Abstract
Introduction:
Head and neck cancer (HNCA) patients have poor nutritional status which clearly bears a negative prognosis in cancer. There is no study and consensus on nutritional assessment tools and diet structure for such patients. This study intends to determine the prevalence of malnutrition and formulate a diet chart keeping in view the general food habit and economic condition of HNCA patients of North East (NE) region.
Aim:
To find out an affordable dietary intervention for HNCA patients based on dietary principles. To assess the role of nutritional assessment tools in these group of patients.
Materials and Methods:
This is a 1-year prospective interventional study on HNCA patients attending the Dept of ENT of a teaching hospital. The outcome of the nutritional intervention using a specific diet were assessed using clinical, laboratory and anthropomorphic assessment tools and indices like Prognostic Nutritional Index (PNI) and Nutritional Assessment Index (NAI).
Results:
The study diet provided appropriate amounts of nutrients to HNCA patients as evident from improvements in anthropomorphic parameters and nutritional indices. Clinically, Hemoglobin percentage (Hb%), Body Mass Index (BMI), Mid Arm Circumference (MAC) and triceps skin fold thickness (TST) were found to be reliable malnutrition markers.
Conclusion:
Nutritional Assessment Index has been found to be the best index to evaluate malnutrition. The daily requirement of nutrients for HNCA patients can be satisfactorily met by adopting specific diet chart presented in our study. As no structured diet plan are available in literature, our diet chart can act as a template diet appropriate for HNCA patients of this region.
Keywords: Dietary intervention, Head and neck cancer, Nutritional assessment index
INTRODUCTION
Head and neck cancer (HNCA) is a major health problem in India, accounting for 30–40% cancers at all sites. In the North-East India region, which comprises of the states of Assam, Manipur, Mizoram, Tripura and Nagaland, the incidence is as high as 54.48%.[1] Similar to all cancers, HNCA too alters the intake, gastrointestinal (GI) function and storage of nutrients by the body. The underlying etiology of malnutrition in such patients is multifactorial including abnormalities in GI function resulting from tumor-related mechanisms or anticancer therapies in addition to tumor-induced metabolic abnormalities.[2] More than 50% of the patients with advanced head and neck cancer have markedly impaired nutrition and a significant involuntary weight loss at the time of diagnosis.[3] HNCA is associated with dysphagia and odynophagia and they may also have underlying chronic malnutrition at presentation due to alchool, tobacco abuse and unhealthy dietary habit as seen in many patients in routine practice.
As, there is no study or consensus on tools of nutritional assessment and diet structure for HNCA patients particularly so for North – East region, this study intends to find out a good diagnostic tool for malnutrition and to formulate a diet chart based on dietary principles keeping in view the local food habit and economic condition.
Aims and objectives
To devise an affordable diet appropriate for head and neck cancer patients having poor nutrition in North–Eastern region.
To assess the role of clinical and laboratory nutritional indices in the evaluation of malnutrition.
MATERIALS AND METHODS
This one year prospective study was conducted in the Department of ENT of a tertiary teaching hospital. HNCA patients who fulfilled the inclusion criteria were selected for the study after obtaining informed consent. Ten age and sex matched healthy control subjects were also included for the study.
Inclusion criteria
Histologically confirmed cases of squamous cell carcinoma of head and neck region in patients of 40–70 years age-group.
Exclusion criteria
Patients requiring total parenteral nutrition or unable to take food orally and patients with cardiac, renal, hepatic or other systemic diseases. Patients with premalignant lesions and lymphoma of head and neck.
The nutritional status of the patients in HNCA group and the control group were assessed from history, physical examination, anthropomorphic measurements and laboratory tests. The anthropomorphic parameters considered in the study were: Percentage of body weight loss, Body mass index (BMI), triceps skin fold thickness (TST), mid-arm circumference (MAC). The percentage of body weight loss was calculated as (New weight/Old weight × 100) – 100. With subjects wearing light clothes, body weight and body height were measured to the nearest 0.1 kg and 0.001 m, respectively. BMI was calculated as weight (kg)/height (mts)2. Measuring tape and weighing scale was used for height and weight measurement. For TST, the skin thickness over the tricep muscle was measured using a self retaining speculum, measured upto the nearest 0.2 mm. Average of three readings were considered. MAC was measured on the left arm, using standard measuring tape in the mid-point of the line joining the acromio-clavicular joint and olecranon fossa.
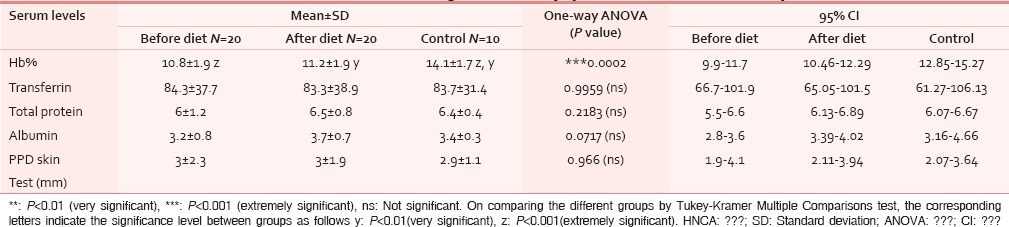
Laboratory parameters considered were Hb%, hematocrit, RBC count and total lymphocyte count (TLC), done using Sysmex 500i Autoanalyser machine. Serum transferrin and serum prealbumin were measured using Immulyte autoanalyser. Serum albumin were done using photometric color test using Automated Wet Chemistry Analyser (Beckman Coulter Au 400) and Serum Total Protein using Olympus System Calibrator. Late hypersensitivity skin tests (PPD) was done by injecting intradermally 0.1ml of PPD containing 5TU on the flexor aspect of the forearm which was read at 72 hours. Induration of diameter ≥10 mm was considered positive (good response), 5 mm or less as negative (poor response) and 6–9 mm as equivocal. We also calculated the Prognostic Nutritional Index (PNI = 10 Alb. +0.005 TLC) where Alb. is serum albumin level (g/100 ml) and Nutritional Assessment Index (NAI = 2.64 AC + 0.6 PA + 3.76 RBP + 0.017 PPD - 53.8) where AC is mid-arm circumference; PA is prealbumin and RBP is retinol-binding protein. NAI was categorised into three groups, good (NAI greater than or equal to 60), intermediate (greater than or equal to 40), and poor (less than 40).[4]
The food taken by the patients during the study were according to a specific diet chart [Table 1] based of daily requirement of protein, calorie and fat appropriate for cancer patients. This diet was administered during the entire treatment period of chemotherapy and or radiotherapy. All the nutritional parameters were measured once at the start of treatment and at the end of treatment. Importance was laid on including foods which were locally available, cheaper, easier to cook and ingest. Data were recorded in Diet Questionnaire and Clinical proformas. The experimental data obtained were analysed using Student's paired t–test and Tukey-Kramer Multiple Comparisons test and significance determined. This study has the Institutional Ethical Clearance for study.
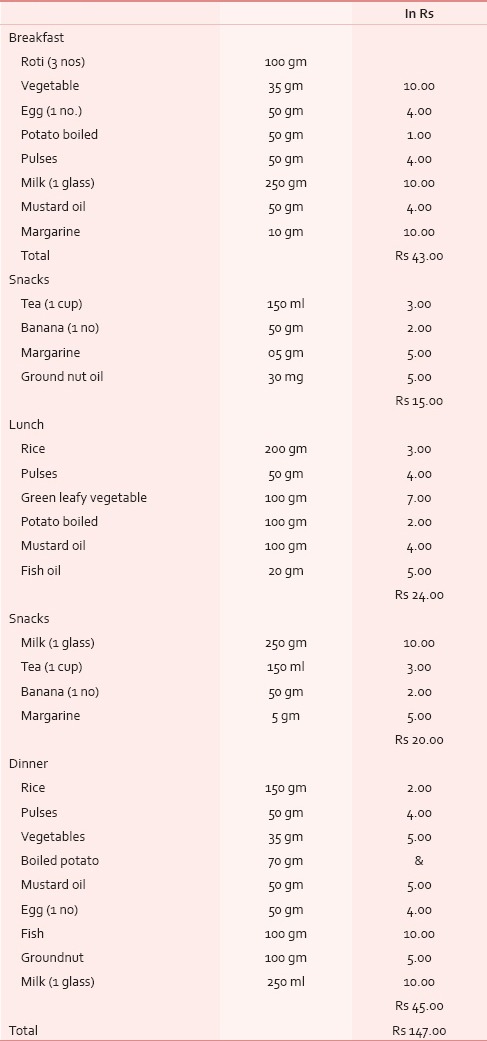
Table 1.
Study diet chart and with cost price (approx)
OBSERVATIONS AND RESULTS
Demographic pattern
Out of the total 26 cases of head and neck cancer taken up for study, 20 cases fulfilled the inclusion criteria and was offered the Study Diet. The prevalence of malnutrition was 76.92%, seen most commonly in 51–60 years age – group with median age of 56 years and male:female ratio of 13:7. In the control group, male female ratio is 7:3. The commonest cancer site was orophayngeal cancer (10 cases) followed by 5 cases each of esophageal and laryngeal cancer. Out of the 6 HNSCC cases excluded from the study, co-morbidities was seen in 3 cases (11.5%): 2 cases had hepatic disorder and 1 case had pulmonary tuberculosis. The remaining three cases dropped out of study due to non-compliance.
Diet composition
The composition of carbohydrate, protein and fat in the study diet was 434 g, 133 g and 81 g respectively while that calculated from the usually taken regular daily diet was 306 g, 63 g and 20.5 g in average respectively. The calories obtained from carbohydrate, protein and fat from the study diet was 1737 Kcal, 534 Kcal, 634 Kcal respectively, making a total of 2906 Kcal. In the non-study diet its 1257 Kcal, 245 Kcal, 185 Kcal with a total of 1687 Kcal. In the diet of control subjects, the availability of carbohydrate, protein and fat was 380 g, 77.7 g and 31.6 g respectively, giving a total calorie of 2103.7 Kcal. The approximate cost per day calculated for the study diet was Rs 147 [Table 1].
Clinical assessment of nutrition
Pallor and geographic tongue was noted in 50% of cases (N = 10). Calf tenderness and dry eye suggesting Vit A deficiency was found in 13 cases. In the control group, pallor, loss of appetite and skin changes were seen in 30% of cases each [Figure 1].
Figure 1.
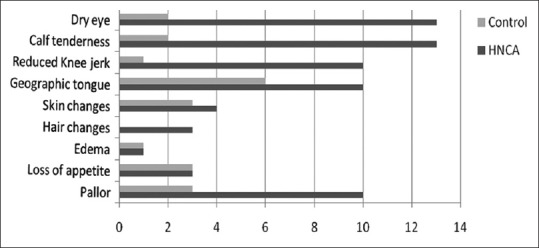
Clinical presentation
Anthropomorphic assessment
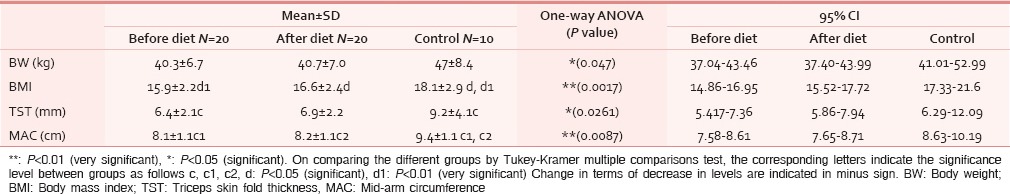
1) Body weight: 60% of HNCA patients having study diet showed statistically significant gain in percentage of body weight. The average gain in weight was 3.5%. Whereas, 30% cases had loss of body weight with 10% showing no change. 2) BMI: Statistically significant improvement in BMI was seen in 22.22% cases in post study diet. The mean BMI in HNCA group was 15.26. 3) TST: 64.3% of cases improved after receiving the study diet which was similar to the control group (66.6%). These results were also statistically significant. 4) MAC: 15% of HNCA cases had normal MAC which showed no change post diet [Table 2].
Table 2.
Students t-test results showing anthropometric parameters in HNCA patients
Laboratory parameters
1) Anemia: Twelve cases (60%) had low Hb levels showing marked anemia in 7 cases and early anemia in 5 cases. After dietary intervention with the study diet, Hb level showed improvement in 15 cases but was not enough to overcome marked anemia [Figure 2]. 2) Serum transferrin: Iron deficiency was seen in eight patients but no significant increase was noted in post study-diet. 3) Serum total protein: 75% cases showed hypoproteinemia out of which 33.33% cases improved after having study diet. 4) PPD Skin Test: Induration of less than 6 mm (immunocompromised state) was seen in 85% cases. After receiving the study diet, there were lesser number of immunocompromised patients (55%) [Table 3].
Figure 2.
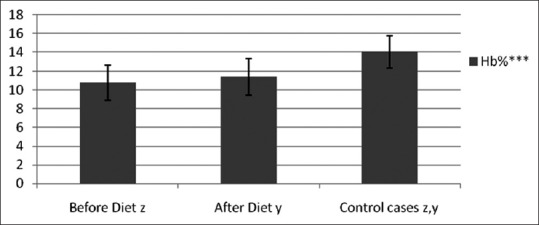
Hemoglobin level (***: P<.001, extremely significant)
Table 3.
Students t-test with P value showing laboratory parameters in HNCA patients
Nutritional status indices
1) NAI: Only 10% cases had good nutrition which increased to 45% after having the study diet [Figures 3 and 4].
Figure 3.
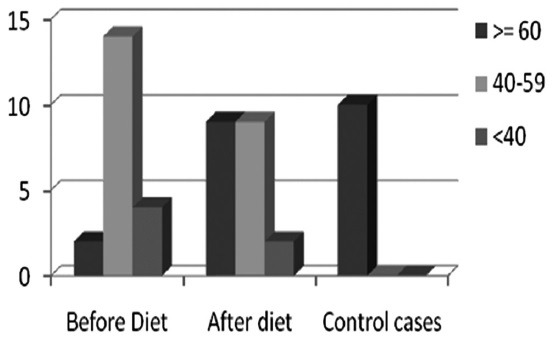
NAI scores in three groups of patients
Figure 4.
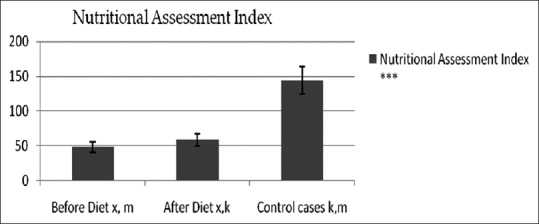
Tukey-Kramer Multiple Comparisons tests on NAI
2) Prognostic Nutritional Index: The mean PNI in HNCA group was 66.78 ± 18.91 which improved to 74.63 ± 16.19 after having the study diet. The PNI for control group was 69.24 ± 17.73. 70% cases had score of <40 that is, poor nutrition whereas after starting the study diet, cases with poor nutrition (score of <40) dropped to 40% in HNCA group and 30% in control group. However, this difference was not statistically significant (P = 0369).
DISCUSSION
Nutritional status of HNCA patients is often the result of many inter-related factors influenced by food intake—its quantity, quality and physical health of patient. Piquet MA et al., showed the effects of early nutritional intervention in patients undergoing oropharyngeal radiotherapy.[5] Various studies have reported the commonest age group of HNCA to be sixth decade (31.13%)[6,7,8] with mean age of 62.6 years for women and 68.8 years for men.[9] Our study revealed that the mean age is much younger (56 years) with 35% cases in the 51–60 years age group. The youngest patient is 35 years and the eldest 70 years. This high prevalence in young males can be attributed to habits of tobacco, lime, betel and smoking from early in life. Oropharyngeal carcinoma is the commonest head and neck site for HNCA patients of NE region.[1] Studies have shown that the incidence of malnutrition in cancer patients, range from 40 to 80%,[10] most frequently seen in GI or head and neck area[11], which is mainly of protein-calorie deficiency.[12] Another study also reported that non-hematological cancer presented with higher malnutrition than hematological disease.[13] Numereous studies have indicated that the prevalence of malnutrition is highest in HNCA patients with 25–50% at presentation.[11,14,15] The prevalence seen in our study was found to be still higher at 76.92%. This seems to be mainly due to poor food consumption both qualitatively and quantitatively by such patients who are generally ignorant about nutritional diet, prone to alcohol and tobacco habit and have poor oral and dental hygiene. Moreover, they also suffer from tumour related problems, odynophagia, dysphagia, trismus, post-radiotherapy mucositis, dysgeusia, xerostomia, chemotherapy-induced nausea, vomiting and post-surgical functional and anatomic impairments of chewing and swallowing.
Dietary status
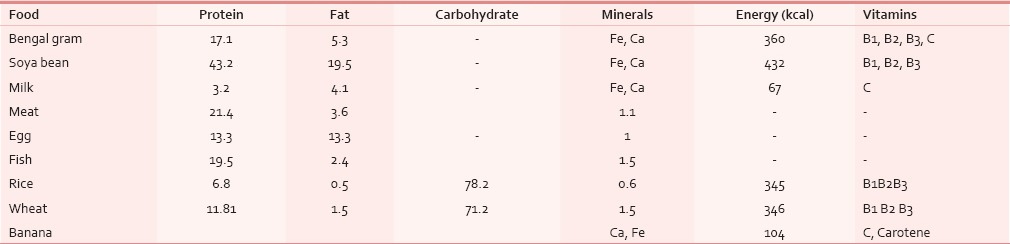
As per Indian Council of Medical Research (ICMR) reports, the recommended approximate total energy contribution by protein, fat and carbohydrate in an Indian diet are 7–15%, 10–30% and 65–80% respectively, with a daily energy requirement for a man of 60 kg with moderate activity of 2425 Kcal/day. But our study showed that the HNCA patients were taking about 1685 Kcal through their usual diet. In order to correct this deficit, the diet devised for our HNCA patients was constituted maintaining the protein, carbohydrate and fat ratio so as to provide a total energy of about 2900 kcal [Table 4]. Protein: The ICMR recommended protein requirement for a normal Indian adult is 1.0 g protein/kg body weight.[16] We doubled this to meet the requirements of HNCA patient of our study. High protein foods like meat, milk, egg, fish, pulses, cereals and soyabean were added in their diet. These foods doubled the energy supply maintaining the total energy contribution from protein at 7–15% along with supplying essential vitamins and minerals. The foods were palatable, digestible, affordable and easily available. Milk was added as it is the most complete of all foods. Fat: We also increased the content of fat from 20.4 g to 70.5 g per day by using groundnut oil in food preparation. Ground nut oil is easily available, cheaper and provides considerable PUFA, linoleic acid and essential fatty acid (EFA) which are precursors for growth and reduces cholesterol and low density lipoproteins (LDL). We followed the ICMR daily recommendation that more than or equal to 20% of total energy should come from fat. Safflower and sunflower oil were not used due to their high cost. Fish which is widely available here was included in our diet as it provided protein in addition to fish oil which is a high source of MUFA, PUFA and Vit K. Nutritional intervention with Fish Oil per day provided a benefit over standard of care, resulting in the maintenance of weight and muscle mass during chemotherapy. Our diet maintained the WHO recommendation that 50% of fat should come from vegetable oils. Carbohydrate: The carbohydrate intake for the HNCA group was increased by 129 g following the ICMR recommended daily intake of 50–70% of total energy from carbohydrates. Cereals like rice and wheat constituted the bulk of the diet which also supplied B group Vitamins, minerals and protein. Among fruits, banana was chosen as it is easily available, cheap and has good nutritive value [Table 5]. Micronutrients: The Vitamin A requirement was fulfilled from milk, fish and green leafy vegetables which protects against epithelial cancer. The study diet also had other vitamins, minerals and trace elements like zinc, copper, Cobalt, Chromium, Mo and antioxidants [Table 5] so that no food supplements or vitamin preparations needed to be prescribed to the HNCA group during the study period.
Table 4.
Comparative composition and calorie contribution from carbohydrate, protein and fat
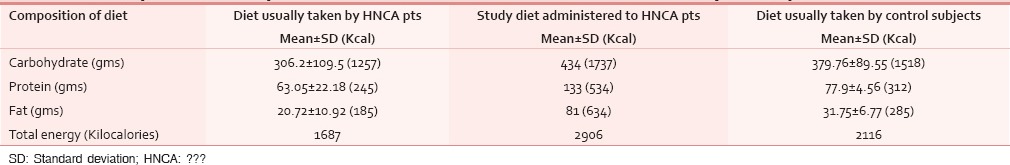
Table 5.
Nutritive value in study group (per 100 gms)
Anthropomorphic assessment of nutrition
Mick et al., reported that for Stage III/IV head and neck cancer treated with multiple modalities—the strongest independent predictor of survival was pretreatment weight loss.[17] We found that both body weight and BMI increased in HNCA group after having the study diet. More than 60% cases showed an average gain in body weight of 3.5% indicating positive nutritional outcome after following our study diet. This was due to increased supply of protein which helped in body building, repair and maintenance. The mean BMI of patients in our study is similar to that of Sanz Oritz (2008), 15.26 and 22.1 respectively.[9] Although, Bruzgielewicz A et al., using BMI measures reported malnutrition in 41% patients,[18] some studies did highlight the limitations of using BMI as the sole measure of nutritional status.[19,20]
The increase in TST seems to be a result of increased fat content in study diet but there was no corresponding change in MAC in HNCA group. Both these parameters were not statistically significant. Turedi A and Garófolo A reported that TST measurement was better in both detecting and establishing malnutrition.[13,21]
Laboratory assessment
The improvement in anemia seen in HNCA patients showed that the study diet satisfactorily supplemented the iron loss and serum transferrin level. There was also significant improvement in protein status as evidenced with increase in serum albumin. The increased dietary protein helped to maintain osmotic pressure and synthesis of plasma proteins and hormones. Low serum albumin is a powerful predictor of surgically related morbidity and thus is of great value in the clinical setting. It is an important part of the general evaluation. Albumin is useful in the assessment of advanced HNCA patients at diagnosis and for stratifying patients taking part in randomized trials.[22] There has also been an improvement in immunity status from the study diet. The well-established interaction between malnutrition and infection seems to be due to modifications of the immunological defense observed in protein-energy malnutrition causing impaired immuno-competence, decreased cell-mediated immunity (anergy)[23] and depressed T-cell proliferation. Malnutrition also decreases the synthesis of acute phase proteins which helps in survival during stress, infection and injury.
Nutritional indices
The improvement in NAI score was significant indicating that the dietary intervention improved the nutritional status from 10 to 45% cases. However, the decrease in malnutrition level as assessed by PNI was found to be not significant. Although Iwasa et al., (1983) proposed the use of NAI for assessing postoperative complication and nutrition, our study reports its first use for nutritional assessment of HNCA patients. Prognostic Nutritional Index (PNI) is widely used method in oncology for esophageal cancer,[24] but our study finds NAI as a better tool for assessment than PNI. Considering the various outcome parameters, we are of opinion that the study diet improved the nutritional status of HNCA patients. It provided both macro and micro-nutrients from food that are easily available at a cost of about Rs. 147 per day which is affordable for the patients of this region.
Limitation of this study
We encountered difficulties in enrolling greater number of patients as because this study calls for greater motivation from the HNCA patients to follow a particular diet during their treatment and also many HNCA patients presented late in their disease or dropped out during treatment owing to their inability to take foods orally. Therefore, further multicentric studies on this subject is required in future.
CONCLUSION
The HNCA patients have a much greater daily requirement of nutrients during their treatment period than that what they usually get from their regular meals. This deficit can be overcome by adopting a certain diet as described in our study. This diet also replaces the need and use of any extra food supplements or vitamin preparations. Our study-diet had the appropriate composition of all essential nutrients which was evident from the positive results seen in the anthropomorphic parameters and nutritional indices. The ingredients of this diet are also easily available locally around the year, is affordable and meets the nutritive value and the requirment for cancer patient.
Among clinical parameters, Hb%, BMI, MAC and TST were reliable malnutrition markers. However, NAI has been found to be the best tool in diagnosis of malnutrition. We suggest that NAI should be routinely used in the work-up of HNCA patients. Our diet chart may be used as template for dietary advice in HNCA patients particularly in cases with malnutrition. This study will help to guide clinicians and oncologists to conduct the nutritional assessment in HNCA patients and their dietary advice. As no literature on the structured dietary plan for such patients of North-East India are available, we hope that the present study can be a starting reference point, a preliminary research on this neglected subject of “nutrition of head neck cancer patients of North-East region” where no study on this matter has been undertaken till date.
Financial support and sponsorship
Indian Council of Medical Research, New Delhi.
Conflict of interest
There are no conflict of interest.
ACKNOWLEDGEMENT
We are thankful to Principal and Head of Department of ENT, Sichar Medical Colllege for helping us to conduct this study.
REFERENCES
- 1.Bhattacharjee A, Chakraborty A, Purkaystha P. Prevalence of head and neck cancer in the North – East India – An institutional study. Indian J Otolaryngol Head Neck Surg. 2006;58:15–9. doi: 10.1007/BF02907731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Meyenfeldt M. Cancer-associated malnutrition: An introduction. Eur J Oncol Nurs. 2005;9:S35–8. doi: 10.1016/j.ejon.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Chasen MR, Bhargava R. A descriptive review of the factors contributing to nutritional compromise in patients with head and neck cancer. Support Care Cancer. 2009;17:1345–51. doi: 10.1007/s00520-009-0684-5. [DOI] [PubMed] [Google Scholar]
- 4.Iwasa M. Nutritional assessment of patients with esophageal cancer. “Nutritional Assessment Index (NAI)” to estimate nutritional conditions in pre-and postoperative period. Nihon Geka Gakkai Zasshi. 1983;84:1031–41. [PubMed] [Google Scholar]
- 5.Piquet MA, Ozsahin M, Larpin I, Zouhair A, Coti P, Monney M, et al. Early nutritional intervention in oropharyngeal cancer patients undergoing radiotherapy. Support Care Cancer. 2002;10:502–4. doi: 10.1007/s00520-002-0364-1. [DOI] [PubMed] [Google Scholar]
- 6.Manjari M, Popli R, Paul S, Gupta VP, Kaholon SK. Prevalence of oral cavity, Pharynx, larynx, nasal cavity malignancies in Amritsar, Punjab. Indian J Otolaryngol Head Neck Surg. 1996;48:189–96. [Google Scholar]
- 7.Thakur S, Chaturvedi VN, Singh AK, Puttewar MP, Raizada RM. Pattern of ear, nose, pharynx, larynx and esophagus (ENPLO) cancers in rural based hospital. Indian J Otolaryngol Head Neck Surg. 2001;53:93–9. doi: 10.1007/BF02991498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaturvedi VN, Raizada RM, Jain SK, Tyagi NK. Cancer of ear, nose, pharynx, Larynx and esophagus in a rural hospital. J Vivekananda Inst Med Sci. 1987;10:63–7. [Google Scholar]
- 9.Sanz Ortiz J, Moreno Nogueira JA, García de Lorenzo y, Mateos A. Protein energy malnutrition (PEM) in cancer patients. Clin Transl Oncol. 2008;10:579–82. doi: 10.1007/s12094-008-0253-8. [DOI] [PubMed] [Google Scholar]
- 10.Ollenschlager G, Viell B, Thomas W, Konkol K, Burger B. Tumor anorexia: Causes, assessment, treatment. Recent Results Cancer Res. 1991;121:249–59. doi: 10.1007/978-3-642-84138-5_28. [DOI] [PubMed] [Google Scholar]
- 11.Lees J. Incidence of weight loss in head and neck cancer patients on commencing radiotherapy treatment at a regional oncology centre. Eur J Cancer Care (Engl) 1999;8:133–6. doi: 10.1046/j.1365-2354.1999.00156.x. [DOI] [PubMed] [Google Scholar]
- 12.Guo CB, Ma DQ, Zhang KH. Nutritional status of patients with oral and maxillofacial malignancies. J Oral Maxillofac Surg. 1994;52:559–62. doi: 10.1016/0278-2391(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 13.Garófolo A, Lopez FA, Petrilli AS. High prevalence of malnutrition among patients with solid non-hematological tumors as found by using skinfold and circumference measurements. Sao Paulo Med J. 2005;123:277–81. doi: 10.1590/S1516-31802005000600005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isenring EA, Capra S, Bauer JD. Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal, head or neck area. Br J Cancer. 2004;91:447–52. doi: 10.1038/sj.bjc.6601962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mekhail TM, Adelstein DJ, Rybicki LA, Larto MA, Saxton JP, Lavertu P. Enteral nutrition during the treatment of head and neck carcinoma: Is a percutaneous endoscopic gastrostomy tube preferable to a nasogastric tube? Cancer. 2001;91:1785–90. [PubMed] [Google Scholar]
- 16.Indian Council of Medical Research, “Nutrient Requirements and Recommended Dietary Allowances for Indians,” 1990. [Accessed on 2011 Feb 11]. Available from: http://www.icmr.nic.in .
- 17.Capuano G, Gentile PC, Bianciardi F, Tosti M, Palladino A, Di Palma M. Prevalence and influence of malnutrition on quality of life and performance status in patients with locally advanced head and neck cancer before treatment. Support Care Cancer. 2010;18:433–7. doi: 10.1007/s00520-009-0681-8. [DOI] [PubMed] [Google Scholar]
- 18.Bruzgielewicz A, Hamera M, Osuch-Wójcikiewicz E. Nutritional status of patients with cancer of larynx and hypopharyx. Otolaryngol Pol. 2009;63:141–6. doi: 10.1016/S0030-6657(09)70095-2. [DOI] [PubMed] [Google Scholar]
- 19.Isenring E, Cross G, Daniels L, Kellett E, Koczwara B. Validity of the malnutrition screening tool as an effective predictor of nutritional risk in oncology outpatients receiving chemotherapy. Support Care Cancer. 2006;14:1152–6. doi: 10.1007/s00520-006-0070-5. [DOI] [PubMed] [Google Scholar]
- 20.Desbrow B, Bauer J, Blum C, Kandasamy A, McDonald A, Montgomery K. Assessment of nutritional status in hemodialysis patients using patient-generated subjective global assessment. J Ren Nutr. 2005;15:211–6. doi: 10.1053/j.jrn.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Turedi A, Demir C, Dilek I. Assessment of malnutrition in adult acute leukemia cases. Asian Pac J Cancer Prev. 2010;11:703–7. [PubMed] [Google Scholar]
- 22.Salas S, Deville JL, Giorgi R, Pignon T, Bagarry D, Barrau K, et al. Nutritional factors as predictors of response to radio-chemotherapy and survival in unresectable squamous head and neck carcinoma. Radiother Oncol. 2008;87:195–200. doi: 10.1016/j.radonc.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Chandra RK. Nutrition, immunology, and infection: Present knowledge and future directions. Lancet. 1983;1:688–91. doi: 10.1016/s0140-6736(83)91980-3. [DOI] [PubMed] [Google Scholar]
- 24.Gurski RR, Schirmer CC, Rosa AR, Brentano L. Nutritional assessment in patients with squamous cell carcinoma of the esophagus. Hepatogastroenterology. 2003;50:1943–7. [PubMed] [Google Scholar]


