Supplemental Digital Content is available in the text
Abstract
Cerebrovascular risk factors and white matter (WM) damage lead to worse cognitive performance in Alzheimer dementia (AD). This study investigated WM microstructure using diffusion tensor imaging in patients with mild to moderate AD and investigated specific fiber tract involvement with respect to predefined cerebrovascular risk factors and neurobehavioral data prediction cross-sectionally and after 18 months. To identify the primary pathoanatomic relationships of risk biomarkers to fiber tract integrity, we predefined 11 major association tracts and calculated tract specific fractional anisotropy (FA) values. Eighty-five patients with AD underwent neurobehavioral assessments including the minimental state examination (MMSE) and 12-item neuropsychiatric inventory twice with a 1.5-year interval to represent major outcome factors. In the cross-sectional data, total cholesterol, low-density lipoprotein, vitamin B12, and homocysteine levels correlated variably with WM FA values. After entering the biomarkers and WM FA into a regression model to predict neurobehavioral outcomes, only fiber tract FA or homocysteine level predicted the MMSE score, and fiber tract FA or age predicted the neuropsychiatric inventory total scores and subdomains of apathy, disinhibition, and aberrant motor behavior. In the follow-up neurobehavioral data, the mean global FA value predicted the MMSE and aberrant motor behavior subdomain, while age predicted the anxiety and elation subdomains. Cerebrovascular risk biomarkers may modify WM microstructural organization, while the association with fiber integrity showed greater clinical significance to the prediction of neurobehavioral outcomes both cross-sectionally and longitudinally.
INTRODUCTION
Although Alzheimer disease (AD) is considered as a neurodegenerative disorder with characteristic amyloid and tau protein accumulation,1 most AD patients are elders with coexisted cardiovascular risk factors. The presence of diabetes mellitus,2 midlife hypertension,3,4 or hyperlipidemia4 have been found to predispose to the onset of AD, resulting into cerebral microvascular damages that modulate the clinical manifestations.5 Specific to the measurable cardiovascular-related biomarkers, the clinical significance of atherogenic low-density lipoprotein (LDL)-cholesterol,6 apolipoprotein E4 (ApoE4) allele,7 or elevated hemoglobin A1C8–10 have been reported. Meanwhile, hyperhomocysteinemia in AD has been associated with silent brain infarcts, cortical atrophy,11,12 and greater deep or periventricular white matter hyperintensities (WMHs),13 all of which can lead to faster neurobehavioral decline.14
Accumulating evidence supports cerebral white matter (WM) alterations in normal aging.15,16 Meanwhile, a number of studies have reported a loss of regional WM volume, WM degeneration, or increased WMHs in AD that not only represent strong predictors to differentiate AD from controls,17 but also modify the clinical symptoms.13,18 Other studies emphasize the coexisting cerebrovascular risk factors19–22 that are related to the WM-microstructural alterations. Among these, elevated sugar levels,23 advanced age,24 ApoE4 status,25 or presence of hypertension and stroke26 were most important.
As the brain is organized into segregated networks, large-scale functional connectivity can be altered if the major fiber tracts are disrupted. Recent diffusion tensor imaging techniques provide in vivo quantification of WM integrity by measuring fractional anisotropy (FA) values.27 It is believed to represent such factors as myelination, axonal density, and/or integrity.28 The development of WM parcellation algorithms with approximation of 3D WM bundle trajectories using probabilistic maps allows for the estimation of major fiber integrity29 and made possible the quantification of specific fiber involvement. With automated tract-specific FA and clinical parameter correlations, the influence of serological cerebrovascular biomarkers with related tract integrity can be modeled.
Based on a literature review, we hypothesized that the major bundle fiber FA may predict the cross-sectional or longitudinal neurobehavioral scores in AD patients while the measurable serological biomarkers, traditionally recognized as “cerebrovascular risk factors,” may show an impact on the WM bundles integrity. To validate this hypothesis, we measured homocysteine, total cholesterol (TC), triglycerol, high-density lipoprotein, LDL, creatinine, folate, vitamin B12, and hemoglobin A1C levels to serve as serological biomarkers of interest. To identify the primary pathoanatomic relationships of the risk biomarkers to fiber tract integrity, we predefined 11 major association tracts and calculated the tract specific FA values which are known to reflect bundle integrity with high sensitivity and specificity.30 The neurobehavioral assessments were arranged twice with an interval of 1.5 years to explore the major clinical outcome factors.
MATERIAL AND METHODS
Inclusion and Exclusion Criteria
This was a single-center, prospective, and observational study. The patients were recruited from the Department of Neurology of Chang Gung Memorial Hospital from 2011 to 2013. All of the patients underwent comprehensive neurological and neuropsychological assessments with consensus rendered at a multidisciplinary conference.13,31 AD was diagnosed according to the International Working Group criteria for AD.32 Patients with a clinical dementia rating score of 0.5 or 1 were enrolled and defined as having mild-stage AD, and those with a clinical dementia rating score of 2 as moderate-stage AD. With regards to treatment, all of the patients received an acetylcholinesterase inhibitor (donepezil 5 mg for 1 month and 10 mg thereafter).
The exclusion criteria were: renal function impairment (reference level of normal creatinine was defined as <1.5 mg/dL); abnormal liver function test; and clinical stroke evidenced by history or neuroimaging. As the aim of this study was to explore the association of fiber integrity with biomarkers, patients with clinical stroke were excluded to avoid focal fiber disruption directly caused by vascular injury.
All subjects who fulfilled the inclusion criteria were followed up every 3 months at the clinic, with neurobehavioral data recorded at enrollment and after 1.5 years. Eighty five patients and 45 age-matched controls33 completed the study. The hospital's Human Ethics Committee approved this study.
Cerebrovascular Risk Confounders
Cerebrovascular serological biomarkers included hemoglobin A1C, homocysteine, TC, triglycerol, high-density lipoprotein, LDL, folate, vitamin B12, and creatinine. The APOE genotype was determined by polymerase chain reaction-restriction fragment length polymorphism assay and restriction enzyme HhaI.34 APOE4 carriers were defined as having 1 or 2 APOE4 alleles.
Neurobehavioral Assessment
Two trained neuropsychologists administered the 30-item minimental state examination (MMSE). For the behavioral observations, we used the 12-item version of the neuropsychiatric inventory (NPI),35 with scores ranging from 0 to 144.
Diffusion Tensor Imaging and Major Fiber Tract FA Calculation
Magnetic resonance imaging was performed using a 3.0T scanner (Excite, GE Medical System, Milwaukee, WI) equipped with echo-planar capability. Single-shot echo-planar sequences with gradients applied in 25 noncollinear directions were used for diffusion tensor imaging acquisition. Axial images were acquired using the following parameters: repetition time 7000 ms, echo time 72 ms, field of view 240 mm × 240 mm, 128 × 128 matrix, which led to an in-plane resolution of 1.875 mm. Data were subsequently interpolated to 1 × 1 mm. Thirty contiguous slices of 5-mm thickness were obtained without gaps. A b value of 1000 s/mm2 was used. The WM parcellation algorithm and calculation of 11 major bundles followed the procedure by Hua et al.29 Details of the fiber tracts are shown in Figure 1. The mean global FA calculation followed the method by Hsu et al.36
FIGURE 1.
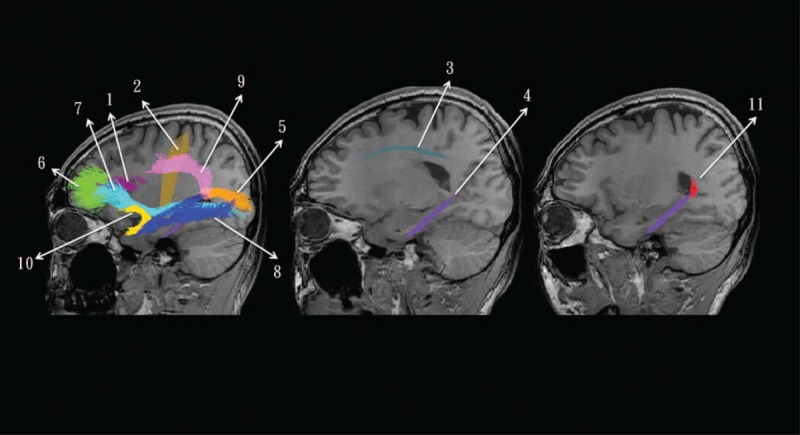
Demonstration of 11 major association fibers tracts overlaid on the sagittal view of 3D T1-weighted images. The mean fractional anisotropy value within each major fiber bundle was averaged to represent the tract-specific fiber integrity. The numbers with related tract names are listed as follows: 1 = forceps major, 2 = forceps minor, 3 = anterior thalamic radiation, 4 = corticospinal tract, 5 = cingulum, 6 = hippocampal cingulum, 7 = inferior fronto-occipital fasciculus, 8 = inferior longitudinal fasciculus, 9 = superior longitudinal fasciculus, 10 = uncinate fasciculus, and 11 = temporal branch of the superior longitudinal fasciculus.
Statistical Analysis
All values were expressed as mean ± standard deviation. In cross-sectional analysis, the Student t-test was used to compare differences between 2 groups. To assess the appropriateness of using parametric statistics for these analyses, we used the Kolmogorov–Smirnov test to examine the normality, and P values greater than 0.05 indicated no significant deviations from normality. To assess the relationships between continuous variables including cerebrovascular risk factors, clinical parameters and neuroimaging parameters, Pearson correlation coefficients, or Spearman rank correlation coefficients were calculated with a corresponding 2-sided significance test at the 0.05 significance level. Stepwise regression was carried out to determine the best predictors of neurobehavioral score. Each model used significant serum biomarkers, WM tract FA data and age (in years) as independent variables. The selection of significant serum biomarkers was based on the results from the correlation analysis.
In the longitudinal study, the MMSE and NPI scores of the AD patients at baseline and after 1.5 years were analyzed using the paired t-test. Stepwise regression was carried out using the significant variables found in the cross-sectional study and age (in years) as independent variables to predict follow-up neurobehavioral data.
All statistical analyses were conducted using the Statistical Package for Social Sciences software package (version 15 for Windows, SPSS Inc., Chicago, IL). A 2-tailed P value less than 0.05 with Bonferroni correction to avoid type I errors in multiple comparisons was considered to be statistically significant.
RESULTS
Neurobehavioral Comparisons Between the Patients and Controls
Comparisons of neurobehavioral data between the 45 age-matched controls (age = 75.4 ± 8.4 years, 24 males) and 85 AD patients (age = 74.5 ± 8.8 years, 41 males) were significant (MMSE: controls = 27.3 ± 3.3, AD = 18.0 ± 6.8, P < 0.001; total NPI score: controls = 3.33 ± 5.3, AD = 7.2 ± 9.0, P < 0.001). The significant NPI subdomains between the controls and AD included delusion (controls: 0, AD = 0.73 ± 2.05, P = 0.002) and mood (controls = 0.16 ± 0.6, AD = 0.93 ± 2.07, P = 0.002).
Factors Related to Neurobehavioral Data in the Patients
Among the 85 patients, 66 had mild-stage AD (MMSE = 20.3 ± 5.4, total NPI score = 6.3 ± 7.6) and 19 had moderate-stage AD (MMSE = 9.8 ± 5.2, total NPI score = 10.2 ± 12.4). The demographic data and predefined cerebrovascular risk factors with the correlation results with the MMSE or NPI are shown in Table 1. Only folate and education levels showed significant relationships with MMSE scores. The MMSE scores did not correlate significantly with total NPI score; however, they were significantly correlated with the NPI subdomains of elation (degrees of freedom [DOF] = 83; r = −0.275, P = 0.01), apathy (DOF = 83; r = −0.279, P = 0.01), disinhibition (DOF = 83; r = −0.288, P = 0.008), and aberrant motor behavior (DOF = 83; r = −0.268, P = 0.014).
TABLE 1.
Clinical Data of 85 Alzheimer Dementia Patients
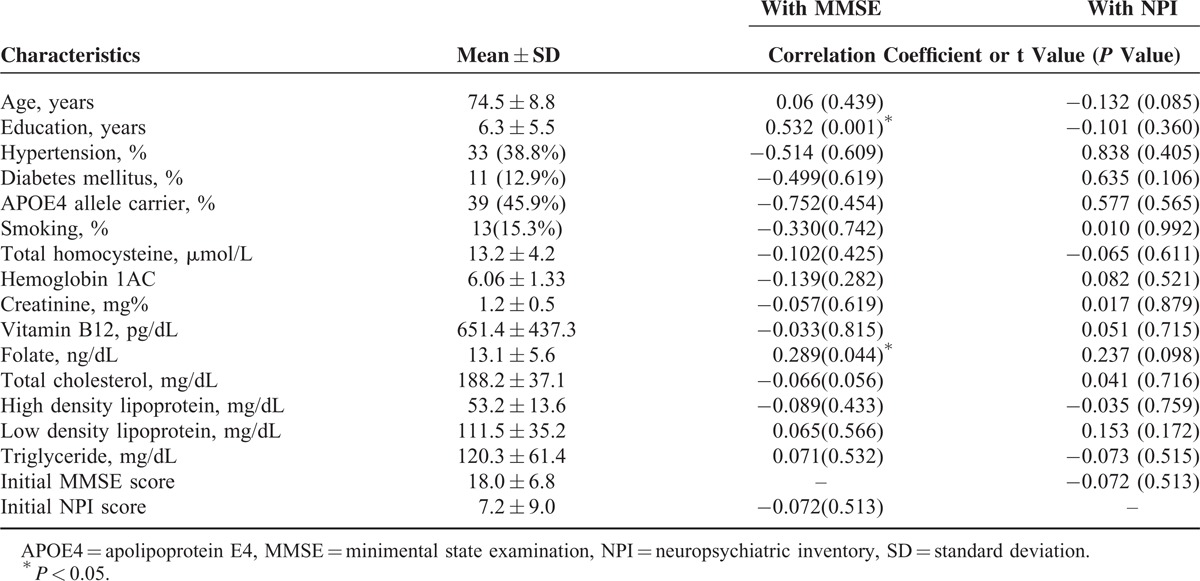
Demographic Data Affecting Fiber Tract FA
The descriptive data of 11 fiber tract FA of the patients and controls are provided in supplementary Table 1, http://links.lww.com/MD/A335. In the patients, the mean global FA was significantly positively correlated with the MMSE scores (Figure 2A; DOF = 83; r = 0.394, P = 0.002) and inversely with age (Figure 2B; DOF = 83; r = −0.341, P = 0.001) at enrollment. The correlation between NPI and mean global FA was not significant (r = −0.200, P = 0.067, DOF = 83). However, for the patients with behavioral changes (ie, NPI > 0, DOF = 61; r = −0.271, P = 0.032), an inverse correlation with mean global FA was found (Figure 2C). Significant correlations between the 11 major tract FA values in the patients were found after adjusting for MMSE (partial r between 0.43 and 0.87, Figure 2D), age (partial r between 0.44 and 0.89, Figure 2E), or NPI (partial r between 0.47 and 0.89, Figure 2F).
FIGURE 2.
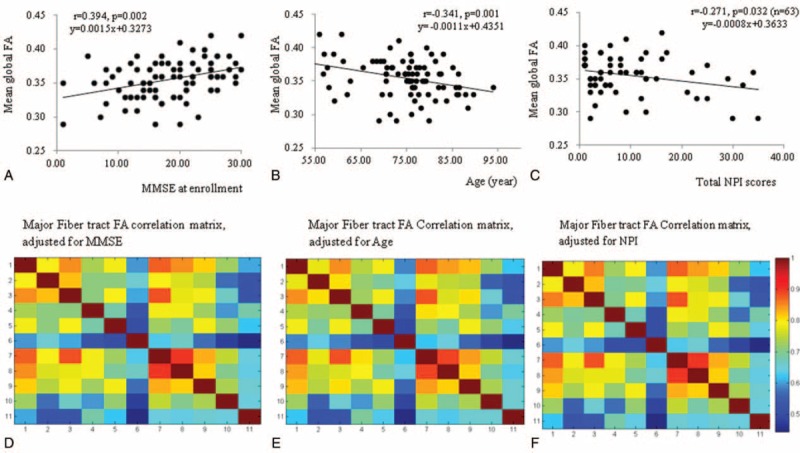
Relationships between mean global fractional anisotropy (FA) with (A) minimental state examination score (MMSE), (B) age, and (C) neuropsychiatric inventory (NPI) scores (n = 63 showing NPI > 0) at enrollment. Correlation matrix between 11 major fiber bundles FA adjusted for (D) MMSE score, (E) age, and (F) NPI. The color bar represents correlation coefficient ranges, and the color within the correlation matrix indicates correlation coefficient values. The degree of freedom for correlation analysis was 84. 1 = forceps major, 2 = forceps minor, 3 = anterior thalamic radiation, 4 = corticospinal tract, 5 = cingulum, 6 = hippocampal cingulum, 7 = inferior fronto-occipital fasciculus, 8 = inferior longitudinal fasciculus, 9 = superior longitudinal fasciculus, 10 = uncinate fasciculus, and 11 = temporal branch of the superior longitudinal fasciculus.
Factors Predicting the Neurobehavioral Data in Regression Analysis in the Patients
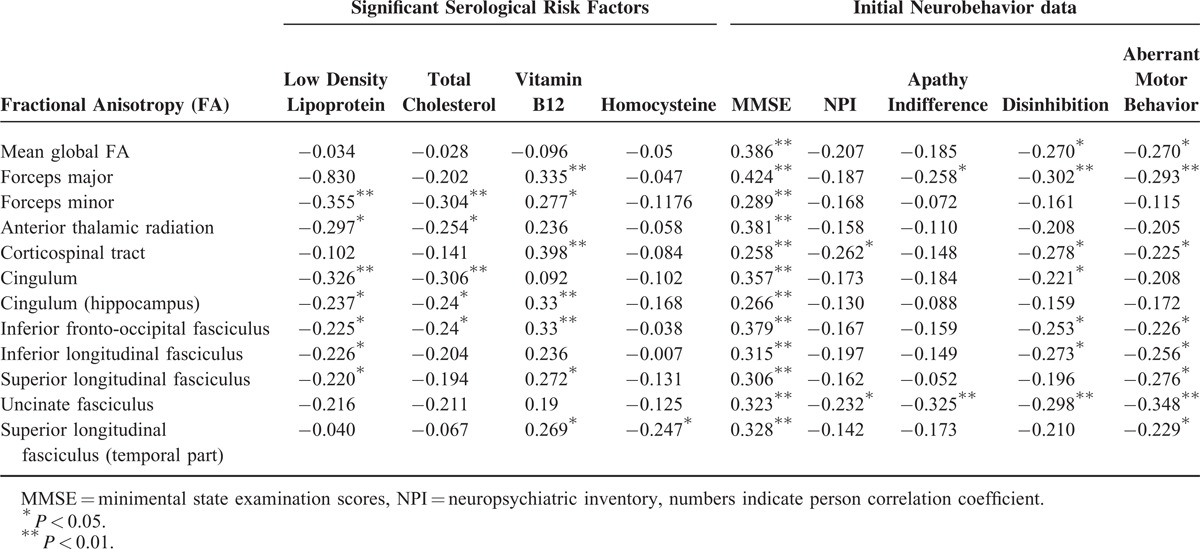
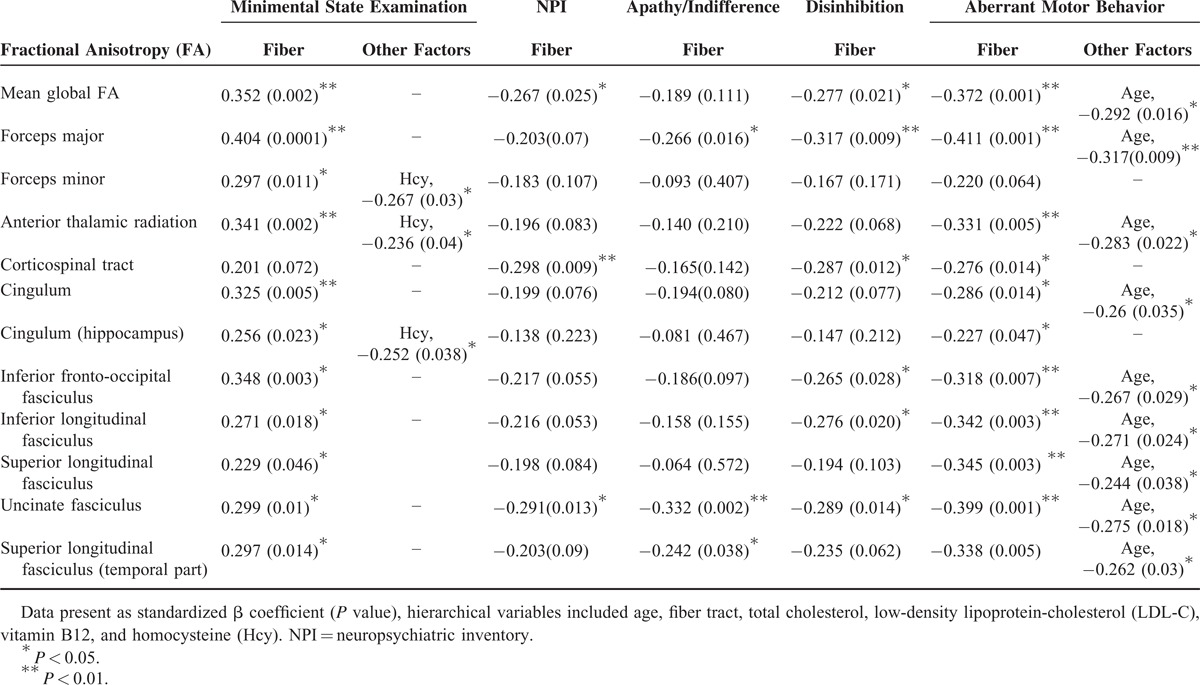
Among the serological biomarkers, only LDL, TC, vitamin B12, and homocysteine showed significant relationships with fiber tract FA (Table 2). The relationships between tract FA and neurobehavior data are also listed in Table 2. There were significant positive correlation between tract FA and MMSE and inverse correlation with total or 3 NPI subdomains.
TABLE 2.
Significant Relationships Between Serological Risk Factors and Major White Matter Tracts Integrity of 85 Alzheimer Dementia Patients
To test our hypothesis that the effect of cerebrovascular biomarkers on neurobehavioral scores would be via the influence of WM tract integrity, we used the neurobehavioral data (ie, MMSE, NPI, or the 3 significant NPI subdomains) separately as dependent variables and a hierarchical regression model to explore the independent roles of age, fiber tract, TC, LDL-C, vitamin B12, and homocysteine (Table 3). The independent roles of the mean global FA and tract FA values to predict the MMSE score were repeatedly found. Among the serological biomarkers, only homocysteine level was found to coexist with the forceps minor, anterior thalamic radiation, or hippocampal cingulum FA in predicting the MMSE score. In the total NPI score, the mean global FA, corticospinal tract, and uncinate fasciculus FA showed clinical significance (Table 3). The fiber tract FA predicted variably the 3 significant NPI subdomains, while the effect of age was found to contribute to fiber tract FA for the aberrant motor behavior subdomain. Of specific note is the unicinate fasciculs FA in predicting total or 3 subdomains NPI scores.
TABLE 3.
Regression Model for Neurobehavior Prediction of 85 Alzheimer Dementia Patients
Longitudinal Neurobehavioral Data
After 1.5 years of follow-up, a significant decline in the MMSE score (mean 1.6 ± 4.9, t = −2.94, P = 0.004), increases in NPI total score (mean 2.4 ± 10.4, P = 0.03), the subdomains of apathy (mean 1.3 ± 3.8, P = 0.002), and sleep disturbance (mean 1.94 ± 0.52, P = 0.001) were found in the patients. The aforementioned domains were also significantly different from the controls (P < 0.001). Eleven patients initially in the mild group were reclassified into the moderate group (n = 30, MMSE = 7.8 ± 6.5, NPI total score = 14.4 ± 13.3) while 55 patients remained in the mild-stage group (MMSE = 18.5 ± 4.7, NPI total score = 8.23 ± 8.7). Although not statistically significant, the age of the patients showed a trend which was related to the changes in the MMSE (r = 0.213, P = 0.052, DOF = 83). The age of the patients was not significantly related to changes in NPI total scores (r = −0.178, P = 0.103, DOF = 83). Changes between MMSE and NPI total scores were also not correlated (r = −0.065, P = 0.59, DOF = 83).
Stepwise Regression Model for Longitudinal Neurobehavioral Prediction
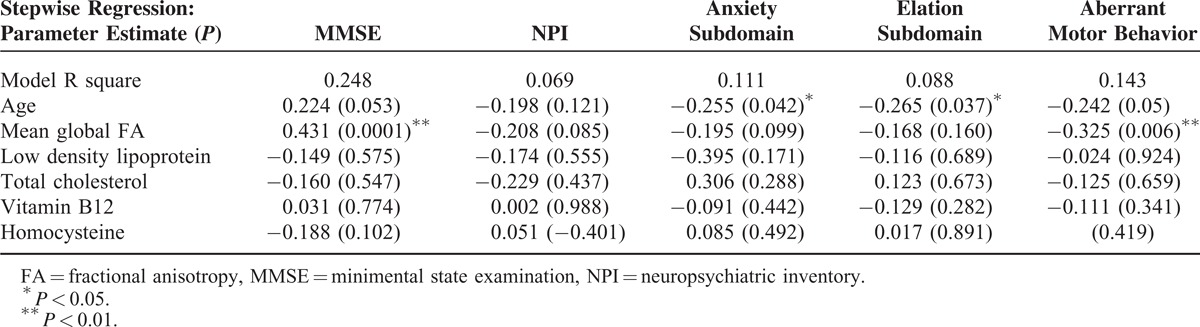
The factors predictive of MMSE or NPI total or subdomain scores after 1.5 years are listed in Table 4. We entered age, mean global FA, LDL, TC, vitamin B12, and homocysteine into the regression model as independent variables and the follow-up MMSE or NPI as the dependent variables. The rationale of not using individual WM FA values was based on the observation that the mean global FA value predicted the MMSE and NPI score at enrollment, and to avoid false positive results by multiple comparisons. The results showed that the mean global WM FA value independently predicted the follow-up MMSE scores and aberrant motor behavior subdomain, while the age of the patients predicted the anxiety and elation subdomains. None of the independent factors predicted NPI total scores. None of the factors were related to the NPI total scores decline.
TABLE 4.
Regression Model for Neurobehavior Predictions of 85 Alzheimer Dementia Patients After 1.5 years
DISCUSSION
Major Findings
The cross- and longitudinal-study explored the roles of cerebrovascular biomarkers with related fiber tract integrities and neurobehavior test scores correlation in AD. There were 3 major findings. Firstly, from the cross-sectional analysis, we found significant correlations between fiber tract integrity with LDL, TC, vitamin B12, or homocysteine levels. Secondly, as significant relationships were also found between fiber tract FA and neurobehavior scores, all the aforementioned biomarkers were entered into the regression model with the fiber tract FA to explore the independent role of these factors. Only the homocysteine level, along with the fiber tract FA, remained significant for MMSE scores prediction while none of the biomarkers showed clinical significance to the NPI scores. Finally, the regression model with related neurobehavior analysis after 18 months follow-up supports the clinical significance of mean fiber tract integrity in MMSE and aberrant motor behavior score prediction. Meanwhile, the age of the patients predicted anxiety and elation scores. Our study results in AD validated the modification of the cerebrovascular risk factors on specific WM bundles integrity and partially supported the hypothesis that major bundle fiber-FA and homocysteine showed greater clinical impacts to neurobehavior performances whether in cross-sectional or longitudinal observation.
WM Integrity With Related Neurobehavioral Scores
There are 2 different perspectives to look at the WM changes in the brain. The quantification of WM damages within the anatomical regions measured the regional “lesion loads,”37 as opposed to the assessment of fiber tracts integrity that measured the structural connectivity.30 As we assumed that the functional status of the brain reflected large-scale connectivity networks, measurement of tract integrity may be more straightforward for clinical symptoms correlations than the quantification of lesional load per se.
In the cross-sectional data, our study found functional segregation of fiber tract networks that determined the MMSE and NPI scores. Among the highly coherent network for the neurobehavior symptoms, the uncinate fasciculus is found to relate to all of the significant neurobehavior symptoms here. The human uncinate fasciculus represents the largest corticocortical WM pathway that connects directly to the frontal and temporal lobes. The early involvement of uncinate fasciculus was found in elderly with positive-amyloid imaging,38 prodromal AD,39 and early AD.40–42 Meanwhile, the relationships between uncinate fasciculus with memory task performance43 and behavioral symptoms in patients with AD44,45 have been established. The fiber integrity of the superior longitudinal fasciculus and inferior frontal-occipital fasciculus have been reported to be related to executive and memory dysfunction in patients with AD.46 Related to the MMSE scores and in aberrant motor behavior scores here, the aforementioned bundles and others with related clinical significance were further established in our study.
Cerebrovascular Risk Factors That Affect WM Fiber Integrity
In AD, changes in WM tract integrity can be the consequence of aging, endothelial damage,47–49 blood–brain barrier damage,27 impaired cerebral auto-regulation,50 prothrombotic changes with hypo-perfusion,51 or cerebral hypo-perfusion.31 Our cross-sectional analysis suggested the importance of lipid profiles, B12, and homocysteine levels, in that they were highly related to major tract integrity.
Homocysteine is considered as a risk factor for cerebral small vessel disease, but the direct correlation between homocysteine with WM integrity was only found in the temporal part of superior longitudinal fasciculus in this study. The possible associations between homocysteine and neurobehavioral performance may be mediated directly by neurotoxicity. Alternatively, the changes may reflect cerebral hypoperfusion31 or endothelial dysfunction52,53 that were mediated by homocysteine and were known to affect the WM integrity.
Significant correlations between B12 and WM FA were also found in this study but the link between B12 and clinical scores is not established here. Several studies conducted before showed the supplements containing vitamin B12 and B9 did not improve cognitive performance in nearly 3000 volunteers,54 in women with cerebrovascular risk factors,55 or in mild to moderate AD patients.56 Therefore, although vitamin B12 supplements are considered to be a homocysteine-lowering strategy, our study result suggested a greater clinical impact of homocysteine, unrelated to B12 protection, in cognitive performances. Meanwhile, although the levels of homocysteine were entered into the regression models with B12 levels, only the homocysteine showed clinical significance. This observation may strengthen the pathophysiological link between hyperhomocysteinemia with WMHs loads in cognitive prediction.13
Specific to the lipid profile, our study found that LDL and TC levels were associated with fiber integrity. However, the levels were not correlated to any of the neurobehavioral data. Higher LDL and TC profiles may also result in endothelial dysfunction and proinflammatory cascades in the endothelial atherogenic process.57 The consequences are cerebral hypoperfusion, WM damage or Wallerian degeneration.58 As higher lipid levels in midlife are associated with a greater risk of developing AD,59 it is possible that cholesterol modulation therapy may show a clinical benefit. A longitudinal study may help to elucidate the dynamic changes of neurobehavioral data in relation to cholesterol modulation therapy, and whether other lipid components also participate in fiber tract integrity.
Independent Dimensions of Cognition and Behavior Symptoms
In AD, behavioral symptoms are highly individualized, and they tend to co-occur, fluctuate, recur, or proceed cognitive changes.60,61 Although the behavioral changes in AD are more commonly seen in the advanced stage,35,62 the MMSE scores were only related to the scores of elation, apathy, disinhibition, and aberrant motor behavior here. The correlation was not significant to other NPI subdomains, other studies have reported treating psychiatric and cognitive dimensions differently during disease evolution.63–65 Therefore, whether all neurobehavior scores should be treated equal is still an open question. As there are 12 behavior domains in the NPI, the best way to accommodate different behavior models at different clinical stages require further discussion. Despite our findings of specific fiber bundle involvement, most of the NPI subdomains with related fiber tract information were not established in this study. Concerning the broad variance of NPI symptoms, the co-occurrence of behavioral symptoms, modulation of psycho-social factors, and the statistics model used here, the exploration of the functional trajectory specific to the behavioral symptoms in AD requires a more sophisticated approach.
Limitations
There are 4 possible limitations. Firstly, we analyzed the influence of biomarkers on WM integrity; however, data on gray matter were not included in the predictive model. As AD is a neurodegenerative disorder targeting gray matter in the early stage, changes in WM may also reflect consequences from specific gray matter region atrophy. The decision was based on the putative mechanism with regards to the cerebrovascular risk factors to the WM integrity. In addition, a lack of longitudinal diffusion tensor imaging may limit the findings as to whether serological biomarkers also influence the longitudinal WM bundle integrity. Secondly, changes of apathy and sleep disturbance scores were most significant in the longitudinal data while the possible parameters that explain these 2 behavior changes were not established here. It is possible that the behavior changes might reflect changes in gray matter rather than WM alteration. Further studies using a multiparametric imaging model may help to understand the clinical weightings of gray matter atrophy and WM microstructural changes. Thirdly, there may be selection bias in this study. We excluded those with stroke or neuroimaging evidence of stroke, which may imply that cases having extreme biomarker levels were already excluded. In addition, the study was conducted in Taiwan where the population is ethnically Chinese. Whether the study results also apply to other populations may require more data. Although we considered possible cerebrovascular-related variables, factors other than those discussed here may have confounded the study results. Finally, although typically assumed to be negligible, microstructural bundle analysis may be affected by the amount of image smoothing, coregistration setting, or template use. A probabilistic approach tends to yield a lower FA compared with the manual tracing technique, and therefore interpretation of the absolute FA values from the probabilistic approach requires great care. The role of the atlas-based method is to highlight the fiber tracts with probable abnormalities which may show clinical relationships with the measured biomarkers. Further studies using manual regions of interest with tractography tracing are warranted.
CONCLUSION
The results of this study extend current knowledge how cerebrovascular risk factors may modify the WM integrity and related to the neurobehavior outcomes in AD. The fiber tract integrity of the major association bundles, age, or homocysteine may contribute in different degrees which may predict the cross-sectional or longitudinal neurobehavioral data.
Footnotes
Abbreviations: AD = Alzheimer disease, DOF = degrees of freedom, FA = fractional anisotropy, LDL = low-density lipoprotein, MMSE = minimental state examination, NPI = neuropsychiatric inventory, TC = total cholesterol, WM = white matter, WMH = white matter hyperintensity.
M-KW and Y-TL contributed equally to this work.
This work was supported by grants CMRPG8D0771, CMRPG8C0571, and CMRPG8B1491 from the Chang Gung Memorial Hospital.
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol 2014; 13:614–629. [DOI] [PubMed] [Google Scholar]
- 2.Ott A, Stolk RP, van Harskamp F, et al. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999; 53:1937–1942. [DOI] [PubMed] [Google Scholar]
- 3.Skoog I, Lernfelt B, Landahl S, et al. 15-year longitudinal study of blood pressure and dementia. Lancet 1996; 347:1141–1145. [DOI] [PubMed] [Google Scholar]
- 4.Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ 2001; 322:1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullen KM, Kocsi Z, Stone J. Microvascular pathology in the aging human brain: evidence that senile plaques are sites of microhaemorrhages. Neurobiol Aging 2006; 27:1786–1796. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Cao D, Desmond R, et al. Cognitive performance and plasma levels of homocysteine, vitamin B12, folate and lipids in patients with Alzheimer disease. Dement Geriatr Cogn Disord 2008; 26:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 1993; 261:921–923. [DOI] [PubMed] [Google Scholar]
- 8.Exalto LG, van der Flier WM, Scheltens P, et al. Dysglycemia, brain volume and vascular lesions on MRI in a memory clinic population. J Diabetes Complications 2014; 28:85–90. [DOI] [PubMed] [Google Scholar]
- 9.Fukazawa R, Hanyu H, Sato T, et al. Subgroups of Alzheimer's disease associated with diabetes mellitus based on brain imaging. Dement Geriatr Cogn Disord 2013; 35:280–290. [DOI] [PubMed] [Google Scholar]
- 10.Roberts RO, Knopman DS, Cha RH, et al. Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. J Nucl Med 2014; 55:759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vermeer SE, van Dijk EJ, Koudstaal PJ, et al. Homocysteine, silent brain infarcts, and white matter lesions: The Rotterdam Scan Study. Ann Neurol 2002; 51:285–289. [DOI] [PubMed] [Google Scholar]
- 12.Seshadri S, Wolf PA, Beiser AS, et al. Association of plasma total homocysteine levels with subclinical brain injury: cerebral volumes, white matter hyperintensity, and silent brain infarcts at volumetric magnetic resonance imaging in the Framingham Offspring Study. Arch Neurol 2008; 65:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang CW, Chang WN, Lui CC, et al. Impacts of hyper-homocysteinemia and white matter hyper-intensity in Alzheimer's disease patients with normal creatinine: an MRI-based study with longitudinal follow-up. Curr Alzheimer Res 2010; 7:527–533. [DOI] [PubMed] [Google Scholar]
- 14.Tu MC, Huang CW, Chen NC, et al. Hyperhomocysteinemia in Alzheimer dementia patients and cognitive decline after 6 months follow-up period. Acta Neurol Taiwan 2010; 19:168–177. [PubMed] [Google Scholar]
- 15.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001; 70:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wardlaw JM. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study: the Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001; 70:2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salat DH, Greve DN, Pacheco JL, et al. Regional white matter volume differences in nondemented aging and Alzheimer's disease. Neuroimage 2009; 44:1247–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeCarli C, Grady CL, Clark CM, et al. Comparison of positron emission tomography, cognition, and brain volume in Alzheimer's disease with and without severe abnormalities of white matter. J Neurol Neurosurg Psychiatry 1996; 60:158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan EV, Marsh L, Mathalon DH, et al. Age-related decline in MRI volumes of temporal lobe gray matter but not hippocampus. Neurobiol Aging 1995; 16:591–606. [DOI] [PubMed] [Google Scholar]
- 20.Tanabe JL, Amend D, Schuff N, et al. Tissue segmentation of the brain in Alzheimer disease. AJNR Am J Neuroradiol 1997; 18:115–123. [PMC free article] [PubMed] [Google Scholar]
- 21.Double KL, Halliday GM, Kril JJ, et al. Topography of brain atrophy during normal aging and Alzheimer's disease. Neurobiol Aging 1996; 17:513–521. [DOI] [PubMed] [Google Scholar]
- 22.Smith CD, Snowdon DA, Wang H, et al. White matter volumes and periventricular white matter hyperintensities in aging and dementia. Neurology 2000; 54:838–842. [DOI] [PubMed] [Google Scholar]
- 23.DeCarli C, Murphy DG, Tranh M, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology 1995; 45:2077–2084. [DOI] [PubMed] [Google Scholar]
- 24.Fotenos AF, Snyder AZ, Girton LE, et al. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology 2005; 64:1032–1039. [DOI] [PubMed] [Google Scholar]
- 25.Hirono N, Yasuda M, Tanimukai S, et al. Effect of the apolipoprotein E epsilon4 allele on white matter hyperintensities in dementia. Stroke 2000; 31:1263–1268. [DOI] [PubMed] [Google Scholar]
- 26.Hirono N, Kitagaki H, Kazui H, et al. Impact of white matter changes on clinical manifestation of Alzheimer's disease: a quantitative study. Stroke 2000; 31:2182–2188. [DOI] [PubMed] [Google Scholar]
- 27.Deppe M, Duning T, Mohammadi S, et al. Diffusion-tensor imaging at 3 T: detection of white matter alterations in neurological patients on the basis of normal values. Invest Radiol 2007; 42:338–345. [DOI] [PubMed] [Google Scholar]
- 28.Rorschach HE, Lin C, Hazlewood CF. Diffusion of water in biological tissues. Scanning Microsc Suppl 1991; 5:S1–S9.discussion S9–10. [PubMed] [Google Scholar]
- 29.Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 2008; 39:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cercignani M. Strategies for Patient–Control Comparison of Diffusion MR Data, Jones DK. editor. London: Oxford University Press; 2010. [Google Scholar]
- 31.Huang CW, Chang WN, Huang SH, et al. Impact of homocysteine on cortical perfusion and cognitive decline in mild Alzheimer's dementia. Eur J Neurol 2013; 20:1191–1197. [DOI] [PubMed] [Google Scholar]
- 32.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang CC, Chang YY, Chang WN, et al. Cognitive deficits in multiple system atrophy correlate with frontal atrophy and disease duration. Eur J Neurol 2009; 16:1144–1150. [DOI] [PubMed] [Google Scholar]
- 34.Del Bo R, Comi GP, Bresolin N, et al. The apolipoprotein E epsilon4 allele causes a faster decline of cognitive performances in Down's syndrome subjects. J Neurol Sci 1997; 145:87–91. [DOI] [PubMed] [Google Scholar]
- 35.Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314. [DOI] [PubMed] [Google Scholar]
- 36.Hsu JL, Leemans A, Bai CH, et al. Gender differences and age-related white matter changes of the human brain: a diffusion tensor imaging study. Neuroimage 2008; 39:566–577. [DOI] [PubMed] [Google Scholar]
- 37.de Leeuw FE, Barkhof F, Scheltens P. White matter lesions and hippocampal atrophy in Alzheimer's disease. Neurology 2004; 62:310–312. [DOI] [PubMed] [Google Scholar]
- 38.Molinuevo JL, Ripolles P, Simo M, et al. White matter changes in preclinical Alzheimer's disease: a magnetic resonance imaging-diffusion tensor imaging study on cognitively normal older people with positive amyloid beta protein 42 levels. Neurobiol Aging 2014; 35:2671–2680. [DOI] [PubMed] [Google Scholar]
- 39.Hiyoshi-Taniguchi K, Oishi N, Namiki C, et al. The uncinate fasciculus as a predictor of conversion from aMCI to Alzheimer disease. J Neuroimaging 2014. [DOI] [PubMed] [Google Scholar]
- 40.Karow DS, McEvoy LK, Fennema-Notestine C, et al. Relative capability of MR imaging and FDG PET to depict changes associated with prodromal and early Alzheimer disease. Radiology 2010; 256:932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alves GS, O’Dwyer L, Jurcoane A, et al. Different patterns of white matter degeneration using multiple diffusion indices and volumetric data in mild cognitive impairment and Alzheimer patients. PLoS One 2012; 7:e52859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Remy F, Vayssiere N, Saint-Aubert L, et al. White matter disruption at the prodromal stage of Alzheimer's disease: relationships with hippocampal atrophy and episodic memory performance. Neuroimage Clin 2015; 7:482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato T, Maruyama N, Hoshida T, et al. Correlation between uncinate fasciculus and memory tasks in healthy individual using diffusion tensor tractography. Conf Proc IEEE Eng Med Biol Soc 2012; 2012:424–427. [DOI] [PubMed] [Google Scholar]
- 44.Hahn C, Lim HK, Won WY, et al. Apathy and white matter integrity in Alzheimer's disease: a whole brain analysis with tract-based spatial statistics. PLoS One 2013; 8:e53493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li W, Muftuler LT, Chen G, et al. Effects of the coexistence of late-life depression and mild cognitive impairment on white matter microstructure. J Neurol Sci 2014; 338:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peelle JE, Powers J, Cook PA, et al. Frontotemporal neural systems supporting semantic processing in Alzheimer's disease. Cogn Affect Behav Neurosci 2014; 14:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zuliani G, Cavalieri M, Galvani M, et al. Markers of endothelial dysfunction in older subjects with late onset Alzheimer's disease or vascular dementia. J Neurol Sci 2008; 272:164–170. [DOI] [PubMed] [Google Scholar]
- 48.Rentzos M, Michalopoulou M, Nikolaou C, et al. Serum levels of soluble intercellular adhesion molecule-1 and soluble endothelial leukocyte adhesion molecule-1 in Alzheimer's disease. J Geriatric Psychiatry Neurol 2004; 17:225–231. [DOI] [PubMed] [Google Scholar]
- 49.Nielsen HM, Londos E, Minthon L, et al. Soluble adhesion molecules and angiotensin-converting enzyme in dementia. Neurobiol Dis 2007; 26:27–35. [DOI] [PubMed] [Google Scholar]
- 50.Hoth KF, Haley AP, Gunstad J, et al. Elevated C-reactive protein is related to cognitive decline in older adults with cardiovascular disease. J Am Geriatr Soc 2008; 56:1898–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wright CB, Sacco RL, Rundek T, et al. Interleukin-6 is associated with cognitive function: the Northern Manhattan Study. J Stroke Cerebrovasc Dis 2006; 15:34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baro L, Fonolla J, Pena JL, et al. n-3 Fatty acids plus oleic acid and vitamin supplemented milk consumption reduces total and LDL cholesterol, homocysteine and levels of endothelial adhesion molecules in healthy humans. Clin Nutr 2003; 22:175–182. [DOI] [PubMed] [Google Scholar]
- 53.Hassan A, Hunt BJ, O'Sullivan M, et al. Homocysteine is a risk factor for cerebral small vessel disease, acting via endothelial dysfunction. Brain 2004; 127:212–219. [DOI] [PubMed] [Google Scholar]
- 54.van der Zwaluw NL, Dhonukshe-Rutten RA, van Wijngaarden JP, et al. Results of 2-year vitamin B treatment on cognitive performance: secondary data from an RCT. Neurology 2014; 83:2158–2166. [DOI] [PubMed] [Google Scholar]
- 55.Kang JH, Cook N, Manson J, et al. A trial of B vitamins and cognitive function among women at high risk of cardiovascular disease. Am J Clin Nutr 2008; 88:1602–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aisen PS, Schneider LS, Sano M, et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA 2008; 300:1774–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 2007; 115:1285–1295. [DOI] [PubMed] [Google Scholar]
- 58.Alexander AL, Lee JE, Lazar M, et al. Diffusion tensor imaging of the brain. Neurotherapeutics 2007; 4:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kivipelto M, Helkala EL, Laakso MP, et al. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med 2002; 137:149–155. [DOI] [PubMed] [Google Scholar]
- 60.Holtzer R, Tang MX, Devanand DP, et al. Psychopathological features in Alzheimer's disease: course and relationship with cognitive status. J Am Geriatr Soc 2003; 51:953–960. [DOI] [PubMed] [Google Scholar]
- 61.Spalletta G, Baldinetti F, Buccione I, et al. Cognition and behaviour are independent and heterogeneous dimensions in Alzheimer's disease. J Neurol 2004; 251:688–695. [DOI] [PubMed] [Google Scholar]
- 62.Harwood DG, Barker WW, Ownby RL, et al. Relationship of behavioral and psychological symptoms to cognitive impairment and functional status in Alzheimer's disease. Int J Geriatr Psychiatry 2000; 15:393–400. [DOI] [PubMed] [Google Scholar]
- 63.Bakker TJ, Duivenvoorden HJ, van der Lee J, et al. Prevalence of psychiatric function disorders in psychogeriatric patients at referral to nursing home care – the relation to cognition, activities of daily living and general details. Dement Geriatr Cogn Disord 2005; 20:215–224. [DOI] [PubMed] [Google Scholar]
- 64.Lam LC, Tang NL, Ma SL, et al. Apolipoprotein epsilon-4 allele and the two-year progression of cognitive function in Chinese subjects with late-onset Alzheimer's disease. Am J Alzheimers Dis Other Demen 2006; 21:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ito T, Meguro K, Akanuma K, et al. Behavioral and psychological symptoms assessed with the BEHAVE-AD-FW are differentially associated with cognitive dysfunction in Alzheimer's disease. J Clin Neurosci 2007; 14:850–855. [DOI] [PubMed] [Google Scholar]


