Abstract
Background:
Mild cognitive impairment (MCI) accompanies brain atrophy in neuroimaging investigations. The aim of this study was to compare MCI patients with the normal population for hippocampal volume (HV) and hippocampal angle (HA), and to assess the correlation between HV and HA.
Materials and Methods:
In a case-control study on 2014, in Kashani Hospital (Isfahan, Iran), 20 MCI patients were compared with 20 normal controls for HV and HA. Subjects were diagnosed with MCI or normal control, based on neuropsychiatry interview, which was confirmed by neuropsychiatry unit cognitive assessment tool (NUCOG). All magnetic resonance imaging scans were processed using the Free-Surfer software package for HV assessment. The HA was measured on the most rostral slice in which the uncal sulcus could be identified on a coronal plane. The data were analyzed using multiple analysis of co-variance and Pearson correlation.
Results:
The mean (standard deviation [SD]) score of NUCOG in control and case group were 91.05 (3.01) and 82.42 (3.57), respectively. Comparison of HV and HA scores in two groups, showed that mean (SD) HV and HA were not different between control and case groups, significantly, (P = 0.094 and P = 0.394, respectively). There was a negative correlation between the adjusted HV and the HA in case (r = −0.642, P = 0.004), and control groups (r = −0.654, P = 0.003).
Conclusion:
HV and HA were not different between MCI patients and normal controls; however, HA is correlated with HV negatively and may be used as an alternative factor because of more feasibility and availability in clinical settings in compared to HV.
Keywords: Alzheimer disease, hippocampus, magnetic resonance imaging, mild cognitive impairment
INTRODUCTION
Alzheimer disease (AD) is nearly the most common cause of cognitive decline in the elderly with increasing many social and financial burdens to the individual.[1] It can result in major or mild neurocognitive disorders with insidious onset and gradual progression of behavioral and cognitive symptoms.[2] Accurate and early diagnosis of patients most at risk of progression to dementia due to AD is very important and hence we need to develop sensitive and strong biomarkers including imaging techniques to quantify disease progress.[3] Mild cognitive impairment (MCI) or mild neurocognitive disorder leads to prominent changes in cognitive function, and is a prodromal phase that describes a state between the typical cognitive changes due to aging and early dementia, especially due to AD.[3,4] Some studies have concluded that 80% of patients with MCI, progress to dementia due to AD after about 6 years of follow-up.[5]
Alzheimer disease affects many brain structures, but the main and earliest region of damage is in medial temporal lobe (MTL) structures especially the entorhinal cortices and hippocampus.[6,7] Various techniques have been used to show structural deficits in brain magnetic resonance imaging (MRI).[8,9,10,11] A significant atrophy of the hippocampus and entorhinal cortex has been considered a hallmark of AD,[12,13] accordingly, hippocampal volume (HV) has been assessed frequently as a reliable biomarker in AD.[14,15,16] On the other hand, recent National Institute on Aging and Alzheimer's Association criteria, indicate that AD can be diagnosed at the predementia/prodromal or MCI stage, by assessment of some core imaging biomarkers, including MTL atrophy.[3,17] However, HV assessment has not been used extensively in MCI as much as AD, because this method needs special equipment and is difficult to obtain in clinical settings.[18] Assessing of HV by automatic techniques requires special computer facilities and need careful tuning of the parameters.[14,15] In manual segmentations by clinical experts, which is the gold-standard for assessment of HV, exact segmentation of structures is difficult, and a robust method for direct volumetric quantification is not available.[12,19]
There is no approved drug for modifying the course of AD, so it is more effective to begin the treatments before the onset of symptoms.[18] However, it is difficult to identify the transitions from the asymptomatic to the symptomatic predementia phase and from the MCI to dementia onset.[20,21] Considering this, using of a single, reliable and available neuroimaging technique for diagnosing of MCI and predicting of progression to dementia due to AD is very important. The most vulnerable brain regions in AD patients are subiculum and Cornu Ammonis-1 (CA) subfield of the hippocampus, which decreases with progression of AD.[22,23] In a previous study, Hayashi et al., showed that atrophy of hippocampus CA1 subfield leads to more adduction of the hippocampus on usual coronal MR images; and with increasing of hippocampal adduction, the angle between the hippocampus and subiculum increases.[19] They evaluated hippocampal angle (HA) enlargement in patients with AD and concluded that in these patients, the HA is affected by hippocampal atrophy and serves as a new marker of AD that could be useful in the routine clinical setting.[19]
In this study, we compared MCI patients with the normal population for HV and HA, as a newer marker, to assess whether these biomarkers could diagnose MCI patients as an early stage of AD. We also assessed the correlation between HV and HA in MCI patients and normal population.
MATERIALS AND METHODS
Study design and participants
This is a case-control study, which was carried out on 2014. The study followed the Declaration of Helsinki on Biomedical Research Involving Human Subjects and was approved by the Ethics Committee from the Isfahan University of Medical Sciences. All participants or their family provided written or oral informed consent.
Subjects were selected from patients who referred to the neuropsychiatry clinic of Kashani Hospital (Isfahan, Iran). All subjects met the following inclusion criteria: (1) ≥60 years aged; (2) being capable of reading and writing; (3) diagnosed with MCI; (4) written or oral informed consent of patient or family. Subjects also met none of the following exclusion criteria: (1) History of any neurodegenerative or cerebrovascular disease, or another neurological or systemic disease or condition likely contributing to cognitive decline; (2) any incidental finding other than Alzheimer's disease presentation in MRI; (3) history of any other major psychiatric disorder.
Patients were diagnosed with MCI, based on neuropsychiatry interview which was confirmed by neuropsychiatry unit cognitive assessment tool (NUCOG). NUCOG scores between 75 and 86.5 was used to confirm the diagnosis.[24,25] Control subjects were age-, sex- and education-matched cognitively normal volunteers, based on the clinical interview, who had no abnormal findings on MRI and no past psychiatric or neurological history. Subjects with NUCOG score of more than 86.5 were considered as healthy population with no cognitive disorder.[24,25] A total of 143 individuals screened for case group and 20 met all inclusion and no exclusion criteria. Then, another 62 individuals were screened for matching control group and 20 healthy subjects were selected [Figure 1].
Figure 1.
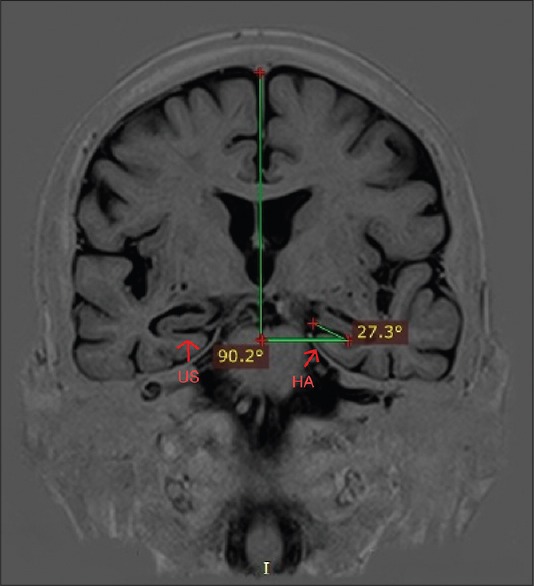
Measuring of hippocampal angle on a coronal magnetic resonance image. HA: Hippocampal angle; US: Uncal sulcus
Procedures and variables assessment
All subjects underwent psychiatrist examination. Baseline profile of subjects, including: Age, sex, years of education, smoking, history of diabetes mellitus, hypertension, hyperlipidemia, family history of Alzheimer's disease, body mass index, serum total cholesterol, fasting blood glucose, triglyceride and creatinine were assessed. Clinical severity of the neurocognitive disorder was assessed using the NUCOG.[24] MRI was performed using a 1.5 Tesla MRI Siemens Avanto scanner system (Siemens, Henkestr Erlangen). The following protocol was administered for obtaining T1-weighted magnetization-prepared, rapid gradient echo (MP-RAGE) scans, with thickness of 1.2 mm: Repetition time = 25 ms, echo time = 3.61 ms, flip angle = 8°, field of view = 240 mm2 × 240 mm2, matrix size = 192 × 192, voxel dimensions = 1.3 mm3 × 1.3 mm3 × 1.2 mm3, number of excitations = 1, and number of slices = 160. Regions of interests for volumetric measurement were based on previous findings and comprised of the hippocampus.
Volumetric measurement
All MRI scans were processed using the Free-Surfer software package, available at http://surfer.nmr.mgh.harvard.edu. Multiple MP-RAGE MRI acquisitions for each participant were motion corrected, averaged and normalized to create a single image volume with relatively high contrast to noise. This averaged volume was used to locate the grey/white matter boundary (white matter surface) and this, in turn, was then used to locate the grey/cerebrospinal fluid boundary (grey matter surface).[26]
Measurement of the hippocampal angle
We used a measuring method which was introduced by Hayashi et al., in a previous study.[19] We defined a coronal plane vertical to a line, which connects the anterior and posterior commissures, then a horizontal line was drawn orthogonal to the falx cerebri on this plane. In the next step, the uncal sulcus line was drawn between the deepest point of the uncal sulcus and the point nearest to the side of the ambient cistern in the uncal gyrus facing the uncal sulcus. Finally, the angle between the horizontal line and the uncal sulcus line was measured as the HA on the most rostral slice in which the uncal sulcus could be identified [Figure 2].
Figure 2.
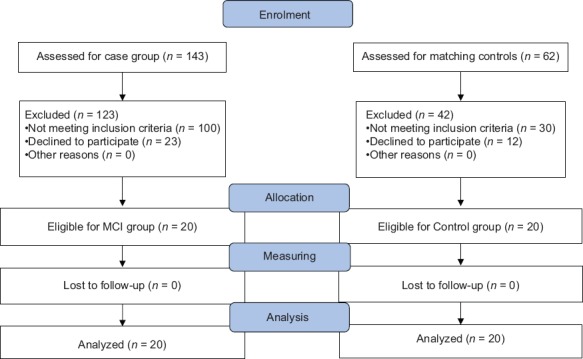
Consort statement
Blinding
The investigators who performed HA measurements or processed MRI scans with software for HV, were blinded to the clinical information and other measurements.
Statistical analysis
Independent t-tests and Chi-square tests were used to compare baseline variables. Differences between patients and controls were tested using multiple analysis of co-variance. Pearson correlation was used to identify the strength and direction of any correlation between HA and brain volumes. Data were analyzed by SPSS version 20.0 (SPSS Inc., Chicago, Illinois, USA). A P < 0.05 was considered significant. Continuous variables were expressed as mean ± standard deviation (SD).
RESULTS
Baseline profile
In 40 subjects who were analyzed, the mean (SD) age was 65.88 (4.31) years, ranging from 60 to 76 years old. There were 32 (80%) male and 8 (20%) female. The mean (SD) score of NUCOG in control and case group were 91.05 (3.01) and 82.42 (3.57), respectively.
Comparison of baseline profile of subjects, including: Age, sex, years of education, smoking, history of diabetes mellitus, hypertension, hyperlipidemia, family history of Alzheimer's disease, body mass index, serum total cholesterol, fasting blood glucose, triglyceride and creatinine revealed no statistically significant differences between two groups [Table 1].
Table 1.
Demographics and clinical characteristics of subjects (n=40)
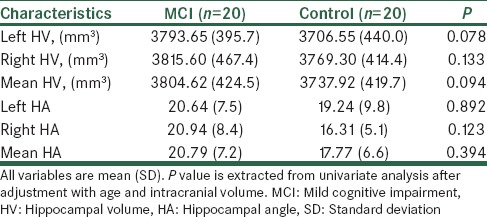
Mean (SD) of HV in control and case groups were 3737.92 (419.75) mm3 and 3804.62 (424.50) mm3, respectively. Mean (SD) of HA in control and case groups were 17.77 (6.60) and 20.79 (7.27), respectively. Comparison of HV and HA scores in two groups was performed after adjustment with age and intracranial volume. The results showed that mean (SD) HV and HA were not different between control and case groups, significantly, (P = 0.094 and P = 0.394, respectively), [Table 2].
Table 2.
Comparing of HV and HA between two groups
Correlations
Pearson's correlation coefficient analysis, showed that there was a negative correlation between the adjusted HV and the HA (r = −0.642, P = 0.004), in case group. This correlation was also seen in control group (r = −0.654, P = 0.003) [Table 3].
Table 3.
Correlations between HV and HA in two groups

The comparison of correlations in two groups showed that the rate of correlations was not different significantly, (P = 0.960).
In the case group, we recognized a positive correlation between the HV and the NUCOG score (r = 0.413, P = 0.032); the correlation between the HA and NUCOG score was negative but not significant (r = -0.090, P = 0.653).
DISCUSSION
This study compared the HV and HA in MCI patients with the normal population. The result showed that there were not any significant differences between two groups.
In previous studies, the comparison of HV between MCI and normal control groups had inconsistent results. In contrast to our study, several previous studies have shown that HV is significantly lower in MCI patients in compare with normal control.[26,27,28] However, other recent studies revealed that HV difference was not significant between two groups.[29,30]
Nevertheless, some studies indicated that HV is a sensitive factor for the diagnosis of only amnestic but not nonamnestic-MCI.[31,32] Our MCI patients also, consisted of both types of amnestic and nonamnestic-MCI, which might be the reason for nonsignificant results of this study. However, in a recent study, Wang et al., showed that even with amnestic-MCI, the HV difference between the MCI and normal control groups was not significant. They concluded that newer methods such as diffusion kurtosis imaging of the hippocampus were more sensitive in the diagnosis of MCI.[33] Hence, HV may be not sufficient for early discrimination of MCI patients as a single factor.
In clinical setting, obtaining of HV is time-consuming and needs special equipment, so using of an available and suitable method for clinical practice is very important. This study showed that HV is correlated with HA negatively, in both case and control groups. It means that with reducing of HV through the progression of the disease, the HA increases. With progression of AD, CA1 subfield of hippocampus decreases. With decreasing of CA1, the lateral surface of the hippocampal head transforms inward, and the inner surface deforms outward. These changes cause a long horizontal elliptical to a long vertical elliptical shape alteration in the hippocampus, which results in the outward rotation, and, therefore, HA enlargement.[22,23]
In one previous study also, Hayashi et al., showed that by hippocampal atrophy in AD, the HA increases, and it could be a new useful marker of AD in the routine clinical setting. They used this method in AD patients, and emphasized that more evaluation is needed to determinate the suitability of the HA as a marker in early-stage of AD;[19] in this study, we showed that this marker is correlated to HV even in early stages of disease. Our sample size was also larger (40 vs. 22), which was a limitation for that study.[19]
In our study, HA was not different between two groups, which was predictable because of nonsignificant differences in HV. However, the correlation of HV and HA shows that this marker could be used instead of measuring of HV, wherever HV assessment is considered for discrimination between different stages of AD, from predementia state to dementia due to AD. HA measurement is easy and more suitable for clinical setting because it do not need special equipment and can be assessed faster.
In this study, there was a significantly positive correlation between the HV and the NUCOG score in the case group. This is consistent with previous studies, which indicated that decreases of HV may leads to cognitive impairment. Steffens et al., and Sawyer et al., showed that decrease in both right and left HV is associated with decrease in MMSE score.[34,35] In a recent study also, Peng et al., revealed that there is a close relationship between HV and cognitive performances in patients with amnestic MCI.[36]
Limitations
This study had some limitations. First, in our study, MCI patients were consisted of both amnestic and nonamnestic groups and this matter could lead to nonsignificant differences of HV with normal controls. Second, measurement of HA is manually and it can differ when sizing by different persons. Hence, it is very important that the measurement should be done by an expert physician on this method to minimize the errors.
CONCLUSION
Hippocampal volume and HA were not different between MCI patients and normal controls and may be not sufficient as a single factor for discrimination of MCI. However, HA is correlated with HV negatively and may be used as an alternative factor because of more feasibility and availability in clinical settings in compared to HV.
Financial support and sponsorship
This work was supported by Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We like to express our gratitude to Shafa Imaging Center, Isfahan, Iran, for their generous contribution.
REFERENCES
- 1.Ferrer I. Defining Alzheimer as a common age-related neurodegenerative process not inevitably leading to dementia. Prog Neurobiol. 2012;97:38–51. doi: 10.1016/j.pneurobio.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. 5th ed. Arlington, VA: American Psychiatric Association; 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 3.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez OL. Mild cognitive impairment. Continuum (Minneap Minn) 2013;19:411–24. doi: 10.1212/01.CON.0000429175.29601.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 6.Pereira JL, Downes A, Gorgulho A, Patel V, Malkasian D, De Salles A. Alzheimer's disease: The role for neurosurgery. Surg Neurol Int. 2014;5:S385–90. doi: 10.4103/2152-7806.140191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubois B, Albert ML. Amnestic MCI or prodromal Alzheimer's disease? Lancet Neurol. 2004;3:246–8. doi: 10.1016/S1474-4422(04)00710-0. [DOI] [PubMed] [Google Scholar]
- 8.Burggren AC, Zeineh MM, Ekstrom AD, Braskie MN, Thompson PM, Small GW, et al. Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein E e4 carriers. Neuroimage. 2008;41:1177–83. doi: 10.1016/j.neuroimage.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lerch JP, Pruessner J, Zijdenbos AP, Collins DL, Teipel SJ, Hampel H, et al. Automated cortical thickness measurements from MRI can accurately separate Alzheimer's patients from normal elderly controls. Neurobiol Aging. 2008;29:23–30. doi: 10.1016/j.neurobiolaging.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Whitwell JL, Shiung MM, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, et al. MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology. 2008;70:512–20. doi: 10.1212/01.wnl.0000280575.77437.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hua X, Leow AD, Lee S, Klunder AD, Toga AW, Lepore N, et al. 3D characterization of brain atrophy in Alzheimer's disease and mild cognitive impairment using tensor-based morphometry. Neuroimage. 2008;41:19–34. doi: 10.1016/j.neuroimage.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lötjönen J, Wolz R, Koikkalainen J, Julkunen V, Thurfjell L, Lundqvist R, et al. Fast and robust extraction of hippocampus from MR images for diagnostics of Alzheimer's disease. Neuroimage. 2011;56:185–96. doi: 10.1016/j.neuroimage.2011.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du AT, Schuff N, Amend D, Laakso MP, Hsu YY, Jagust WJ, et al. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2001;71:441–7. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chupin M, Gérardin E, Cuingnet R, Boutet C, Lemieux L, Lehéricy S, et al. Fully automatic hippocampus segmentation and classification in Alzheimer's disease and mild cognitive impairment applied on data from ADNI. Hippocampus. 2009;19:579–87. doi: 10.1002/hipo.20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolz R, Aljabar P, Hajnal JV, Hammers A, Rueckert D Alzheimer's Disease Neuroimaging Initiative. LEAP: Learning embeddings for atlas propagation. Neuroimage. 2010;49:1316–25. doi: 10.1016/j.neuroimage.2009.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lötjönen JM, Wolz R, Koikkalainen JR, Thurfjell L, Waldemar G, Soininen H, et al. Fast and robust multi-atlas segmentation of brain magnetic resonance images. Neuroimage. 2010;49:2352–65. doi: 10.1016/j.neuroimage.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, et al. Revising the definition of Alzheimer's disease: A new lexicon. Lancet Neurol. 2010;9:1118–27. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 18.Lee SJ, Ritchie CS, Yaffe K, Stijacic Cenzer I, Barnes DE. A clinical index to predict progression from mild cognitive impairment to dementia due to Alzheimer's disease. PLoS One. 2014;9:e113535. doi: 10.1371/journal.pone.0113535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi T, Wada A, Uchida N, Kitagaki H. Enlargement of the hippocampal angle: A new index of Alzheimer disease. Magn Reson Med Sci. 2009;8:33–8. doi: 10.2463/mrms.8.33. [DOI] [PubMed] [Google Scholar]
- 20.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Csernansky JG, Wang L, Swank J, Miller JP, Gado M, McKeel D, et al. Preclinical detection of Alzheimer's disease: Hippocampal shape and volume predict dementia onset in the elderly. Neuroimage. 2005;25:783–92. doi: 10.1016/j.neuroimage.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 23.Hsu YY, Schuff N, Du AT, Mark K, Zhu X, Hardin D, et al. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J Magn Reson Imaging. 2002;16:305–10. doi: 10.1002/jmri.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walterfang M, Siu R, Velakoulis D. The NUCOG: Validity and reliability of a brief cognitive screening tool in neuropsychiatric patients. Aust N Z J Psychiatry. 2006;40:995–1002. doi: 10.1080/j.1440-1614.2006.01923.x. [DOI] [PubMed] [Google Scholar]
- 25.Barekatain M, Walterfang M, Behdad M, Tavakkoli M, Mahvari J, Maracy MR, et al. Validity and reliability of the Persian language version of the neuropsychiatry unit cognitive assessment tool. Dement Geriatr Cogn Disord. 2010;29:516–22. doi: 10.1159/000313981. [DOI] [PubMed] [Google Scholar]
- 26.Desikan RS, Cabral HJ, Hess CP, Dillon WP, Glastonbury CM, Weiner MW, et al. Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer's disease. Brain. 2009;132:2048–57. doi: 10.1093/brain/awp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi F, Liu B, Zhou Y, Yu C, Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer's disease: Meta-analyses of MRI studies. Hippocampus. 2009;19:1055–64. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- 28.Ries ML, Carlsson CM, Rowley HA, Sager MA, Gleason CE, Asthana S, et al. Magnetic resonance imaging characterization of brain structure and function in mild cognitive impairment: A review. J Am Geriatr Soc. 2008;56:920–34. doi: 10.1111/j.1532-5415.2008.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menezes TL, Andrade-Valença LP, Valença MM. Magnetic resonance imaging study cannot individually distinguish individuals with mild cognitive impairment, mild Alzheimer's disease, and normal aging. Arq Neuropsiquiatr. 2013;71:207–12. doi: 10.1590/0004-282x20130003. [DOI] [PubMed] [Google Scholar]
- 30.Hänggi J, Streffer J, Jäncke L, Hock C. Volumes of lateral temporal and parietal structures distinguish between healthy aging, mild cognitive impairment, and Alzheimer's disease. J Alzheimers Dis. 2011;26:719–34. doi: 10.3233/JAD-2011-101260. [DOI] [PubMed] [Google Scholar]
- 31.Siuda J, Gorzkowska A, Opala G, Ochudlo S. Vascular risk factors and intensity of cognitive dysfunction in MCI. J Neurol Sci. 2007;257:202–5. doi: 10.1016/j.jns.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 32.Vos SJ, van Rossum IA, Verhey F, Knol DL, Soininen H, Wahlund LO, et al. Prediction of Alzheimer disease in subjects with amnestic and nonamnestic MCI. Neurology. 2013;80:1124–32. doi: 10.1212/WNL.0b013e318288690c. [DOI] [PubMed] [Google Scholar]
- 33.Wang D, Guo ZH, Liu XH, Li YH, Wang H. Examination of hippocampal differences between Alzheimer disease, amnestic mild cognitive impairment and normal aging: Diffusion kurtosis. Curr Alzheimer Res. 2015;12:80–7. doi: 10.2174/1567205012666141218142422. [DOI] [PubMed] [Google Scholar]
- 34.Steffens DC, McQuoid DR, Payne ME, Potter GG. Change in hippocampal volume on magnetic resonance imaging and cognitive decline among older depressed and nondepressed subjects in the neurocognitive outcomes of depression in the elderly study. Am J Geriatr Psychiatry. 2011;19:4–12. doi: 10.1097/JGP.0b013e3181d6c245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawyer K, Corsentino E, Sachs-Ericsson N, Steffens DC. Depression, hippocampal volume changes, and cognitive decline in a clinical sample of older depressed outpatients and non-depressed controls. Aging Ment Health. 2012;16:753–62. doi: 10.1080/13607863.2012.678478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng GP, Feng Z, He FP, Chen ZQ, Liu XY, Liu P, et al. Correlation of hippocampal volume and cognitive performances in patients with either mild cognitive impairment or Alzheimer's disease. CNS Neurosci Ther. 2015;21:15–22. doi: 10.1111/cns.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]


