Abstract
Hepatocellular carcinoma (HCC) is one of the most malignant cancers and ranks as the third leading cause of cancer-related death in the world. However, some patients with untreated HCC can experience spontaneous regression, a rare phenomenon that has been observed in various malignancies.
Here, we report a unique case with untreated HCC, who first underwent a spontaneous cancer regression after the spontaneous clearing of chronic hepatitis B virus (HBV) infection from the liver as evidenced by hepatitis B virus surface antigen (HBsAg) seroconversion; then developed the recurrent HCC with epithelial-mesenchymal transition (EMT) after 14 years.
We hypothesized that a strengthened immune system in response to HBV infection may have led to immune-mediated spontaneous cancer regression. The later recurrence of HCC may suggest the host's immune system was no longer able to contain HCC since aging and other chronic diseases may have significantly weakened the immune surveillance.
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignancy worldwide, with increasing incidence. The HCC prognosis is usually bleak.1 For instance, the majority (>70%) of HCC patients are not amenable to curative treatment, and the median overall survival is <1 year.2 However, there are rare incidences of spontaneous cancer regression despite no treatment sought and taken.3
Spontaneous regression was termed by Cole and Everson4 in 1956 as a partial or complete involution of a malignant tumor without any specific therapy being applied. Although spontaneous regression of several different malignancies has been documented, it remains rare and intricate in patients with HCC considering the aggressive malignancy. Here we report a unique and rare case who first experienced a spontaneously complete HCC regression following HBV clearance, and later the HCC recurrence with epithelial–mesenchymal transition (EMT) during 17 years of time span.
CASE PRESENTATION
In January 1997, a 56-year-old man presented with right abdominal discomfort. The patient had a 15-year history of chronic liver disease related to hepatitis B virus (HBV). Serum hepatitis B virus surface antigen (HBsAg) was positive and antihepatitis C virus antibody was negative. Serum a-fetoprotein (AFP) was 446 ng/mL (normal, <20 ng/mL). Abdominal ultrasonography examination detected a solid mass measuring 2 × 2 cm in the right liver. A contrast-enhanced computed tomography (CT) scan confirmed a low-density mass in segment 6 (Figure 1A and B). The above findings were highly suggestive of HCC. An early-stage HCC diagnosis was made and the resection-based curative treatment was recommended. But the patient declined the surgical intervention and any other treatment options for the financial reason.
FIGURE 1.
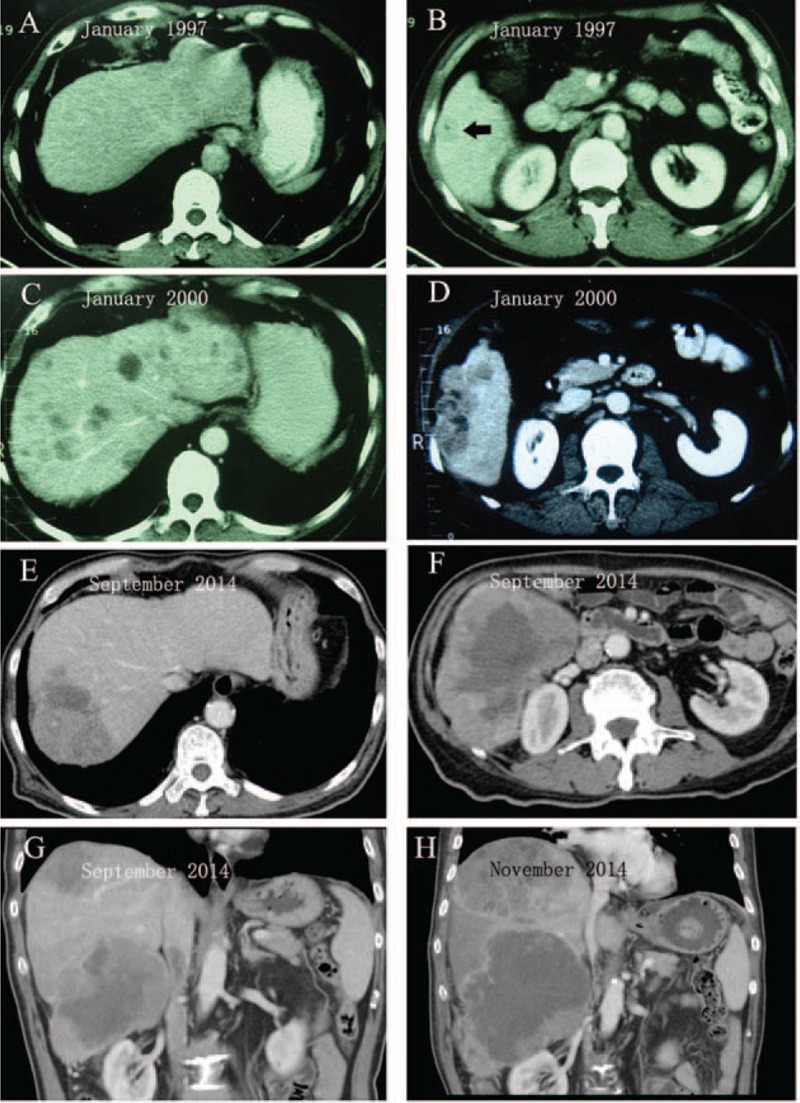
Diagnostic images of the untreated HCC over time as shown by enhanced computed tomography (CT) scan. (A) No lesions in left liver. (B) A low-density mass (black arrow) with 2 cm in size located in segment 6. (C) Multiple small lesions were found in both right and left liver lobes. (D) A heterogeneous hypodense mass 4 cm in size located in segment 6, the same site as in 1997 (B). (E) A heterogeneous hypodense mass located in segment 7. Surprisingly, the multiple small lesions (C) in left liver completely disappeared. (F) A large low-density mass with central necrosis located in segment 6. (G) Coronal image of CT scan showed 2 independent tumors that located in segments 7 and 6, respectively. (H) Coronal image of CT scan showed rapid growth of the 2 tumors. HCC = hepatocellular carcinoma.
Three years later, in January 2000, he was readmitted to the hospital for right abdominal pain. Surprisingly, he became negative for both HBeAg and HBsAg, but positive for anti-HBs, anti-HBe, and anti-HBc. He denied having received any antiviral treatment. Serum AFP was 2100 ng/mL (normal, <20 ng/mL). A new abdominal contrast-enhanced CT scan revealed multiple hepatic masses that were highly suggestive of HCC with multiple intrahepatic metastases (Figure 1C and D). The patient again declined all recommended palliative treatment. One month after discharge, his abdominal pain gradually disappeared. Six months later in July 2000, he visited the hospital for checkup. Surprisingly, no hepatic masses were detected by the ultrasound.
Fourteen years later, this 73-year-old patient represented with increasing abdominal distension and pain in September 2014. Results of blood analysis at the time of admission showed white blood cell count, 26.5 × 109/L (77.0% neutrophils) (normal, 4.0–10.0 × 109/L); hemoglobin count, 110 g/L (normal, 120–150 g/L); platelet count, 112 × 109/L (normal, 100–300 × 109/L); aspartate aminotransferase (AST) 43 IU/L (normal, <40 IU/L); alanine aminotransferase (ALT) 35 IU/L (normal, <40 IU/L); albumin, 25 g/L (normal, 35–50 g/L); total bilirubin, 0.9 mg/dL (normal, 0.1–1.0 mg/dL); direct bilirubin, 0.3 mg/dL (normal, 0–0.4 mg/dL). Serum HBsAg and hepatitis C virus antibody were negative. Serum tumor markers were >1210 ng/mL (normal, <7 ng/mL) for AFP, 2.5 ng/mL (normal, <3.4 ng/mL) for carcinoembryonic antigen (CEA), and 19.73 U/mL (normal, <27U/mL) for CA19-9, respectively. Contrast-enhanced CT showed 2 huge (7 ×5 and 10 × 6 cm), and heterogeneous masses in segments 7 and 6 of the liver (Figure 1E–G), respectively. The original multiple masses in the left liver lobe completely disappeared (Figure 1E). Ultrasonography-guided biopsies revealed 2 different tumor cell populations. The tumor tissue in segment 7 consisted of poorly differentiated cancerous parenchymal hepatocytes (Figure 2A, HE) whereas the tumor in segment 6 was composed of spindle-shaped cells, mesenchymal cells (Figure 2B, HE). In addition, lots of inflammatory cells could be observed in both the tumor sites.
FIGURE 2.
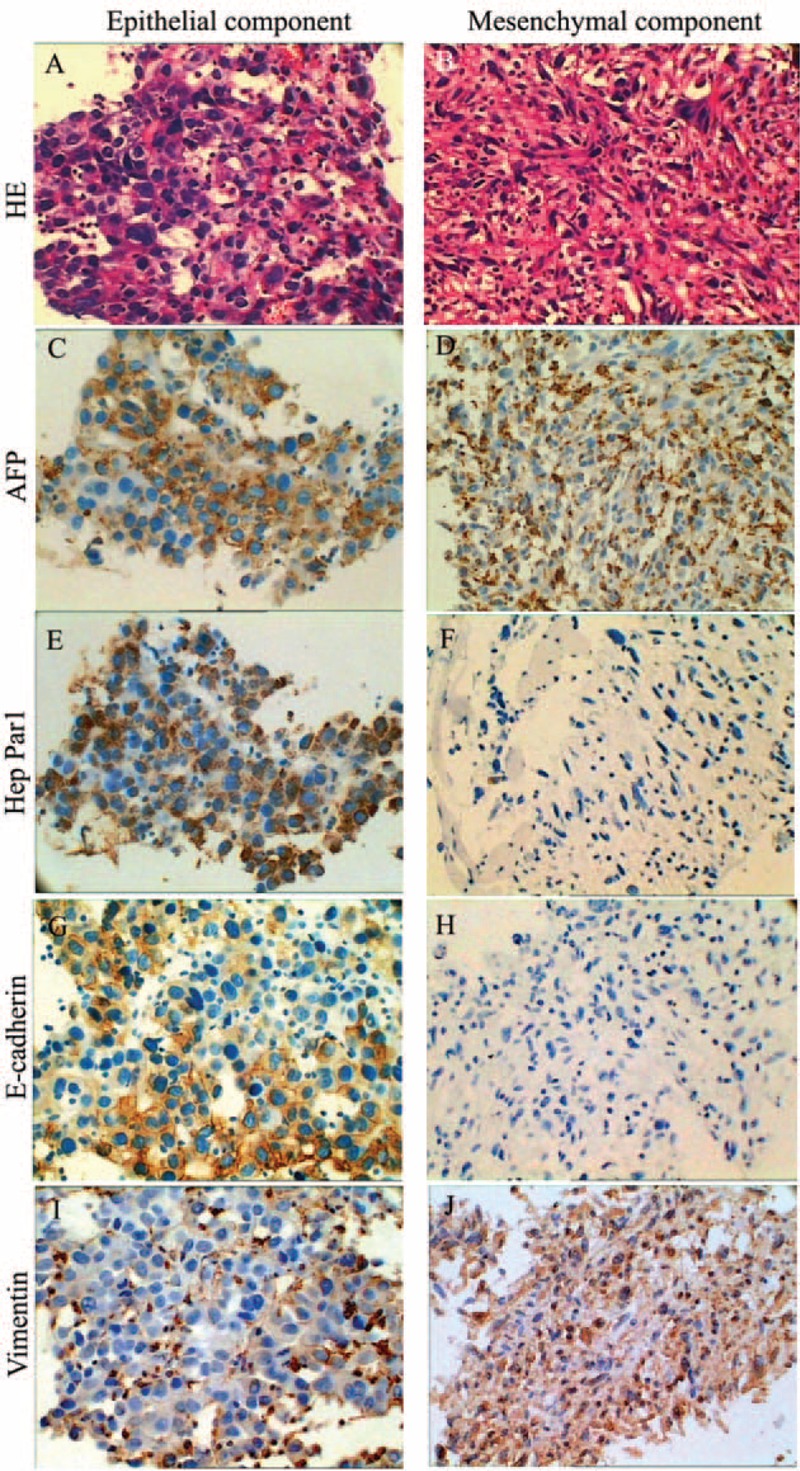
Pathological and immunohistochemical analysis of tumor biopsy from 2 segments. (A) Biopsy revealed poorly differentiated HCC in segment 7. (B) Spindle-shaped cells consisted of a mesenchymal component in the tumor in segment 6. (C)–(J) Tumor cells in both sections were diffusely positive for AFP (C and D). The epithelial component was focally positive for Hep Par1 (E), E-cadherin (G), and vimentin (I). The mesenchymal section was negative for Hep Par1 (F) and E-cadherin (H), and diffusely positive for vimentin (J). AFP = serum a-fetoprotein, HCC = hepatocellular carcinoma.
Tumor cell phenotypes were further analyzed with immunohistochemistry (Figure 2C–J). Strongly positive GPC3 and AFP staining that was in a diffuse distribution pattern in the cytoplasm was detected in both the tumor sections; but while the poorly differentiated parenchymal cells were focally positive for E-cadherin, Hep Par1, and vimentin, the mesenchymal cells was negative for E-cadherin and Hep Par1, and diffusely positive for vimentin. N-cadherin staining was negative on both the sections. In addition, the mesenchymal cells showed a strong positivity for CK18 and negative staining for SMA, CD117, desmin, S100, and CD34. Thus, the tumor in the segment 6 was diagnosed as HCC with sarcomatous change. The patient only accepted symptom-relieving treatment and then discharged himself from the hospital.
In November 2014, the patient was readmitted to our hospital for persistent abdominal pain and a palpable abdominal mass. A latest CT scan (Figure 1H) revealed that the 2 liver masses had significantly increased in size in an interval of 3 months, as measured in maximum axial diameter from 7 to 11 cm (in segment 7) and 10 to 15 cm (in segment 6). The 2 tumors were merged together as a result of rapid growth, which was already occupying nearly the entire right lobe. The patient reached the terminal stage of HCC and received the supportive treatment. He shortly died of aggressive tumor progression that rapidly deteriorated his general conditions.
The case study was approved by the Ethics Committee of PLA 401 Hospital, China. In addition, informed consent was obtained from the patient's son before data collection.
DISCUSSION
Despite the advances in the HCC diagnosis and treatment techniques, the prognosis of HCC remains poor. Median survivals of untreated HCC patients as analyzed by Barcelona Clinic Liver Cancer stages decline progressively as the tumor stage advances. For instance, stage A averages 33 months; B, 17.4 months; C, 6.9 months; and D, 1.8 months.5 However, aggressive HCC does undergo spontaneous regression though rarely. Our case declined both curative and palliative treatment on 2 occasions for financial reason, but survived for more than a decade until recent HCC rebout. The only reasonable explanation for his extremely long survival is the spontaneous regression. There was clear evidence supporting this conclusion. No hepatic masses were found again by the ultrasound in July of 2000 after the multitumors were identified by CT in January of 2000, and the patient had been in the remission for 14 years until September of 2014 when 2 huge hepatic tumors were identified in his right lobe, which were different from the multicancers identified in January of 2000. To date, over 80 cases of apparently spontaneous regression of HCC have been reported in the literature.3,6 However, the underlying mechanisms of this phenomenon are much unknown. A literature review conducted by Huz et al3 in 2012 suggested that the most common factor associated with the spontaneous regression was a systemic inflammatory response including cholangitis, trauma, and elevated cytokine levels. Pathological evidence of inflammatory response for spontaneous regression was also observed. Hicks et al7 showed that spontaneous regression in mice was accompanied by rapid infiltration of leukocytes. And Park et al8 reported a regressing HCC with massive lymphoid infiltration. But it is unclear whether the observed inflammation was the cause of regression or represented a response to the necrosis of tumor cells.
We do not know for sure what triggered the spontaneous regression in our case. Previous reports suggested various factors, such as tumor necrosis,9 long-lasting fever,10 and herbal medicine11 that were associated with spontaneous regression of HCC. In addition, several reports have shown that HCC patients received some palliative treatments such as transarterial chemoembolization (TACE)12 and radiotherapy13 before spontaneous regression occurred. In contrast, our case received neither anticancer drugs nor interventional procedures prior to the regression. But it is worth noting that chronic HBV infection was cleared as evidenced by spontaneous HBsAg seroconversion before the occurrence of spontaneous regression. Spontaneous HBsAg seroconversion is marked by disappearance of HBsAg and appearance of anti-HBs antibody in the serum that is usually preceded by loss of detectable HBVDNA and HBeAg. The frequency of conversion is about 0.49% to 2.26% per year in untreated chronic HBV-infected patients, which is considered as a sign for terminating HBV infection.14,15 Thus, a spontaneous HBsAg seroconversion indicates a strengthening host immune system, which may have contributed to the process of spontaneous regression of HCC.
Chronic HBV infection is associated with over 50% of the HCC patients worldwide and this association is increased to >80% in hyperendemic, especially in China.16 The evidence has shown that HBV antigen might serve as one of the tumor-associated antigens of HCC.17 A robust T-cell response was related to successful control of HBV infection and elimination of HCC expressing HBV antigens.18 However, HBV-specific T cells are usually deleted or dysfunctional in patients with chronic HBV infection or HBV-related HCC.19 Infusing of genetically modified T cells could reconstitute virus-specific T-cell immunity in chronic HBV patients and target tumor cells in HBV-related HCC.20 Therefore, both the spontaneous HBsAg seroconversion and the HCC regression could be triggered by restoration or reinforcement of virus-specific T-cell immunity in our case. It is the first time that a spontaneous tumor regression following spontaneous HBsAg seroconversion was observed in a patient with HCC.
Remarkable increases of white blood cell count and inflammatory cell infiltration in the liver were also observed, which might indicate the thriving host–immune response to the outgrowing tumor in our case. Obviously, the immune system was unable to contain the recurrent HCC progression. A tumor model suggests a dynamic balance between cancer immune surveillance and tumor recurrence/progression during spontaneous regression,21 implying a weakened surveillance may tip the balance toward to the recurrence. In addition, Wilkie et al22 reported that tumor-immune dynamics that was regulated in the microenvironment informed the transient nature of immune-induced tumor dormancy. We can further suggest that spontaneous regression may represent a special status of long-lasting immune-induced tumor dormancy,23,24 not necessarily a complete clearance of the tumor cells in the liver. Therefore, tumor recurrence after spontaneous regression may occur once the balance between host immune system and potential activation of tumor cell growth/division was shifted to lift the previous restriction on expansion of tumor cells, as a consequence of deteriorating general conditions in the aged patient. Our case with 2 distinct episodes of regression and recurrence is consistent with the report that also observed the recurrence of HCC with rapid growth after spontaneous regression.25
Another rare sarcomatous change was also observed in our case. Immunohistochemical analysis showed that sarcomatous components were negative for E-cadherin but diffusely stained with vimentin as well as AFP and CK18. These findings suggest that the detected sarcomatous component could be arisen from the dedifferentiation process of HCC, which is known as EMT. EMT is a process by which an epithelial cell undergoes multiple biochemical changes as it is transformed into a mesenchymal cell phenotype.26 Mobility and the invasive predisposition of cancer cells could be enhanced by EMT both in in vivo and in vitro. A sudden acceleration of disease progression was observed during last 3 months. Clinical manifestations in our case were in accordance with the previous reports that EMT is usually associated with very aggressive tumor behavior.27 Recently, EMT was thought to be as pluripotent as stem cell.28 EMT cells are hypothesized as founder cells of metastases and then disseminating cancer cells can revert back to an epithelial phenotype (mesenchymal–epithelial transition, MET) to establish a full-blown metastasis in distant site.29 Thus, the tumor with low-grade differentiation in segment 7 may derive from an intrahepatic metastasis of the recurred tumor with EMT in segment 6. However, multicentral hepatocarcinogenesis cannot be ruled out.
CONCLUSIONS
In summary, our case presented distinct sequential episodes of HCC with extremely long natural history, which included an initial small HCC, lately advanced HCC with multiple intrahepatic metastases, 14 years of spontaneous regression, and recurrent HCC. Furthermore, 2 additional rare events, spontaneous HBsAg seroconversion and EMT, were also observed. The reason to draw our attention from this case is that it provides valuable clue to researchers who are trying to identify and validate better therapy strategy and therapeutics to treat HCC. Further studies are warranted to explore the roles of immunity (innate and adaptive) in the process of spontaneous regression and the underlying mechanisms.
Footnotes
Abbreviations: AFP = serum a-fetoprotein, ALT = alanine aminotransferase, AST = aspartate aminotransferase, CEA = carcinoembryonic antigen, CT = computed tomography, EMT = epithelial–mesenchymal transition, HBsAg = hepatitis B virus surface antigen, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, TACE = transarterial chemoembolization.
Yang SZ and Zhang W contributed equally to this work.
This study was supported by the National S&T Major Project for Infectious Diseases of China (NO.2012ZX10002-017).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.EI-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142:1264–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang JF, Shu ZJ, Xie CY, et al. Prognosis of unresectable hepatocellular carcinoma: comparison of seven staging systems (TNM, Okuda, BCLC, CLIP, CUPI, JIS, CIS) in a Chinese cohort. PLoS One 2014; 9:e88182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huz JI, Melis M, Sarpel U. Spontaneous regression of hepatocellular carcinoma is most often associated with tumour hypoxia or a systemic inflammatory response. HPB (Oxford) 2012; 14:500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole WH, Everson TC. Spontaneous regression of cancer: preliminary report. Ann Surg 1956; 144:366–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabibbo G, Maida M, Genco C, et al. Natural history of untreatable hepatocellular carcinoma: a retrospective cohort study. World J Hepatol 2012; 4:256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim DH, Park KW, Lee SI. Spontaneous complete regression of multiple metastases of hepatocellular carcinoma: a case report. Oncol Lett 2014; 7:1225–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hicks AM, Riedlinger G, Willingham MC, et al. Transferable anticancer innate immunity in spontaneous regression/complete resistance mice. Proc Natl Acad Sci USA 2006; 103:7753–7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park HS, Jang KY, Kim YK, et al. Hepatocellular carcinoma with massive lymphoid infiltration: a regressing phenomenon? Pathol Res Pract 2009; 205:648–652. [DOI] [PubMed] [Google Scholar]
- 9.Uenishi T, Hirohashi K, Tanaka H, et al. Spontaneous regression of a large hepatocellular carcinoma with portal vein tumor thrombi: report of a case. Surg Today 2000; 30:82–85. [DOI] [PubMed] [Google Scholar]
- 10.Markovic S, Ferlan-Marolt V, Hlebanja Z. Spontaneous regression of hepatocellular carcinoma. Am J Gastroenterol 1996; 91:392–393. [PubMed] [Google Scholar]
- 11.Chien RN, Chen TJ, Liaw YF. Spontaneous regression of hepatocellular carcinoma. Am J Gastroenterol 1992; 87:903–905. [PubMed] [Google Scholar]
- 12.Takeda Y, Togashi H, Shinzawa H, et al. Spontaneous regression of hepatocellular carcinoma and review of literature. J Gastroenterol Hepatol 2000; 15:1079–1086. [DOI] [PubMed] [Google Scholar]
- 13.Nam SW, Han JY, Kim JI, et al. Spontaneous regression of a large hepatocellular carcinoma with skull metastasis. J Gastroenterol Hepatol 2005; 20:488–492. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Yang H, Lee MH, et al. Incidence and determinants of spontaneous Hepatitis B surface antigen seroclearance: a community-based follow-up study. Gastroenterology 2010; 139:474–482. [DOI] [PubMed] [Google Scholar]
- 15.Yuen MF, Hui CK, Cheng CC, et al. Long-term follow-up of interferon alfa treatment in Chinese patients with chronic hepatitis B infection: The effect on hepatitis B e antigen seroconversion and the development of cirrhosis-related complications. Hepatology 2001; 34:139–145. [DOI] [PubMed] [Google Scholar]
- 16.Pujol FH, Navas MC, Hainaut P, et al. Worldwide genetic diversity of HBV genotypes and risk of hepatocellular carcinoma. Cancer Lett 2009; 286:80–88. [DOI] [PubMed] [Google Scholar]
- 17.Gotsman I, Alper R, Klein A, et al. Induction oral immune regulation of hepatitis B virus envelope proteins suppresses the growth of hepatocellular carcinoma in mice. Cancer 2002; 94:406–414. [DOI] [PubMed] [Google Scholar]
- 18.Chun E, Lee J, Cheong HS, et al. Tumor eradication by hepatitis B virus X antigen-specific CD8+ T cells in xenografted nude mice. J Immunol 2003; 170:1183–1190. [DOI] [PubMed] [Google Scholar]
- 19.Gehring AJ, Ho ZZ, Tan AT, et al. Profile of tumor antigen-specific CD8 T cells in patients with hepatitis B virus-related hepatocellular carcinoma. Gastroenterology 2009; 137:682–690. [DOI] [PubMed] [Google Scholar]
- 20.Gehring AJ, Xue SA, Ho ZZ, et al. Engineering virus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J Hepatol 2011; 55:103–110. [DOI] [PubMed] [Google Scholar]
- 21.Hsiao YW, Liao KW, Chung TF, et al. Interactions of host IL-6 and IFN-γ and cancer-derived TGF-β1 on MHC molecule expression during tumor spontaneous regression. Cancer Immunol Immunother 2008; 57:1091–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkie KP, Hahnfeldt P. Tumor-immune dynamics regulated in the microenvironment inform the transient nature of immune-induced tumor dormancy. Cancer Res 2013; 73:3534–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herishanu Y, Solar I, Ben-Ezra J, et al. Complete spontaneous regression of chronic lymphocytic leukemia. J Clin Oncol 2012; 30:e254–e256. [DOI] [PubMed] [Google Scholar]
- 24.Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell 2013; 155:750–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakajima T, Moriguchi M, Watanabe T, et al. Recurrence of hepatocellular carcinoma with rapid growth after spontaneous regression. World J Gastroenterol 2004; 10:3385–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seok JY, Na DC, Woo HG, et al. A fibrous stromal component in hepatocellular carcinoma reveals a cholangiocarcinoma-like gene expression trait and epithelial-mesenchymal transition. Hepatology 2012; 55:1776–1786. [DOI] [PubMed] [Google Scholar]
- 28.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133:704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brabletz T. To differentiate or not: routes towards metastasis. Nat Rev Cancer 2012; 12:425–436. [DOI] [PubMed] [Google Scholar]


