Abstract
Although pneumatic dilation is an accepted method for the treatment of achalasia, this therapy has high recurrence and complication rates, and prolonged follow-up studies on the parameters associated with various outcomes are rare. In this prospective 10-year follow-up study, a satisfactory therapeutic effect was achieved without serious complications. We report the therapeutic experience with pneumatic dilation, having aimed to evaluate the long-term clinical safety and efficacy of pneumatic dilation.
In total, 35 consecutive patients with idiopathic achalasia who underwent pneumatic dilation were followed up at regular intervals in person or by a phone interview over a 10-year period. The mean duration of the follow-up was 43.03 ± 26.34 months (range 6–120 months). Remission was assessed by the dysphagia classification and symptom scores. Patients’ clinical symptom scores were calculated before and at 6 to 36 months, 37 to 60 months, and >60 months after therapy. The influence of the patients’ age, gender, and disease duration on the therapeutic effect was analyzed.
The success rate of the operation was 97.2% (35/36), without massive hemorrhaging, perforation or other serious complications. Dysphagia after the therapy was significantly eased (P < 0.01). In total, 35 patients have been followed up for 6 to 36 months after therapy, 21 cases for 37 to 60 months, and 5 cases for >60 months, and the patients’ symptom scores separately decreased significantly compared with the pretherapy scores (P < 0.01). For these patients, the 6 to 36 months remission rate was 85.7% (30/35), the 37 to 60 months rate was 61.9% (13/21), and the >60 months rate was 40% (2/5). The dilation effect had no relationship to the patient's age, gender, and disease duration (P > 0.05). The patients in 30 cases (85.7%) were successfully treated with a single dilation, in 4 cases (11.4%) with 2 dilations, and in 1 case (2.9%) with 3 dilations.
These results suggest that endoscopic pneumatic dilation is an achalasia therapy with a good response; it is a simple and safe procedure with long-term clinical effectiveness. It is a preferred method in the treatment of achalasia.
INTRODUCTION
Achalasia is a disease of esophageal motor dysfunction,1,2 characterized by an esophageal emptying delay caused by a lower esophageal sphincter (LES) relaxation disorder; the major clinical symptom of achalasia is dysphagia.3 In most patients, regurgitation, chest pain, and weight loss are present as well. The etiology is unknown. The association of achalasia with viral infections and autoantibodies against plexus has been reported; however, the causal relationship is unclear.4,5 The current treatment includes pharmacological agents, endoscopic pneumatic dilation,6–11 botulinum toxin12 or sclerosant substance injections,13 cardiac stent placement,12,14 per oral endoscopic myotomy (POEM),15–17 and surgical myotomy.18–22 Pharmacological therapy is administered primarily as a subordinate treatment. Botulinum toxin could inhibit presynaptic nerve terminals from releasing acetylcholine to reducing LES pressure and facilitate esophageal emptying.23 This method has functioned well recently, whereas the patients should undergo repeated therapies, and some patients develop a primary tolerance. Cardia stent placement uses consistent dilation of a stent to break the muscle fibers for a reduction of pressure, whereas the stent might be displaced or dislodged after the operation and negatively affect the therapy outcome.14 Controlling the depth and length of the cut in POEM is difficult, and the technology requires improvement; the long-term effect should be investigated further. An esophageal Heller myotomy tends to cause extensive injuries; patients remain in the hospital longer, and the procedure is costly and has a high rate of complications.24
Pneumatic dilation is easy to manage, has fewer postoperative complications, and could be repeated in the clinic; it is favored by medical practitioners and patients. We treated achalasia by applying pneumatic dilation that was guided and monitored by endoscopy; our procedure included a long-term follow-up, and we analyzed the comprehensive data of the treated patients to explore the effect and safety of this treatment.
MATERIALS AND METHODS
Patients
The patient cohort comprised 29 males and 7 females, ranging in age from 16 to 60 years (mean age, 37.2 years). With the exception of a single patient who interrupted the treatment, 35 patients (97.2%) underwent successful dilation and were followed until the final interview in 2014. The duration of the disease in the 35 cases included in this study ranged from 2 to 95 months (42.94 ± 27.27 months). Among these cases, 1 patient had been previously treated by Sa's expander in other hospital.
The patients had differing degrees of dysphasic symptoms, as follows; 21 patients had vomiting after eating and significant weight loss (58.3%); 15 patients had chest pain and substernal discomfort (41.7%); and 8 patients had food reflux and nocturnal coughing at night (22.2%).
According to the dysphasic symptoms, which were categorized by the Stooler classification, 4 cases were graded as I (11.1%), 25 cases as II (69.4%), 5 cases as III (13.9%), and 2 cases as IV (5.6%). The Eckardt25 average score was 8.63 points.
Evaluation of Symptoms
Stooler classification (0–IV grade): Grade 0: no difficulty in swallowing food; Grade I: difficulties in swallowing solid food; Grade II: difficulties in swallowing semi-fluid food; Grade III: difficulties in swallowing liquid difficulties; and Grade IV: cannot take food.
Eckardt symptom score standard (0–12 points): depending on whether dysphagia, regurgitation, and chest pain occurred occasionally, daily, or several times during the day, a symptom score of between 0 and 3 was determined. In addition, a symptom score of 0 to 3 was assigned to the degree of weight loss. Thus, a completely asymptomatic patient would have a symptom score of 0 whereas a severely affected patient could have a symptom score of up to 12. The patients were considered to have reached clinical remission if the symptoms had totally disappeared or if they had improved by at least 2 points and did not exceed a score of 3. The Eckardt symptom score standard is summarized and shown in Table 1.
TABLE 1.
Eckardt Symptom Score

The project was approved by the Shanghai Ninth People's Hospital Ethics Committee in China. All the patients gave written informed consent to participate in the study. These data do not contain information that could identify the patients.
Preoperative Inspection
The diagnosis of achalasia was based on the results of a barium esophagogram, a chest CT, and an endoscopic examination, details on these tests were shown in Table 2. All the patients had no obvious symptoms of blood coagulation or cardiopulmonary dysfunction.
TABLE 2.
Results of a Barium Esophagogram, a Chest CT, and an Endoscopic Examination
Preoperative Preparation
To reduce or eliminate tension, fear, and anxiety in the patients.
To procure the understanding and letter of consent from the patients.
To inject anisodamine and diazepam at 10 mg, 15 min before the operation.
Other preparations were similar to those of conventional gastroscopy.
Endoscopic Pneumatic Dilation
The Rigiflex dilator (Boston Inc., Marlborough, Massachusetts, USA) is offered in 3 sizes, according to the diameters of the dilators, that is, a diameter of 3 cm, 3.5 cm, or 4.0 cm, with a length of 10 cm. Considering the body features Chinese patients, we normally selected the 3.5-cm diameter dilator. The pneumatic mechanism should be checked to investigate for leaks before the surgery. During the entire surgical process, we emphasized the following: we directed the endoscope lens at the mouth side of the pneumatic mechanism and observed and monitored the process of dilation. The pneumatic mechanism inflates every 10 s for 1 min, and the process repeated 3 to 4 times. The dilation pressure was initiated at approximately 150 to 188 mm Hg (1 mm Hg = 0.133 kPa), and gradually increased until, the final pressure reached 262 to 300 mm Hg. Finally, the inspection was performed through the endoscope, and the bleeding of the cardia mucosa was managed; we observed carefully whether the patient presented with abdominal pain, shortness of breath, or other abnormal phenomena.
Postoperative Treatment
The patients were allowed only a liquid diet at normal and low temperatures within 2 to 3 h after operation and could consume only a semi-liquid diet for 24 h after surgery; the patients gradually returned to a normal diet. Antacids, mucosal protective agents, and anti-inflammatory treatment were applied to the patients after the treatment.
Postoperative Follow-Up
In total, 35 patients who completed the treatment had been followed-up at regular intervals in person or by a phone interview individually for 6 to 120 months (43.03 ± 26.34 months) after therapy, and their clinical symptom scores were calculated before and at 6 to 36 months, 37 to 60 months, and >60 months after therapy. Patients were considered to have reached clinical remission if symptoms had totally disappeared or if they had improved by at least 2 points, and the total score decreased to below 3 points. Simultaneously, a record was maintained regarding dysphagia in patients whose symptoms had decreased 6 months after treatment.
Statistical Analysis
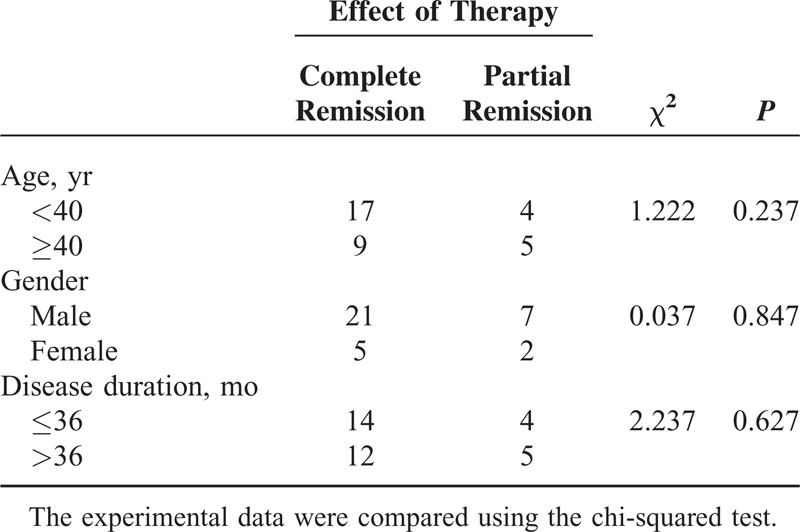
These data were analyzed by SPSS 19.0 statistical software (SPSS Inc., Chicago, IL, USA). The differences in the symptom scores were compared using the t test. The comparisons of the dysphagia symptom between the groups were performed using the Wilcoxon statistical methods, as follows: the influence of the patients’ age, gender, and duration of the disease on the therapeutic effect was analyzed using the chi-square test. A P < 0.01 indicated the statistical value.
RESULTS
With the exception of a single patient who interrupted the treatment, 35 patients (97.2%) underwent successful dilation and were followed until the final interview in 2014. The 35 patients had tolerable retrosternal pain during the dilation. Three patients felt pain postoperatively, which was relieved spontaneously after 2 to 3 days. After the treatment, endoscopic observation of the cardia showed 3 to 4 radial tears, and a small amount of blood. Five patients were treated with norepinephrine topical spray, which stopped bleeding, and the other patients the rest were in spontaneous remission. No massive hemorrhaging, perforation, or other serious complications occurred. The images of achalasia are shown in Figure 1. The symptoms of dysphagia were significantly eased 6 months after the surgery (P < 0.01) (Table 3) without recurrence during the follow-up period. Some patients had mild substernal discomfort and regurgitation. The followed up continued for 6 to 36 months in 35 cases, 37 to 60 months in 21 cases, and >60 months in 5 cases. The separate scores of the symptoms score decreased significantly from the preoperative scores (P < 0.01). For these patients, the 6 to 36 months remission rate was 85.7% (30/35), the 37 to 60 months rate was 61.9% (13/21), and the >60 months rate was 40% (2/5). The patients’ symptom score at 37 to 60 months increased compared to that at 6 to 36 months. There was no significant difference between the scores at >60 months and 37 to 60 months (Table 4). The dilation results at the final interview were not related to the patient's age, gender, and duration of the disease (P > 0.05) (Table 5). Among the 35 patients, 30 (85.7%) were successfully treated with a single dilation, 4 (11.4%) with 2 dilations, and 1 (2.9%) with 3 dilations. The patients required repeated dilations had the procedures within a 6-month follow-up period.
FIGURE 1.
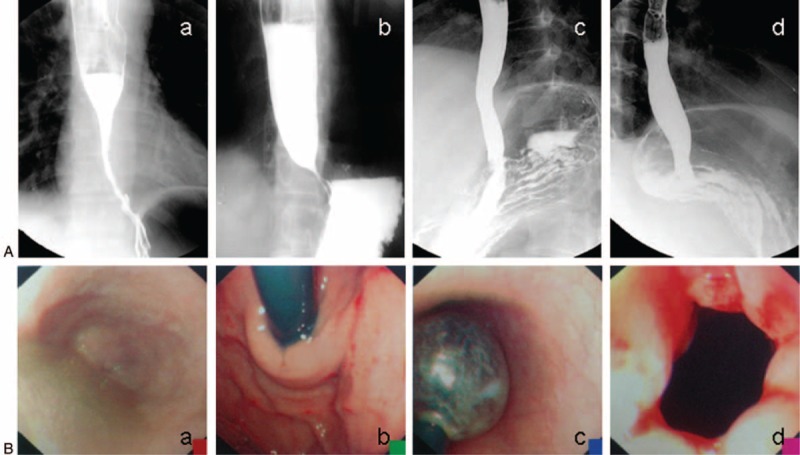
Barium esophagogram and endoscopic images of achalasia. (A) a, b: Image of barium esophagogram before therapy. c, d: Image of the barium esophagogram after therapy. (B) a, b: Endoscopic image of achalasia before therapy. c: Endoscopic image of pneumatic dilation in the treatment for achalasia. d: Endoscopic image of achalasia after therapy.
TABLE 3.
Dysphagic Symptom Comparison, Pre/Posttherapy
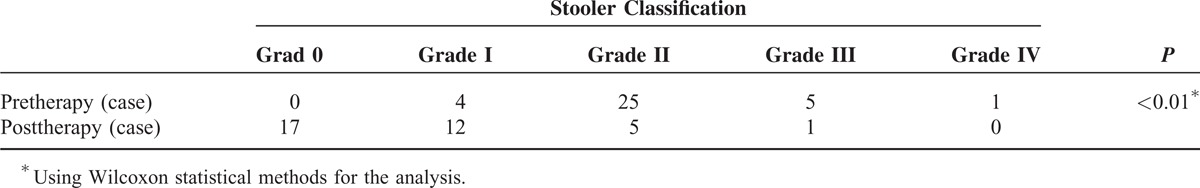
TABLE 4.
Comparison of the Difference in the Symptom Scores, Before/6–36 Months, 37–60 Months, and >60 Months After Therapy
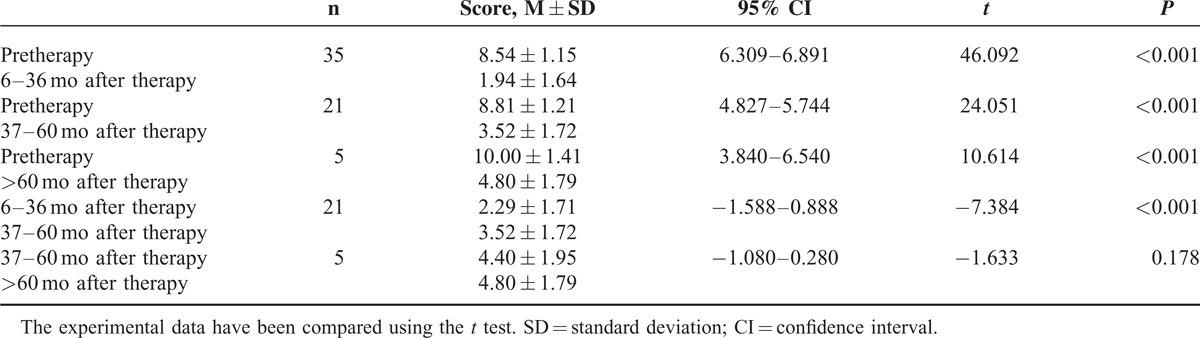
TABLE 5.
Influence of the Patients’ Age, Gender, and Disease Duration on the Therapeutic Effect
DISCUSSION
Pneumatic dilation uses external mechanical force to dilate LES, causing a breach of the muscle fibers to achieve the therapy effect. We adopted dilation oriented and monitored by endoscope to treat achalasia without X-rays, and the operation success rate was 97.2% (35/36). During the treatment, tolerable chest pain occurred, which eased spontaneously; the esophageal mucosa were torn, with bleeding, which could stop spontaneously or remit after topical hemostasis. No upper gastrointestinal massive hemorrhage, esophagus perforation, or other serious complications occurred. One patient in this group interrupted the treatment because the dilator could not be inserted because of the tightly closed cardia and softness of the head of the dilator. This type of patients was rare, and these patients should be treated with a harder dilator, such as Sa's dilator.
In total, 35 patients completed the treatment, and their symptoms of dysphasia were eased significantly 6 months after therapy (P < 0.01); these patients remained in remission. During the follow-up of 6 to 120 months after therapy, 6 to 36 months after therapy, 37 to 60 months after therapy, and >60 months after therapy, the patients’ separate symptom scores decreased significantly (P < 0.01). The score at 37 to 60 months increased compared to that at 6 to 36 months (P < 0.01). There was no significant difference between the score at >60 months and at 37 to 60 months (P > 0.05). These results showed that the therapy had a distinct clinical curative effect until 120 months following the procedure. The effects decreased after 36 months, and tended to be stable 60 months after therapy, whereas the symptoms had continuously improved compared with the pretherapy symptoms. In this group, the 6 to 36 months remission rate was 85.7% (30/35), the 37 to 60 months rate was 61.9% (13/21), and the >60 months rate was 40% (2/5). The study found that the dilation effect had no relationship to the patient's age, gender, and duration of the disease (P > 0.05). However, there were only 5 patients at >60 months after therapy, and additional patient data and expansion of the sample size are required to confirm the research.
In this study, a satisfactory therapeutic effect had been achieved. Our experience included the following conclusions: to guarantee the dilation therapy effect, the head of the endoscope should be positioned at the side of the mouth of the pneumatic dilator to monitor its position and the dilation process, whereas the waist of the pneumatic dilator should remain at the cardia; some patients felt tolerable chest pain; there would be no chest pain from the pneumatic air if the pneumatic dilator slips into the stomach or esophagus, and chest pain signaled successful dilation therapy. Some scholars suggest that breaching and bleeding of the esophageal mucosa is a sign of successful dilation therapy. Furthermore, an insufficient pneumatic dilating force would negatively affect the curative effect, and some studies have emphasized that the dilator diameter is central to the effect. Reaching the full diameter of the pneumatic dilator that is tolerable by the patients is recommended. The pressure reached in this case group was 262 to 300 mm Hg. The main complications of pneumatic dilation include perforation and massive hemorrhaging. The reports of the perforation rate ranged from 1% to 10%, with an average of 2.5% whereas no perforation occurred in this group. Massive hemorrhaging or other serious complications did not occur. Most of patients with perforation had original epiphrenic diverticula of the esophagus or a recurrence after a Heller myotomy. Dilation treatment should be applied carefully to these patients. Initial pressure that is excessively high or rapid is a probably cause of perforation. The statistical clinical case reports showed that the initial air pressure was above 300 mm Hg in all the perforation cases. An abrupt increase in abdominal pressure incurred by nausea, hiccups, coughing, and other factors might increase the pneumatic inner pressure and cause perforation. Thus, the initial dilating pressure should not be excessively high and inflation should be conducted slowly. Patients might be injected with anisodamine and diazepam before therapy and kept calm during the procedure. In the event of the above occurrences, air should immediately be released from the pneumatic dilator. Excessively frequent or lengthy periods of inflation were not necessary. Inflation for 1 min, repeated 3 to 5 times, is recommended to avoid cardia mucosa ischemia necrosis and scar formation.
We reached the following conclusions from our operation experience and the results of this long-term observation: the effects of pneumatic dilation were significant; the procedure was simple, with minor injuries, few complications, and a low recurrence rate; and the therapy could be performed on an outpatient basis. The technique enabled endoscopy for the direct observance and monitoring of the mucosal lesions and bleeding during the dilation to stop the bleeding when necessary and reduce the complications.26 The therapy was preferable and had priority in the treatment of achalasia.
Footnotes
Abbreviations: LES = lower esophageal sphincter, POEM = per oral endoscopic myotomy
This work was supported by grants from the Research Foundation of Shanghai Health Bureau, China (No. 20124271).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Lacy BE, Weiser K. Esophageal motility disorders: medical therapy. J Clin Gastroenterol 2016; 42:652–658. [DOI] [PubMed] [Google Scholar]
- 2.Ghoshal UC, Daschakraborty SB, Singh R. Pathogenesis of achalasia cardia. World J Gastroenterol 2016; 18:3050–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pratap N, Kalapala R, Darisetty S, et al. Achalasia cardia subtyping by high-resolution manometry predicts the therapeutic outcome of pneumatic balloon dilatation. J Neurogastroenterol Motil 2016; 17:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farrokhi F, Vaezi MF. Idiopathic (primary) achalasia. Orphanet J Rare Dis 2016; 2:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoogerwerf WA, Pasricha PJ. Achalasia: treatment options revisited. Can J Gastroenterol 2016; 14:406–409. [DOI] [PubMed] [Google Scholar]
- 6.Benini L, Castellani G, Sembenini C, et al. Gastric emptying of solid meals in achalasic patients after successful pneumatic dilatation of the cardia. Dig Dis Sci 2016; 39:733–737. [DOI] [PubMed] [Google Scholar]
- 7.Ponce J, Garrigues V, Pertejo V, et al. Individual prediction of response to pneumatic dilation in patients with achalasia. Dig Dis Sci 2016; 41:2135–2141. [DOI] [PubMed] [Google Scholar]
- 8.Cusumano A, Bonavina L, Norberto L, et al. Early and long-term results of pneumatic dilation in the treatment of oesophageal achalasia. Surg Endosc 2016; 5:9–10. [DOI] [PubMed] [Google Scholar]
- 9.Dohrmann P, Mengel W. Treatment of achalasia by the endoscopic-pneumatic dilatation method. Prog Pediatr Surg 2016; 25:132–137. [DOI] [PubMed] [Google Scholar]
- 10.Matsuo Y, Sugimura F, Seki A. Long-term prognosis of patients with achalasia treated by cardial dilatation therapy. Gastroenterol Jpn 2016; 27:719–727. [DOI] [PubMed] [Google Scholar]
- 11.Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology 2016; 103:1732–1738. [DOI] [PubMed] [Google Scholar]
- 12.Cai XB, Dai YM, Wan XJ, et al. Comparison between botulinum injection and removable covered self-expanding metal stents for the treatment of achalasia. Dig Dis Sci 2016; 58:1960–1966. [DOI] [PubMed] [Google Scholar]
- 13.Moreto M, Ojembarrena E, Barturen A, et al. Treatment of achalasia by injection of sclerosant substances: a long-term report. Dig Dis Sci 2016; 58:788–796. [DOI] [PubMed] [Google Scholar]
- 14.Zhao JG, Li YD, Cheng YS, et al. Long-term safety and outcome of a temporary self-expanding metallic stent for achalasia: a prospective study with a 13-year single-center experience. Eur Radiol 2016; 19:1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponsky JL, Marks JM, Orenstein SB. Retrograde myotomy: a variation in per oral endoscopic myotomy (POEM) technique. Surg Endosc 2016; 28:3257–3259. [DOI] [PubMed] [Google Scholar]
- 16.Charton JP, Schumacher B, Toermer T, et al. The role of peroral endoscopic myotomy (POEM) in achalasia. Zentralbl Chir 2016; 139:58–65. [DOI] [PubMed] [Google Scholar]
- 17.Bredenoord AJ, Rosch T, Fockens P. Peroral endoscopic myotomy for achalasia. Neurogastroenterol Motil 2016; 26:3–12. [DOI] [PubMed] [Google Scholar]
- 18.Katada N, Sakuramoto S, Yamashita K, et al. Comparison of the Heller-Toupet procedure with the Heller-Dor procedure in patients who underwent laparoscopic surgery for achalasia. Surg Today 2016; 44:732–739. [DOI] [PubMed] [Google Scholar]
- 19.Kaman L, Iqbal J, Kochhar R, et al. Laparoscopic Heller myotomy for achalasia cardia-initial experience in a teaching institute. Indian J Surg 2016; 75:391–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alves A, Perniceni T, Godeberge P, et al. Laparoscopic Heller's cardiomyotomy in achalasia. Is intraoperative endoscopy useful, and why? Surg Endosc 2016; 13:600–603. [DOI] [PubMed] [Google Scholar]
- 21.Gockel I, Junginger T, Eckardt VF. Long-term results of conventional myotomy in patients with achalasia: a prospective 20-year analysis. J Gastrointest Surg 2016; 10:1400–1408. [DOI] [PubMed] [Google Scholar]
- 22.Dobrowolsky A, Fisichella PM. The management of esophageal achalasia: from diagnosis to surgical treatment. Updates Surg 2016; 66:23–29. [DOI] [PubMed] [Google Scholar]
- 23.Jankovic J. Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry 2016; 75:951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tank AK, Kumar A, Babu TL, et al. Resectional surgery in achalasia cardia. Int J Surg 2016; 7:155–158. [DOI] [PubMed] [Google Scholar]
- 25.Eckardt VF, Gockel I, Bernhard G. Pneumatic dilation for achalasia: late results of a prospective follow up investigation. Gut 2016; 53:629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katsinelos P, Kountouras J, Paroutoglou G, et al. Long-term results of pneumatic dilation for achalasia: a 15 years’ experience. World J Gastroenterol 2016; 11:5701–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]


