Abstract
Background:
There are various drugs tried for relieving pain associated with radiation-induced mucositis. This paper aims to study role of honey in relieving pain due to radiation induced mucositis in head and neck cancer patients receiving concomitant chemoradiation.
Materials and Methods:
A randomized controlled trial on 78 subjects (40 in test group and 38 in control group) was undertaken to study the analgesic effect of honey, but the analysis of 69 patients was done as nine patients (four in test and five in control group) were lost to follow-up or left treatment in between the study. All patients were advised to do salt-soda and benzydamine mouth gargles, alternatively every 3 hours. Test group patients additionally received 20 ml honey three times a day during the entire course of radiation treatment and 3 months following radiation therapy (RT).
Results:
Honey significantly reduced the severity of mucositis associated pain and resulted in lesser treatment gaps and a decrease in overall radiotherapy treatment duration. None of the test group and majority of controls (51.5%) had severe pain score during the 7th week of RT. The same pattern was seen in the post-RT period. Mean pain score was significantly different in both groups during all weeks during and upto 6 weeks post-RT (mean score of 3.08 and 6.54 for test and control respectively at 7th week RT, P < 0.001).
Conclusion:
Honey being a cheap, palatable, and natural medicament can be used for decreasing pain associated with radiation-induced mucositis in cancer patients.
Keywords: Cancer, Chemoradiation, Honey, Pain
INTRODUCTION
Concurrent chemoradiation with cisplatin is the standard approach for definitive management of locally advanced head and neck squamous cell carcinoma.[1,2] Radiation-induced mucositis and associated pain are the major and most important causes of morbidity and treatment gaps during the standard management.[3] Pain imparts additional morbidity and economic burden to patients in the form of requiring parenteral analgesia, interruption of radiation therapy (RT) and/or hospitalization, parenteral or tube feeding, all of which have a negative impact on quality of life.
There are various drugs tried for prevention and treatment of pain, but none have achieved satisfactory level. Topical application of honey to the oral cavity and pharynx results in reduction of pain to significantly low levels resulting in lesser analgesic use,[4,5] treatment gaps and weight loss hence overall improving their compliance and tolerability toward radiation schedule. This has helped in completing the radiation treatment protocol within stipulated time period, hence causing the maximum possible effect achieved by RT on the tumor.
Prophylaxis and management of radiation-induced mucositis by honey has been studied in past in phase-II randomized controlled trials, and it has shown encouraging results.[6,7,8] This paper attempts to evaluate the effect of honey on pain due to radiation induced mucositis in our set up.
MATERIALS AND METHODS
Study design
This prospective, randomized open label controlled trial was undertaken in a tertiary care hospital setup to study the analgesic effect of honey in locally advanced head and neck cancer (HNC) patients receiving the concomitant chemoradiation. An open label design was chosen because participants (patients) were necessarily aware of the treatment given and group assigned. The study was approved by the Institutional Ethics Committee, and informed consent was obtained from all participants.
Study population
HNC patients attending Radiation Oncology Clinic from November 2011 to January 2013.
Sample size, enrolment, and study procedures
The study was designed and powered to detect the difference in reduction of pain score by honey. Taking previous studies[9] into consideration, the expected difference between the arms was taken to be 25% (reduction of pain score from 80% to 55% by honey). Considering α (type I error rate) and desired power (1−β, where β < 0.2) to be 0.05 and 80% respectively, the desired sample size comes out to be 35 in each arm. Additionally adding dropout rate of 10% in each arm (four patients in each group), the final sample size came up to total 78 patients. In our set up, approximately 90 patients with HNC requiring upfront RT turn up every year and taking attrition and lost to follow-up into account, so all the patients agreeing to participate in the study were randomized during the first 10 months of the study period which was the period for recruitment. Primary end point was the occurrence of grade ≥3 vomiting, an anaphylactic reaction that is not attributable to entities other than honey beyond doubt.
Inclusion criteria
Patients having age between 18 and 70 years, Karnofsky Performance Status (KPS) scale >70, carcinoma of oral cavity, oropharynx, hypopharynx, and larynx planned to receive radical chemoradiation with directly visible oral or oropharyngeal mucosa in radiation field (Stage III, IVA and IVB), planned to receive RT by conventional fractionation schedule with normal hematological investigations. Renal function test (RFT), liver function test (LFT), chest X-ray.
Exclusion criteria
Patients planned to receive external beam RT (EBRT) by altered fractionation schedules, planned to receive total dose <66 Gy, history of previous treatment of head and neck malignancy with any of the following modalities-surgery, radiotherapy, chemotherapy, no evidence of distant metastasis.
Randomization
Eligible patients were randomly allocated to one of the two treatment groups (experimental and control) using random number table. A total of 78 participants were enrolled and subsequently randomly assigned to either of the group. The participants in the test group and control group were 40 and 38 respectively but due to lost to follow-up and some patients left the treatment, finally 36 participants in the test group and 33 participants in the control group were analyzed [Figure 1].
Figure 1.
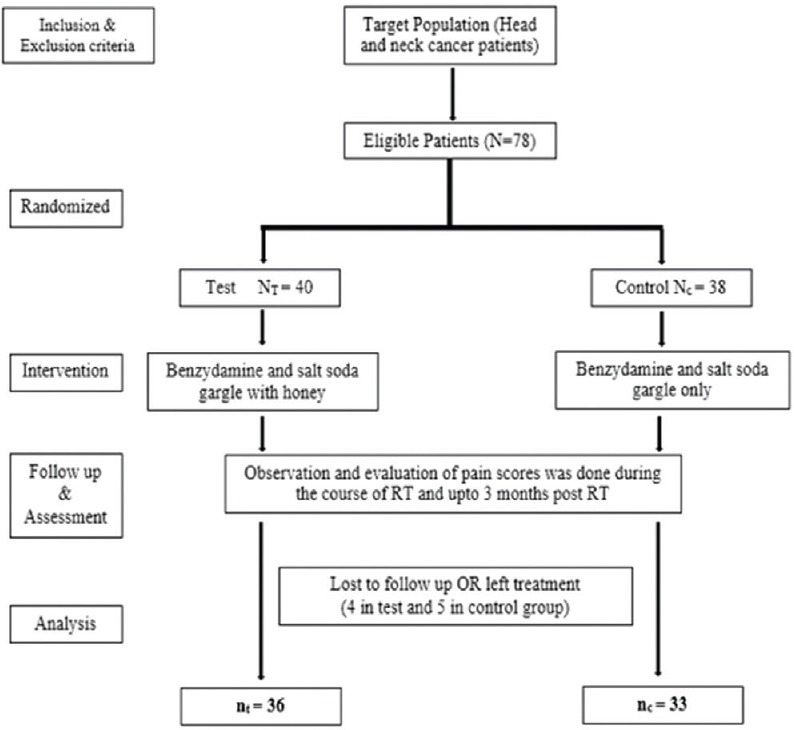
Flow diagram of study design
Treatment plan
Pretreatment evaluation
A complete detailed history of presenting complaints, past history, associated comorbid conditions, family history, personal history, and socioeconomic history with emphasis on personal habits such as betel nut chewing, tobacco, and alcohol consumption was taken. Patient's performance and nutritional status were assessed and documented. Detailed oral, oropharyngeal, laryngeal examination to evaluate the extent of the primary disease with also an emphasis on the oral, and dental hygiene was done. Investigations included complete blood picture (complete hemogram/RFT/LFT), biopsy from the primary tumor and/or fine-needle aspiration cytology of metastatic lymph nodes, dental evaluation, dental prophylaxis, chest X-ray (posteroanterior view), X-ray mandible (panorex/lateral oblique view), and computed tomography scan neck (base of skull to clavicle).
Description of intervention
Test group patients were treated with Cobalt-60 teletherapy unit of 80 cm source to surface distance (Theratron Elite 80, MDS Nordion Ontario, Canada). Initial tumor volume consisted of the primary tumor, involved lymph nodes, and probable sub-clinical disease. The irradiation field was reduced after 46 Gy to spare the spinal cord. Posterior neck electron boost to a dose of 24 Gy in 12 fractions were given in selected cases. The patients were immobilized with thermoplastic masks, in the supine position with shoulder retraction. All patients were treated with two parallel opposed lateral fields. Simulation X-ray film with lead wire markings was taken for verification of radiation portals and necessary corrections according to the standard portals were made before the start of treatment. RT was given as conventional RT. Treatment commenced on Monday, continued up to Friday and Saturday, Sunday being the rest days. Patients received a total of 6600–7000 cGy/33–35 fractions, 200 cGy/fraction over 6.5–7 weeks (47–49 days). Primary and involved nodes received a dose of 6600–7000 cGy. Posterior neck electron boost was given in selected cases with N3 node or nodes in level V, which were not covered in off cord field. The drug cisplatin was used as a single agent concurrently with EBRT in the dose of 40 mg/m2/week for 5–6 cycles. All patients were evaluated by fasting and postprandial blood sugar level. All patients in the test group received 20 ml of honey 15 min before, 15 min after, and 6 h after RT. Patients were advised to rinse honey on the oral mucosa and then to swallow slowly to smear it on the oral and pharyngeal mucosa. Written protocol in their language was given to all patients. Fasting and postprandial blood sugar levels were monitored for all test group patients every week during RT. Along with the honey, the patients were advised to do salt-soda and benzydamine mouth gargles alternatively, every 3 h as the standard oral protocol. They were advised to do this protocol throughout the radiation treatment and 3 months post-RT along with advice on adequate fluid intake, with a high protein diet, oral and dental cares.
Control group
All patients were treated with same radiation and chemotherapy protocols as test group patients. The standard oral protocol of salt-soda and benzydamine gargle every 3 h was done by patients during the course of EBRT and upto 3 months post-RT.
Treatment monitoring
All patients were advised to demonstrate the act of swishing and swallowing honey on Monday of every week. All patients were closely monitored during their entire treatment course every day. Every attempt was made to maintain adequate hydration, protein and caloric intake, and oral hygiene for all the patients during the entire treatment course. Pain scores were documented on a weekly basis using visual analog scale [Figure 2] by two independent observers. Timely interventions in the form of oral or intravenous antibiotics after oral swab/sputum culture sensitivity testing; short course steroids for extensive grade 3 mucositis, gentian violet local application for moist desquamations of skin were administered. Patients with reduced oral intake were put on Nasogastric tube feeds.
Figure 2.
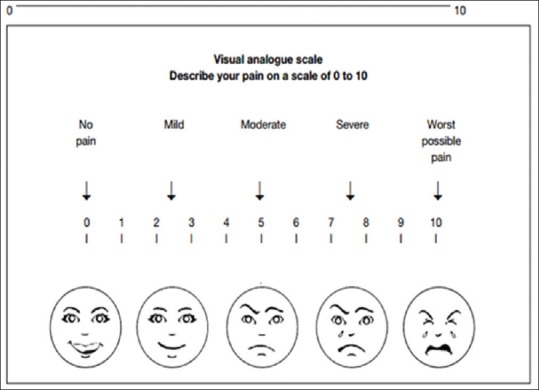
Pain assessment scale
Any delay in causing treatment interruption was noted, and necessary gap correction for radiotherapy was done. Chemotherapy was withheld during radiotherapy interruptions. Radiotherapy was planned to be continued in spite of chemotherapy being discontinued due to chemotherapy-related toxicities.
Data analysis
The data were compiled in (Microsoft Inc., United States) 2010 and software used for analysis was (SPSS version 20 IBM, United States). The data are presented in percentages and statistical tests used for comparing qualitative and quantitative data are Chi-square and Mann–Whitney U-test, respectively.
OBSERVATIONS AND RESULTS
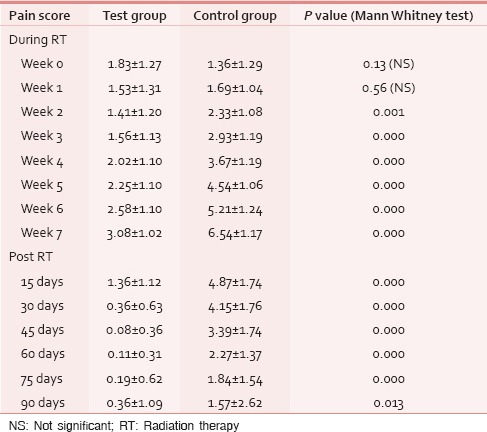
A total of 78 patients with locally advanced HNCs were recruited according to the inclusion and exclusion criteria as mentioned earlier. Sixty-nine patients were analyzed as nine patients (four in test and five in control) discontinued treatment or lost to follow-up. The majority of patients were aged 51–70 years. Both groups were matched for age, gender, literacy, KPS, and grade of the tumor [Table 1]. Smoking was the most prevalent and chewing pain was the least prevalent habit in both groups. All Patients had the locally advanced disease. Approximately, 72% of patients in both groups had T3, T4, and Stage IVA disease [Tables 2 and 3]. Both group patients had received a mean dose of 69.77 Gy (test) 69.75 Gy (control). Majority had received 6 cycles of cisplatin (92.3% in the test and 93.9% in controls). None of the test group and the majority of controls (51.5%) had severe pain score during the 7th week of RT [Table 4]. The Same pattern was seen in the post-RT period [Table 5]. Mean pain score was significantly different in both groups during all weeks during and up to 6 weeks postradiotherapy except at presentation and during 1st week of radiotherapy (mean score of 3.08 and 6.54 for test and control respectively at 7th week RT, P < 0.001) [Table 6].
Table 1.
Characteristics of study population
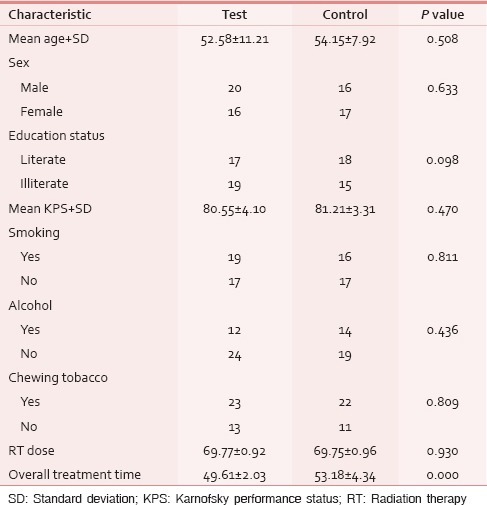
Table 2.
T and N staging
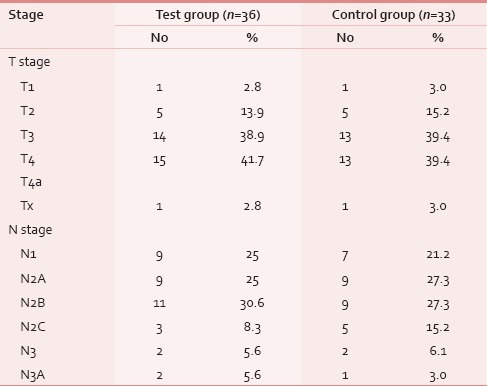
Table 3.
Stage group
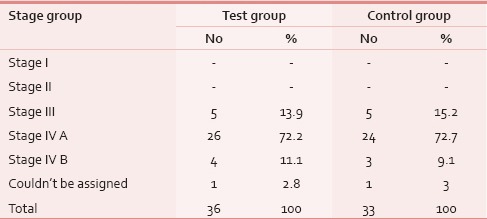
Table 4.
Pain score during RT
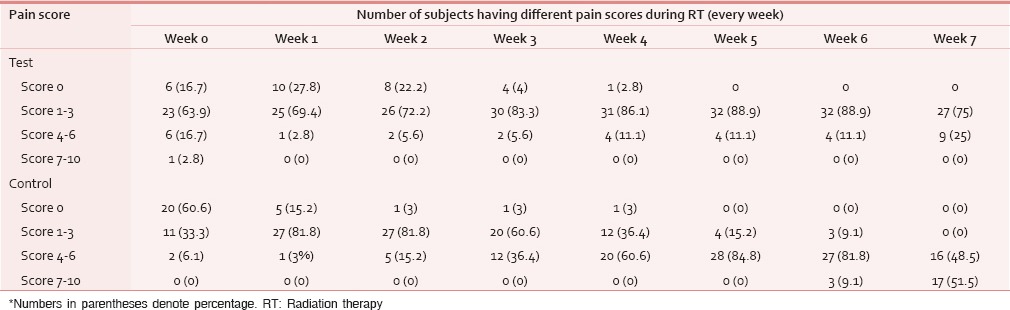
Table 5.
Pain score post RT
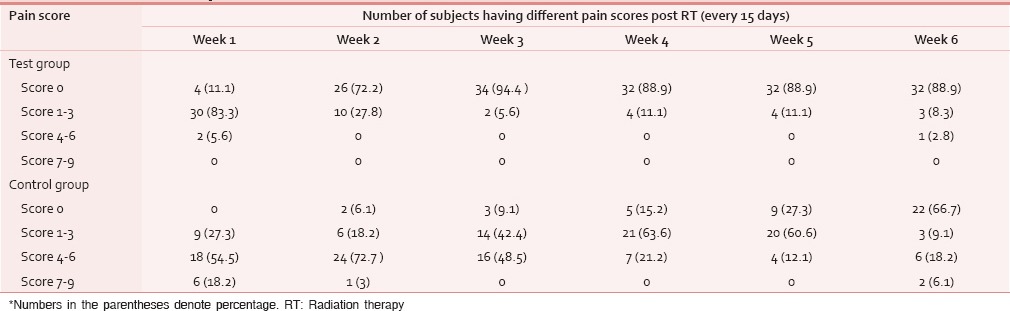
Table 6.
Comparison of mean pain score
DISCUSSION
Historically, locoregionally advanced head and neck tumors were treated with surgery (with or without adjuvant RT) or radiotherapy alone. Concurrent chemoradiation improves survival[10] and organ preservation at the cost of significant toxicities such as grade 3–4 mucositis and mucositis induced pain. Mucositis associated pain is the most debilitating symptom that reduces their oral intake and tolerability to complete the RT schedule and reducing the local control and survival.[11] This study was intended to evaluate the role of honey as a therapeutic measure for radiation-induced pain in HNC patients receiving definitive concurrent chemoradiation by conventional fractionation with weekly cisplatin.
In this study, all patients in both the groups were matched for variables such as age, gender, education status, KPS habits (smoking, chewing paan, tobacco, etc.). Most common site affected in both the groups was oropharynx (36% in test group and 40% in control group) which may be attributed to the fact that though oral cavity cancers are the most common HNC in India, but majority of them undergo surgery, which was an exclusion criteria for our study. Majority (72%) had Stage IVA disease amongst the included stages in the study.
Both groups received a mean dose of 69.7 Gy. Majority had received 6 cycles of cisplatin (90% in the test and 93% in controls). Mean over-the-top was 49.61 days in the test group and 53.18 days in the control group. This difference was statistically significant (P < 0.001) and may be attributed to less treatment breaks in test group due to less pain.
Honey has potent antioxidant, analgesic, and anti-inflammatory properties. Sonis et al. has described that analgesic and anti-inflammatory activity of honey are due to inhibition of the signal amplification by proinflammatory cytokines such as tumor necrosis factor-α, interleukin-1 (IL-1) and IL-6.[11] The analgesic property of honey is well confirmed in our study as shown in the tables. Unequal use of steroids and antibiotic use was two likely biases, but both of them were eliminated as steroids were used in only extensive grade 3 mucositis as predefined criteria and antibiotics were given in patients irrespective of the arms whenever infection was documented and confirmed by oral swab culture. Honey showed minor insignificant side effects such as dyspepsia, nausea, and vomiting which were manageable.
Despite promising and convincing results, our study had some limitations. In our study, we have advised the patients to take honey according to our protocol but they were free to choose any honey from the market as it wasn't provided from us, but they were strictly prohibited to use raw honey or nonpackaged honey to ensure its safety. Patients were also instructed to maintain strict hygiene while using honey to avoid contamination. The microbiological culture was done to assure the safety, whenever a new honey bottle or source was used by the patients but the chemical analysis of the honey was not done which was beyond the scope of the study. Our study had limited study period, smaller sample size and short duration follow-up, and hence multicentric randomised controlled studies with larger number of subjects are needed to further strengthen the analgesic effect of honey for mucositis associated pain in HNC patients receiving concurrent chemoradiation.
CONCLUSION
Honey is effective in reducing pain associated with radiation-induced mucositis. Being a cheap, palatable, and easily available product it can be used for decreasing mucositis associated pain in HNC patients receiving the concomitant chemoradiation.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Glisson B, Cango M, Feigenberg S. Head and neck tumors. In: Pazdur R, Wagman L, Camphausen K, Hoskins W, editors. Cancer management: A multidisciplinary approach. 13th ed. Manhassett: CMP Health Media; 2011. [Google Scholar]
- 2.National Comprehensive Cancer Network Head and Neck Cancers V.2; 2014. [Last accessed on 2015 Mar 12]. Available from: http://www.nccn.org .
- 3.Rosenthal DI. Consequences of mucositis-induced treatment breaks and dose reductions on head and neck cancer treatment outcomes. J Support Oncol. 2007;5 9 Suppl 4:23–31. [PubMed] [Google Scholar]
- 4.Subrahmanyam M. Topical application of honey in treatment of burns. Br J Surg. 1991;78:497–8. doi: 10.1002/bjs.1800780435. [DOI] [PubMed] [Google Scholar]
- 5.Efem SE. Clinical observations on the wound healing properties of honey. Br J Surg. 1988;75:679–81. doi: 10.1002/bjs.1800750718. [DOI] [PubMed] [Google Scholar]
- 6.Biswal BM, Zakaria A, Ahmad NM. Topical application of honey in the management of radiation mucositis: A preliminary study. Support Care Cancer. 2003;11:242–8. doi: 10.1007/s00520-003-0443-y. [DOI] [PubMed] [Google Scholar]
- 7.Motallebnejad M, Akram S, Moghadamnia A, Moulana Z, Omidi S. The effect of topical application of pure honey on radiation-induced mucositis: A randomized clinical trial. J Contemp Dent Pract. 2008;9:40–7. [PubMed] [Google Scholar]
- 8.Rashad UM, Al-Gezawy SM, El-Gezawy E, Azzaz AN. Honey as topical prophylaxis against radiochemotherapy-induced mucositis in head and neck cancer. J Laryngol Otol. 2009;123:223–8. doi: 10.1017/S0022215108002478. [DOI] [PubMed] [Google Scholar]
- 9.Epstein JB, Hong C, Logan RM, Barasch A, Gordon SM, Oberle-Edwards L, et al. A systematic review of orofacial pain in patients receiving cancer therapy. Support Care Cancer. 2010;18:1023–31. doi: 10.1007/s00520-010-0897-7. [DOI] [PubMed] [Google Scholar]
- 10.Pignon JP, le Maître A, Maillard E, Bourhis J. MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, et al. Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100 9 Suppl:1995–2025. doi: 10.1002/cncr.20162. [DOI] [PubMed] [Google Scholar]


