBFGF.[6]
ACKNOWLEDGMENT
This project was financially supported by the Pasteur Institute of Iran as a Ph. D thesis.
Footnotes
Source of Support: This project was financially supported by the Pasteur Institute of Iran as a Ph. D thesis.
Conflict of Interest: None declared.
REFERENCES
- 1.Szymkowski DE. Creating the next generation of protein therapeutics through rational drug design. Curr Opin Drug Discov Devel. 2005;8:590–600. [PubMed] [Google Scholar]
- 2.Jevsevar S, Kunstelj M, Porekar VG. PEGylation of therapeutic proteins. Biotechnol J. 2010;5:113–28. doi: 10.1002/biot.200900218. [DOI] [PubMed] [Google Scholar]
- 3.Mateo C, Lombardero J, Moreno E, Morales A, Bombino G, Coloma J, et al. Removal of amphipathic epitopes from genetically engineered antibodies: Production of modified immunoglobulins with reduced immunogenicity. Hybridoma. 2000;19:463–71. doi: 10.1089/027245700750053959. [DOI] [PubMed] [Google Scholar]
- 4.Walsh G. Second-generation biopharmaceuticals. Eur J Pharm Biopharm. 2004;58:185–96. doi: 10.1016/j.ejpb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Tian H, Guo Y, Gao X, Yao W. PEGylation enhancement of pH stability of uricase via inhibitive tetramer dissociation. J Pharm Pharmacol. 2013;65:53–63. doi: 10.1111/j.2042-7158.2012.01575.x. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, Li X, Zeng Y, Zheng Q, Wu S. Site-directed PEGylation of human basic fibroblast growth factor. Protein Expr Purif. 2006;48:24–7. doi: 10.1016/j.pep.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Pfister D, Morbidelli M. Process for protein PEGylation. J Control Release. 2014;180:134–49. doi: 10.1016/j.jconrel.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Rissanen S, Kumorek M, Martinez-Seara H, Li YC, Jamróz D, Bunker A, et al. Effect of PEGylation on drug entry into lipid bilayer. J Phys Chem B. 2014;118:144–51. doi: 10.1021/jp4105745. [DOI] [PubMed] [Google Scholar]
- 9.Tagalakis AD, Kenny GD, Bienemann AS, McCarthy D, Munye MM, Taylor H, et al. PEGylation improves the receptor-mediated transfection efficiency of peptide-targeted, self-assembling, anionic nanocomplexes. J Control Release. 2014;174:177–87. doi: 10.1016/j.jconrel.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Simon M, Stefan N, Borsig L, Plückthun A, Zangemeister-Wittke U. Increasing the antitumor effect of an EpCAM-targeting fusion toxin by facile click PEGylation. Mol Cancer Ther. 2014;13:375–85. doi: 10.1158/1535-7163.MCT-13-0523. [DOI] [PubMed] [Google Scholar]
- 11.Lee JI, Eisenberg SP, Rosendahl MS, Chlipala EA, Brown JD, Doherty DH, et al. Site-specific PEGylation enhances the pharmacokinetic properties and antitumor activity of interferon beta-1b. J Interferon Cytokine Res. 2013;33:769–77. doi: 10.1089/jir.2012.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Paholak H, Ito M, Sansanaphongpricha K, Qian W, Che Y, et al. ’Living’ PEGylation on gold nanoparticles to optimize cancer cell uptake by controlling targeting ligand and charge densities. Nanotechnology. 2013;24:355101. doi: 10.1088/0957-4484/24/35/355101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onyskiw PJ, Eniola-Adefeso O. Effect of PEGylation on ligand-based targeting of drug carriers to the vascular wall in blood flow. Langmuir. 2013;29:11127–34. doi: 10.1021/la402182j. [DOI] [PubMed] [Google Scholar]
- 14.Kaminskas LM, Ascher DB, McLeod VM, Herold MJ, Le CP, Sloan EK, et al. PEGylation of interferon a2 improves lymphatic exposure after subcutaneous and intravenous administration and improves antitumour efficacy against lymphatic breast cancer metastases. J Control Release. 2013;168:200–8. doi: 10.1016/j.jconrel.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan X, Fabregat D, Yoshimoto K, Nagasaki Y. High PEGylation efficiency of pentaethylenehexamine-end poly (ethylene glycol) (mPEG-N6) for active-ester surface. Colloids Surf B Biointerfaces. 2012;92:25–9. doi: 10.1016/j.colsurfb.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Huang Z, Ye C, Liu Z, Wang X, Chen H, Liu Y, et al. Solid-phase N-terminus PEGylation of recombinant human fibroblast growth factor 2 on heparin-sepharose column. Bioconjug Chem. 2012;23:740–50. doi: 10.1021/bc200550f. [DOI] [PubMed] [Google Scholar]
- 17.Gefen T, Vaya J, Khatib S, Harkevich N, Artoul F, Heller ED, et al. The impact of PEGylation on protein immunogenicity. Int Immunopharmacol. 2013;15:254–9. doi: 10.1016/j.intimp.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Mohs A, Ambrogelly A, Yang X, Haverick M, Cheung JK, Narasimhan C, et al. Effect of pegylation on self-association of IFN-a2b. Mol Pharm. 2014;11:158–63. doi: 10.1021/mp400343b. [DOI] [PubMed] [Google Scholar]
- 19.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–21. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 20.Beenken A, Mohammadi M. The FGF family: Biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–53. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harmer NJ, Pellegrini L, Chirgadze D, Fernandez-Recio J, Blundell TL. The crystal structure of fibroblast growth factor (FGF) 19 reveals novel features of the FGF family and offers a structural basis for its unusual receptor affinity. Biochemistry. 2004;43:629–40. doi: 10.1021/bi035320k. [DOI] [PubMed] [Google Scholar]
- 22.Hughes SE. Differential expression of the fibroblast growth factor receptor (FGFR) multigene family in normal human adult tissues. J Histochem Cytochem. 1997;45:1005–19. doi: 10.1177/002215549704500710. [DOI] [PubMed] [Google Scholar]
- 23.Otsuki T, Yamada O, Yata K, Sakaguchi H, Kurebayashi J, Nakazawa N, et al. Expression of fibroblast growth factor and FGF-receptor family genes in human myeloma cells, including lines possessing t (4;14)(q16.3;q32) and FGFR translocation. Int J Oncol. 1999;15:1205–12. doi: 10.3892/ijo.15.6.1205. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 2006;281:15694–700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng J, Vagnoni KE, Bird IM, Magness RR. Expression of basic fibroblast growth factor, endothelial mitogenic activity, and angiotensin II type-1 receptors in the ovine placenta during the third trimester of pregnancy. Biol Reprod. 1997;56:1189–97. doi: 10.1095/biolreprod56.5.1189. [DOI] [PubMed] [Google Scholar]
- 26.Daviet I, Herbert JM, Maffrand JP. Involvement of protein kinase C in the mitogenic and chemotaxis effects of basic fibroblast growth factor on bovine cerebral cortex capillary endothelial cells. FEBS Lett. 1990;259:315–7. doi: 10.1016/0014-5793(90)80035-h. [DOI] [PubMed] [Google Scholar]
- 27.Hagood SK, McGinn MJ, Sun D, Colello RJ. Characterizing the mitogenic effect of basic fibroblast growth factor in the adult rat striatum. J Neurotrauma. 2006;23:205–15. doi: 10.1089/neu.2006.23.205. [DOI] [PubMed] [Google Scholar]
- 28.Izevbigie EB, Gutkind JS, Ray PE. Angiotensin II and basic fibroblast growth factor mitogenic pathways in human fetal mesangial cells. Pediatr Res. 2000;47:614–21. doi: 10.1203/00006450-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Peyrat JP, Bonneterre J, Hondermarck H, Hecquet B, Adenis A, Louchez MM, et al. Basic fibroblast growth factor (bFGF): Mitogenic activity and binding sites in human breast cancer. J Steroid Biochem Mol Biol. 1992;43:87–94. doi: 10.1016/0960-0760(92)90191-k. [DOI] [PubMed] [Google Scholar]
- 30.Presta M, Maier JA, Ragnotti G. The mitogenic signaling pathway but not the plasminogen activator-inducing pathway of basic fibroblast growth factor is mediated through protein kinase C in fetal bovine aortic endothelial cells. J Cell Biol. 1989;109(4 Pt 1):1877–84. doi: 10.1083/jcb.109.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akita S, Akino K, Imaizumi T, Hirano A. Basic fibroblast growth factor accelerates and improves second-degree burn wound healing. Wound Repair Regen. 2008;16:635–41. doi: 10.1111/j.1524-475X.2008.00414.x. [DOI] [PubMed] [Google Scholar]
- 32.Hayashida K, Akita S. Quality of pediatric second-degree burn wound scars following the application of basic fibroblast growth factor: Results of a randomized, controlled pilot study? Ostomy Wound Manage. 2012;58:32–6. [PubMed] [Google Scholar]
- 33.Muneuchi G, Suzuki S, Moriue T, Igawa HH. Combined treatment using artificial dermis and basic fibroblast growth factor (bFGF) for intractable fingertip ulcers caused by atypical burn injuries. Burns. 2005;31:514–7. doi: 10.1016/j.burns.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 34.Nie K, Li P, Zeng X, Sun G, Jin W, Wei Z, et al. Clinical observation of basic fibroblast growth factor combined with topical oxygen therapy in enhancing burn wound healing. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010;24:643–6. [PubMed] [Google Scholar]
- 35.Fathi E, Nassiri SM, Atyabi N, Ahmadi SH, Imani M, Farahzadi R, et al. Induction of angiogenesis via topical delivery of basic-fibroblast growth factor from polyvinyl alcohol-dextran blend hydrogel in an ovine model of acute myocardial infarction. J Tissue Eng Regen Med. 2013;7:697–707. doi: 10.1002/term.1460. [DOI] [PubMed] [Google Scholar]
- 36.Takehara N, Tsutsumi Y, Tateishi K, Ogata T, Tanaka H, Ueyama T, et al. Controlled delivery of basic fibroblast growth factor promotes human cardiosphere-derived cell engraftment to enhance cardiac repair for chronic myocardial infarction. J Am Coll Cardiol. 2008;52:1858–65. doi: 10.1016/j.jacc.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto M, Sakakibara Y, Nishimura K, Komeda M, Tabata Y. Improved therapeutic efficacy in cardiomyocyte transplantation for myocardial infarction with release system of basic fibroblast growth factor. Artif Organs. 2003;27:181–4. doi: 10.1046/j.1525-1594.2003.06993.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhang HY, Zhang X, Wang ZG, Shi HX, Wu FZ, Lin BB, et al. Exogenous basic fibroblast growth factor inhibits ER stress-induced apoptosis and improves recovery from spinal cord injury. CNS Neurosci Ther. 2013;19:20–9. doi: 10.1111/cns.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu WG, Luo YX. The early protective effects of basic fibroblast growth factor on acute spinal cord injury in rats. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1999;13:291–4. [PubMed] [Google Scholar]
- 40.Rabchevsky AG, Fugaccia I, Turner AF, Blades DA, Mattson MP, Scheff SW. Basic fibroblast growth factor (bFGF) enhances functional recovery following severe spinal cord injury to the rat. Exp Neurol. 2000;164:280–91. doi: 10.1006/exnr.2000.7399. [DOI] [PubMed] [Google Scholar]
- 41.Rabchevsky AG, Fugaccia I, Fletcher-Turner A, Blades DA, Mattson MP, Scheff SW. Basic fibroblast growth factor (bFGF) enhances tissue sparing and functional recovery following moderate spinal cord injury. J Neurotrauma. 1999;16:817–30. doi: 10.1089/neu.1999.16.817. [DOI] [PubMed] [Google Scholar]
- 42.Teng YD, Mocchetti I, Taveira-DaSilva AM, Gillis RA, Wrathall JR. Basic fibroblast growth factor increases long-term survival of spinal motor neurons and improves respiratory function after experimental spinal cord injury. J Neurosci. 1999;19:7037–47. doi: 10.1523/JNEUROSCI.19-16-07037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee TT, Green BA, Dietrich WD, Yezierski RP. Neuroprotective effects of basic fibroblast growth factor following spinal cord contusion injury in the rat. J Neurotrauma. 1999;16:347–56. doi: 10.1089/neu.1999.16.347. [DOI] [PubMed] [Google Scholar]
- 44.Mocchetti I, Rabin SJ, Colangelo AM, Whittemore SR, Wrathall JR. Increased basic fibroblast growth factor expression following contusive spinal cord injury. Exp Neurol. 1996;141:154–64. doi: 10.1006/exnr.1996.0149. [DOI] [PubMed] [Google Scholar]
- 45.Batra J, Robinson J, Mehner C, Hockla A, Miller E, Radisky DC, et al. PEGylation extends circulation half-life while preserving in vitro and in vivo activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) PLoS One. 2012;7:e50028. doi: 10.1371/journal.pone.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Estapé D, van den Heuvel J, Rinas U. Susceptibility towards intramolecular disulphide-bond formation affects conformational stability and folding of human basic fibroblast growth factor. Biochem J. 1998;335(Pt 2):343–9. doi: 10.1042/bj3350343. [DOI] [PMC free article] [PubMed] [Google Scholar]


