Abstract
To assess the prognostic value of primary tumor metabolic activity in patients with high-grade bone sarcomas (BS) or soft tissue sarcomas (STS) using F-18 FDG PET/CT.
A single-site, retrospective study including 92 patients with high-grade BS or STS. Pretreatment F-18 FDG PET/CT scan was performed. Clinical data were registered. Accuracy of maximum standardized uptake value of primary tumor (SUVmax) and tumor-to-background (T/B) uptake ratio as prognostic variables and identification of cut-off values to group patients were determined. Kaplan–Meier survival estimates and log-rank test were used to compare survival distributions. Prognostic variables were assessed using Cox proportional hazards regression analysis.
Forty-one of 92 patients died during follow-up (45%). Average survival was 6.5 years (95% CI 5.8–7.3 years) and probability of 5-year survival was 52%. Accuracy of SUVmax and T/B uptake ratio as prognostic variables in all patients and during subgroup analysis of patients with STS was significant. No significant results for AUCs were registered in patients with BS. Surgery was independently prognostic for survival throughout multivariate regression analysis of all patients (P = 0.001, HR 3.84) and subgroup analysis (BS: P = 0.02, HR 11.62; STS: P = 0.005, HR 4.13). SUVmax was significant as prognostic variable in all patients (P = 0.02, HR 3.66) and in patients with STS (P = 0.007, HR 3.75). No significant results were demonstrated for T/B uptake ratio.
Estimation of primary tumor metabolic activity with pretherapeutic SUVmax using F-18 FDG PET/CT demonstrates independent properties beyond histologic grading for prediction of survival in patients with high-grade STS, but not with high-grade BS.
INTRODUCTION
Bone and soft-tissue sarcomas (BS and STS) are a diverse group of malignant mesenchymal tumors. Sarcomas are rare, only comprising approximately 1% of all cancers.1 However, the diversity of these tumors in terms of histology, aggressiveness, and clinical course1–3 poses challenges in the diagnostic work-up and treatment, with reported 5-year mortality rates as high as 50%.4 With this in mind, the importance of proper staging of disease becomes obvious, as it helps define the prognosis for patients, helps guide their treatment, and allows meaningful comparisons to be done among groups of patients. Both the Musculoskeletal Tumor Society (MSTS)5 and the American Joint Committee of Cancer (AJCC) staging system6,7 for malignant primary bone and soft-tissue lesions are widely accepted, providing prognostic information. Both systems take features of tumor including tumor grade, nodal status, and metastasis to distant organs into account, but require postoperative input of histological data. Also, the substantial diversity in clinical outcome even within the same tumor grade is another issue to address. Consequently, a reliable method to make a preoperative prediction of the disease course supplemental to histological characteristics is warranted.
Traditional anatomical imaging modalities, such as magnetic resonance imaging (MRI) and computed tomography (CT), have limited properties in terms of assessing tumor behavior, which being its biological activity or its potential metastatic course. Consequently, functional imaging with positron emission tomography (PET) – especially with the fluorine-18 radiolabeled glucose analog fluoro-2-deoxy-d-glucose (F-18 FDG) – has emerged as an important imaging modality in the assessment of patients with sarcoma, as it allows noninvasive, three-dimensional visualization, and quantification of tumor glucose metabolism in vivo.8,9 There are several methods for quantifying FDG uptake in tumors on acquired PET data. Being easy accessible parameters, the application of the maximum standardized uptake value (SUVmax) normalized to body weight and tumor-to-background (T/B) uptake ratio has gained popularity.
In general, clinical evidence in sarcoma research – including the application of semiquantitative calculations of tumor FDG uptake – suffers from the low incidence of tumors as well as high intra- and intertumoral heterogeneity in terms of histological features of cellular proliferation, necrosis, noncellular accumulations, and physiological characteristics.10 Even though most studies are retrospective, include few patients and mixed populations, pretreatment estimation of SUVmax of the primary tumor in sarcoma patients has been suggested being a significant prognostic factor for overall and progression-free survival.11–19 However, the literature on the subject is sparse and is even sparser regarding the prognostic value of T/B uptake ratio on F-18 FDG PET in sarcoma patients. Consequently, despite the recognition of the potential benefits of F-18 FDG PET in staging, treatment response evaluation, and oncological outcomes, it has proven difficult to standardize the implementation of this imaging modality in the diagnostic work-up and follow-up of patients with sarcoma.20,21 The present study compares the prognostic value of different methods of semiquantitative calculations of primary tumor metabolic activity using F-18 FDG PET/CT in the initial assessment of a specified group of patients with histologically verified high-grade bone or soft-tissue sarcoma.
METHODS
Study Population and Design
A single-site, retrospective study from July 1, 2002 to December 31, 2012 including 92 consecutive patients (47 males; 45 females; median age 49.8 (11.2–86.3) years; Table 1) referred for further evaluation and/or surgical treatment according to the following criteria: first, histologically verified high-grade BS (N = 37) or STS (N = 55) according to either the French Federation of Cancer Centers Sarcoma Group (FNCLCC) grading system22 or the grading recommendations of the College of American Pathologists,23 second, no previous history of malignancy, third, underwent an onsite preoperative F-18 FDG PET/CT scan for staging, and fourth, minimum follow-up period of 1 year for survived patients. Medical records, imaging examinations, and histopathology were reviewed. The histological classification of the included bone sarcomas was as follows: osteosarcoma N = 20, Ewing sarcoma N = 6, chondrosarcoma N = 5, others N = 6. Regarding the included soft tissue sarcomas the histological subtypes were distributed as follows: myogenic sarcoma N = 16, synovial sarcoma N = 9, malignant peripheral nerve sheath tumor N = 6, liposarcoma N = 5, angiosarcoma N = 4, myxofibrosarcoma N = 3, undifferentiated pleomorphic sarcoma N = 2, and others N = 10. Only patients with Ewing sarcoma/primitive neuroectodermal tumor (PNET) and osteosarcoma received preoperative chemotherapy (these patients also received postoperative chemotherapy). The treatment protocol, which also included radiotherapy for prevention of local recurrence in patients with marginal or intralesional tumor resection, was not changed during the study period. The study was approved by the Danish Data Protection Agency (journal number 2011-41-5734).
TABLE 1.
Clinical Characteristics
F-18 FDG PET/CT: Acquisition and Analysis
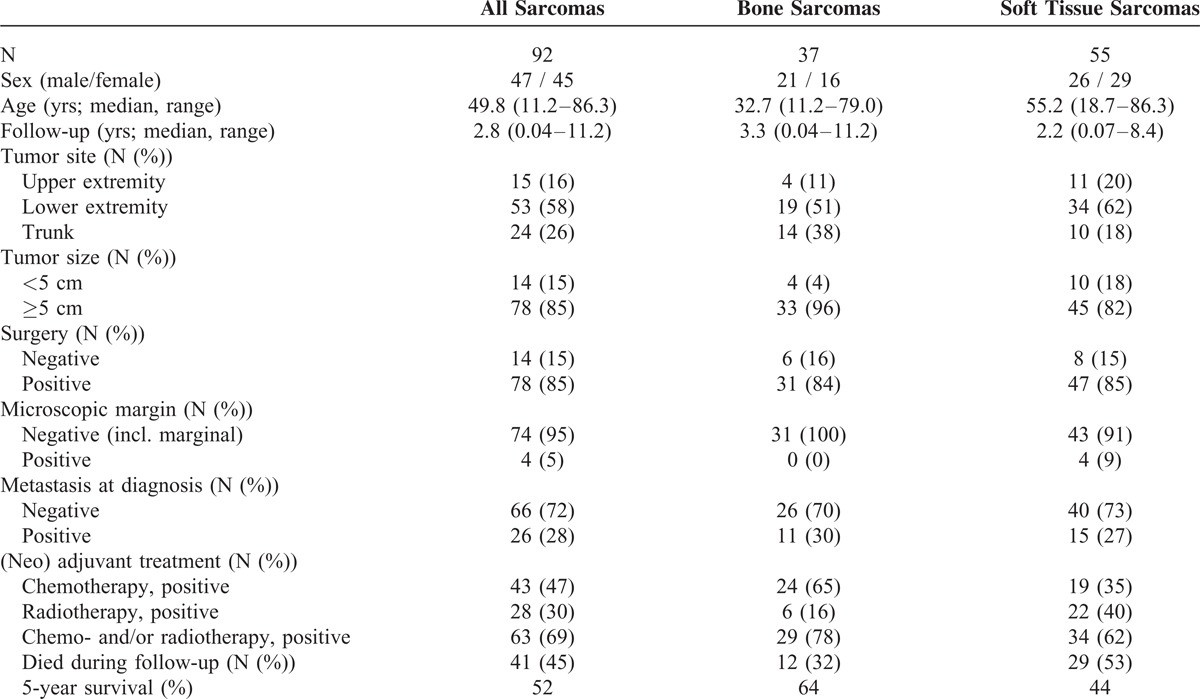
Routine F-18 FDG PET/CT was performed with dedicated PET/CT scanners (GE Discovery LS, GE Healthcare, Waukesha, WI; Siemens Biograph Sensation 16, Siemens Biograph 40 TruePoint, Siemens Biograph 64 TruePoint, Siemens Biograph mCT-S 64, Siemens Medical Solutions, Knoxville, TN) according to the European Association of Nuclear Medicine procedure guidelines for tumor PET imaging,24 which stresses the importance of standardization of patient preparation including factors affecting plasma glucose levels and FDG plasma clearance. Patients fasted for a minimum of 6 h before FDG injection. Before June 1, 2010 a dose of 400 MBq (10.8 mCi) F-18 FDG was injected intravenously 60 min before the scan; hereafter, a dose of 4 MBq (0.108 mCi)/kg body weight was used. The PET emission scan was performed for 2.5– 5 min per bed position depending on the scanner type and the body mass index of the patients. Patients were scanned from the base of the skull to the distal side of the tumor, at least including the proximal femora. Covering the same area, a contrast-enhanced CT was performed (500 mL Ioxitalamat solution 12.6 mg/mL administered orally 30 min before the scan and intravenous Optiray 300 mg/mL at 1.5–2.5 mL/s with a delay of 60–80 s). CT data were used for attenuation correction of the PET emission data. No separate low-dose CT scan for attenuation correction was performed in order to reduce the risk of movement artifacts due to a prolonged examination time.25 The acquired PET and CT data were reconstructed in 3 dimensions. During the study period our institution switched from a standard iterative reconstruction (AW-OSEM: 4 iterations and 8 subsets with 4 mm Gaussian postfilter) of PET images toward using a point spread function reconstruction (3 iterations and 21 subsets followed by a 2 mm Gaussian postfilter). Images (PET, CT, and fused) were reviewed applying a dedicated workstation and software (syngo.via, Siemens Healthcare, Forchheim, Germany). Interpretations were done by a specialist in nuclear medicine and a specialist in diagnostic radiology in consensus. A volume of interest (VOI) was drawn including primary tumor, and SUV normalized to body weight was calculated as the VOI activity (MBq/mL)/((injected dose (MBq)/body weight (g)). SUVmax was the single maximum pixel value in the VOI. For calculation of T/B uptake ratio, SUVmax was determined in a 1 cm3 VOI located in tumor-free femoral muscular tissue on the contralateral side of the primary tumor, and T/B uptake ratio was defined as SUVmax in primary tumor/SUVmax in background tissue (Figure 1).
FIGURE 1.
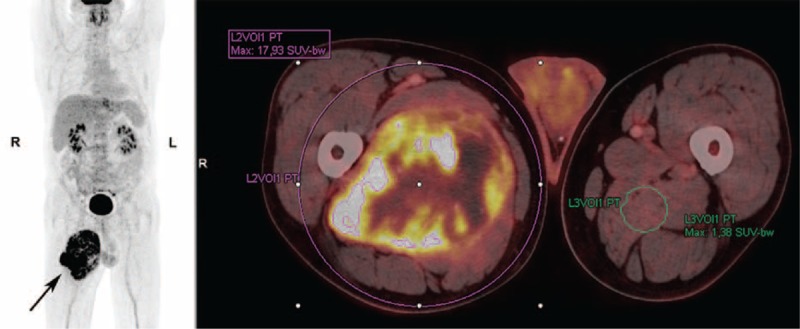
53-year-old male with high-grade soft tissue sarcoma (pleomorphic liposarcoma; tumor grade III (FNCLCC grading system)) located in the adductor musculature on the right femur (arrow). The patient underwent a preoperative F-18 FDG PET/CT scan for staging, which showed no metastatic disease. For semiquantitative calculations of tumor and background-tissue metabolic activity, volumes-of-interest were drawn on the acquired PET-images, and SUVmax of the primary tumor and T/B uptake ratio was determined. CT = computed tomography; FNCLCC = French Federation of Cancer Centers Sarcoma Group; PET = positron emission tomography; SUVmax = maximum standardized uptake value.
Clinical Endpoints
Overall survival was set as clinical endpoint. Survival time was defined as the period from the date of the preoperative F-18 FDG PET/CT scan to the date of death; for survived patients the date on which data regarding patient survival were obtained from the Danish Centralized Civil Register (April 11, 2014).
Statistics
Statistical analyses were performed using IBM SPSS Statistics 19.0 (SPSS Inc., IBM, Somers, NY) and MedCalc version 12.7.1.0 (MedCalc Software, Ostend, Belgium) on data registered in the following groups: all included patients, patients with BS, and patients with STS. Measurements of the accuracy of SUVmax and T/B uptake ratio as prognostic variables and identification of optimal discriminating cut-off values were performed through receiver operating characteristic (ROC) curve analysis. Patients were grouped according to the cut-off values. All deaths were considered an event in the survival analysis and the patients were censored at the end of their follow-up. Kaplan–Meier survival estimates and the log-rank test were used to compare the degree of equality of survival distributions. Prognostic variables with related hazard ratios (HR) were assessed applying Cox proportional hazards regression analysis. The following categorical variables were included: sex, tumor size, surgery, metastasis at diagnosis, SUVmax, and T/B uptake ratio. P-values <0.05 were considered statistically significant.
RESULTS
Clinical characteristics are presented in Table 1. Median follow-up period was 2.8 years (range 0.04–11.2 years). A total of 41 of 92 patients died during follow-up (45%; 12 BS and 29 STS patients). Average survival for all included patients was 6.5 years (95% CI 5.8–7.3 years) and the probability of 5-year survival was 52%. The probability of 5-year survival and average survival for patients with BS was 64% and 7.8 years (95% CI 6.2–9.4 years), while being 44% and 4.5 years (95% CI 3.5–5.5 years) for patients with STS.
ROC Curve Analysis
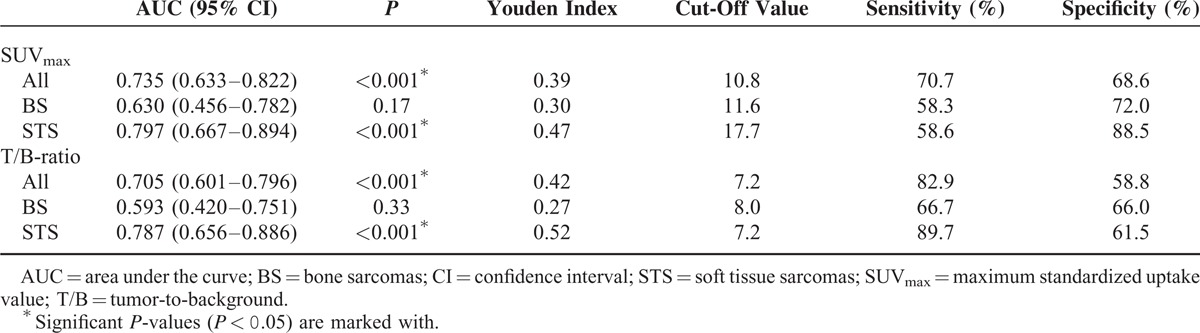
ROC curve analysis of overall survival with area under the curve (AUC) data and optimal cut-off values are presented in Figure 2 and Table 2. The AUC for SUVmax was greater than for T/B uptake ratio when looking on all patients (0.735 vs. 0.705) as well as during subgroup analysis of patients with BS (0.630 vs. 0.593) or STS (0.797 vs. 0.787) only. The AUCs were significant for SUVmax as well as T/B uptake ratio in the groups including all patients and patients with STS. No significant results for the estimated AUC were registered in subgroup analysis of patients with BS.
FIGURE 2.
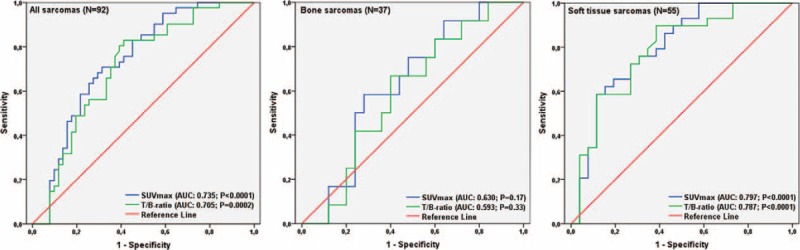
ROC curve analysis of all included patients (N = 92) and subgroups of patients with BS (N = 37) or STS (N = 55), with measurements of the accuracy of SUVmax of the primary tumor and T/B uptake ratio as prognostic variables. BS = bone sarcoma; ROC = receiver operating characteristic; STS = soft tissue sarcoma; SUVmax = maximum standardized uptake value.
TABLE 2.
ROC Curve Analysis
Kaplan–Meier Survival Estimates
Kaplan–Meier survival data for ungrouped data and grouped data according to the estimated optimal cut-off values are presented in Figure 3 and Table 3. When dividing all patients into 2 groups below and above cut-off value for SUVmax (10.8), 12 out of 47 and 29 out of 45 patients died during follow-up, respectively. Probabilities of 5-year survival were 71% and 29%, and average survival was 8.6 years (95% CI 7.3–9.8 years) and 3.1 years (95% CI 2.2–4.0 years) in the 2 groups (P < 0.001). When dividing all patients into 2 groups below and above cut-off value for T/B uptake ratio (7.2), 7 out of 37 and 34 out of 55 patients died during follow-up. Probabilities of 5-year survival were 78% and 33%, and average survival was 9.2 years (95% CI 7.9–10.5 years) and 4.6 years (95% CI 3.3–6.0 years), respectively (P < 0.001).
FIGURE 3.
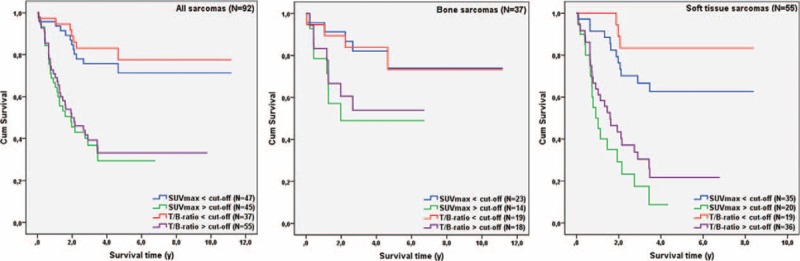
Kaplan–Meier survival curves for all included patients (N = 92) and subgroups of patients with BS (N = 37) or STS (N = 55). Data were grouped according to the optimal discriminating cut-off value for SUVmax of the primary tumor and T/B uptake ratio determined with ROC curve analysis. BS = bone sarcoma; ROC = receiver operating characteristic; STS = soft tissue sarcoma; SUVmax = maximum standardized uptake value.
TABLE 3.
Survival Data (Ungrouped and Grouped Data (Above/Below Optimal Cut-Off Value))
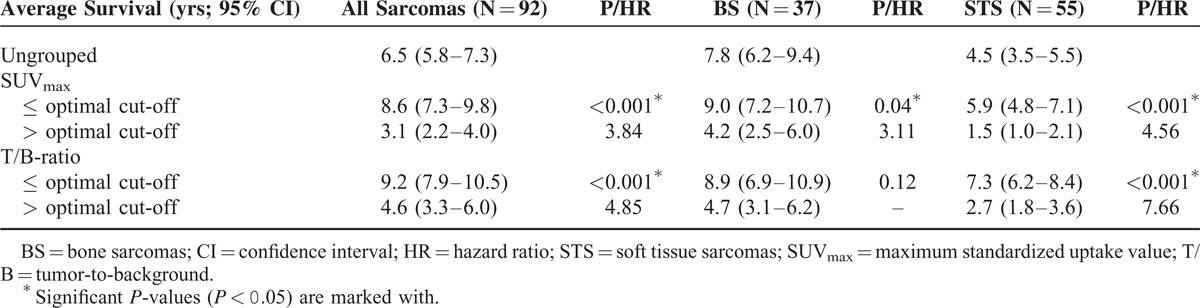
When analyzing data from patients with BS, 5 out of 23 and 7 out of 14 patients died during follow-up, when patients were grouped according to optimal cut-off value for SUVmax (11.6). Estimated 5-year survival was 74% below cut-off and 49% above, with an average survival of 9.0 years (95% CI 7.2–10.7 years) and 4.2 years (95% CI 2.5–6.0 years), respectively (P = 0.04). No significant differences were registered during subgroup analysis of patients with BS when they were grouped according to the optimal discriminating cut-off value for T/B uptake ratio (8.0).
In subgroup analysis of patients with STS probabilities of 5-year survival, when data were grouped below (12 out of 35 patients died during follow-up) and above (17 out of 20 patients died during follow-up) the optimal cut-off values for SUVmax (17.7) were 63% and 9%, respectively. Significant differences in average survival were registered, as average survival was 5.9 years (95% CI 4.8–7.1 years) below the cut-off value and 1.5 years (95% CI 1.0–2.1 years) above (P < 0.001). Regarding T/B uptake ratio, probabilities of 5-year survival below and above the optimal cut-off value (7.2) were 83% and 22%. Three out of 19 and 26 out of 36 patients died during follow-up, respectively. Average survival was 7.3 years (95% CI 6.2–8.4 years) below cut-off and 2.7 years (95% CI 1.8–3.6 years) above (P < 0.001).
Prognostic Values of SUVmax and T/B Uptake Ratio
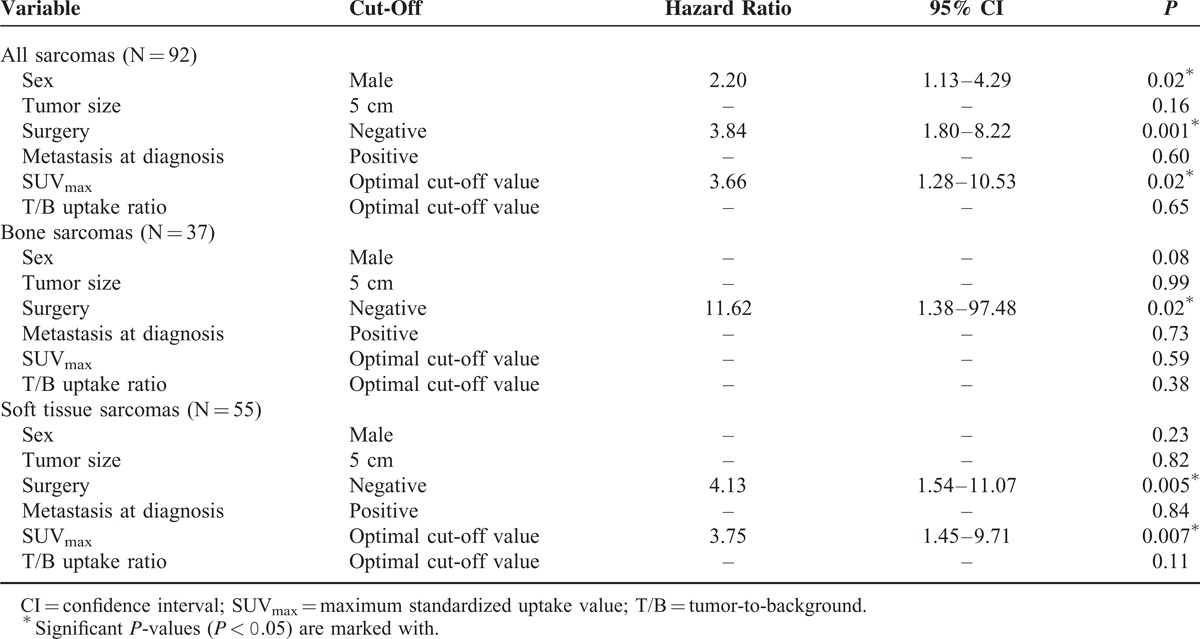
Data from Cox proportional hazards regression analysis with variables affecting overall survival in multivariate analyses are presented in Table 4. Performed surgery was the only variable which was significant as a prognostic variable for survival throughout analysis of all included patients (P = 0.001; HR: 3.84) as well as subgroup analysis of patients with BS (P = 0.02; HR: 11.62) or STS (P = 0.005; HR: 4.13). While no significant results were demonstrated for T/B uptake ratio, SUVmax of the primary tumor above optimal discriminating cut-off value was significant as prognostic variable when analyzing all included patients (P = 0.02; HR: 3.66) and during subgroup analysis of patients with STS (P = 0.007; HR: 3.75).
TABLE 4.
Variables Affecting Overall Survival in Multivariate Analyses
DISCUSSION
Despite the unspecific nature of the F-18 labeled glucose analog FDG, with obvious limitations in discriminating inflammatory from malignant tissue as well as in the correct delineation of tumor boundaries, PET applying this tracer in an oncological setting appears to be one of the most powerful biomarkers introduced for clinical trials as well as for assessments of the individual patient. The statistical weakness of quantitative F-18 FDG PET imaging markers, including SUVmax and T/B uptake ratio, is well known and thoroughly described.26 However, the opportunity of an easy applicable, noninvasive quantification of tumor metabolic activity is in many circumstances appealing, giving further opportunities for the guidance of treatment, definition of prognosis, as well as comparisons between groups – this also being the case in patients with sarcomas.
Due to the general low incidence of sarcomas, our study does not diverge from most previous reports in terms of its retrospective study design. Neither does it ignore the extensive variation in tumor origin and localization, tumor size and burden, as well as intra- and intertumoral properties. However, it tries to make the study population more homogenous in terms of tumor grading, as one of the inclusion criteria was histologically verified high-grade sarcoma. Consequently, it gives the opportunity to identify possible prognostic variables beyond tumor grading, which is considered one of the most important variables when predicting survival in sarcoma patients. In a sarcoma setting the study also embraces a relatively large number of patients both with BS or STS, giving the opportunity of meaningful subgroup analyses of patients. Despite the use of several PET/CT scanners and slight on-site changes in the F-18 FDG PET/CT scan protocol in terms of reconstruction algorithm and injected dose of the tracer during an inclusion period of more than 10 years, we consider our results valid and reproducible.27
Regarding SUVmax of the primary tumor – even though included patients are not completely comparable – our data follow the footsteps of most previous studies reporting this F-18 FDG PET imaging marker as a significant independent prognostic variable in terms of overall survival in patients with STS.14–16,19 However, conflicting results were reported by Choi et al,12 which failed to identify SUVmax as a prognostic factor for progression-free survival in 55 patients with STS. Possible explanations for this reported divergence were the aforementioned heterogeneity of included histological subtypes, variation in tumor localization, and the inability of SUVmax to reflect the characteristics of the entire tumor, that is, the total volume and total activity of metabolically active tumor cells. Also, the differences in clinical endpoint should be noted. In our study, SUVmax remained its significant prognostic value when looking on all included patients with high-grade sarcoma, probably due to the strong diagnostic properties of SUVmax in STS (the majority of included patients), as no significant results were seen during subgroup survival analysis of patients with high-grade BS only. The latter supports a study by Costelloe et al,13 which reported no significant association between pretherapeutic SUVmax of the primary tumor and mortality in 31 patients with high-grade osteosarcoma. However, in the same population, they found SUVmax after chemotherapy to be a significant prognostic variable for overall survival. This variable was not included in our study.
SUVmax purely represents the maximum value of a single voxel in the acquired imaging data, and concerns have been raised as this variable does not reflect important prognostic tumor properties, such as tumor heterogeneity as well as tumor size and burden. Consequently, volume-based F-18 FDG PET imaging markers in terms of metabolic tumor volume (MTV) and total lesion glycolysis (TLG) have been introduced in an attempt to overcome these limitations of SUVmax, with several studies reporting significant prognostic properties in terms of prediction of treatment response and survival in a diversity of solid tumors.28–32 However, in patients with sarcoma reported data are conflicting. One study reported the superiority of TLG to SUVmax as a reliable predictor of progression-free survival in STS.12 However, another study by Hong et al16 concluded that the aforementioned volume-based F-18 FDG PET imaging markers may not provide additional prognostic information in STS, and importantly, that SUVmax in their study population was an independent prognostic factor for overall survival. They explained the results as a possible consequence to tumor necrosis, which lowers the values of volume-based F-18 FDG PET imaging markers, but does not affect the maximum value in a single voxel (ie, SUVmax). Eary et al10 demonstrated the prognostic impact of quantification of tumor F-18 FDG spatial heterogeneity using an image analysis algorithm, where they assess the extent to which the spatial distribution of F-18 FDG uptake within the tumor follows a certain idealized pattern. This method of assessing sarcomas has until date not achieved a major impact in the routine diagnostic work-up and follow-up of patients with sarcomas, and thus was not included in the data analysis in the present study. However, there is increased evidence for the prognostic value of primary tumor asphericity in pretherapeutic FDG PET for risk stratification in patients with head and neck cancer.33 By our knowledge, no such data exist for patients with high-grade sarcomas. Further studies are needed to explore the prognostic value of this array of different F-18 FDG PET imaging markers.
The tumor-to-background uptake ratio failed to demonstrate any significant independent prognostic properties in terms of survival during multivariate regression analysis, both when looking on all included patients as well as during subgroup analysis. The present study is by our knowledge the first to report survival data examining this variable in patients with high-grade sarcomas. Schulte et al34,35 proposed T/B uptake ratio to be a promising tool for the estimation of biologic activity of skeletal lesions and soft tissue neoplasms, but no correlation with survival was performed. Several biases affecting the tumor-to-background activity ratio in PET have been reported,36 such as the effect of tumor size, attenuation, nonuniformity of tissues, tumor location, and limited spatial resolution. These could all be factors contributing to the lack of significant prognostic value of T/B uptake ratio in our study population.
CONCLUSION
Semiquantitative estimation of primary tumor metabolic activity in terms of pretherapeutic SUVmax using F-18 FDG PET/CT demonstrates independent prognostic properties beyond histologic grading for prediction of overall survival in patients with high-grade soft tissue sarcoma, but interestingly fails to do so in patients with high-grade bone sarcoma. In the studied setting, we do not recommend the application of tumor-to-background uptake ratios as a prognostic variable.
Footnotes
Abbreviations: AJCC = American Joint Committee of Cancer, AUC = area under the curve, BS = bone sarcoma, CI = confidence interval, CT = computed tomography, F-18 = fluorine-18, FDG = fluoro-2-deoxy-D-glucose, FNCLCC = French Federation of Cancer Centers Sarcoma Group, HR = hazard ratio, MRI = magnetic resonance imaging, MSTS = Musculoskeletal Tumor Society, MTV = metabolic tumor volume, PET = positron emission tomography, PNET = primitive neuroectodermal tumor, ROC = receiver operating characteristic, STS = soft tissue sarcoma, SUVmax = maximum standardized uptake value, T/B = tumor-to-background, TLG = total lesion glycolysis, VOI = volume of interest.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. World Health Organisation Classification of Tumours of Soft Tissue and Bone. 4th edLyon: IARC Press; 2013. [Google Scholar]
- 2.Coindre JM, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 2001; 91:1914–1926. [DOI] [PubMed] [Google Scholar]
- 3.Skubitz KM, D’Adamo DR. Sarcoma. Mayo Clin Proc 2007; 82:1409–1432. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29. [DOI] [PubMed] [Google Scholar]
- 5.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 1980. 106–120. [PubMed] [Google Scholar]
- 6.Edge S, Byrd DR, Compton CC, et al. AJCC: soft tissue sarcoma. AJCC Cancer Staging Manual 7th ed2010; New York, NY: Springer, 291–298. [Google Scholar]
- 7.Edge S, Byrd DR, Compton CC, et al. AJCC: bone. AJCC Cancer Staging Manual 7th ed2010; New York, NY: Springer, 281–290. [Google Scholar]
- 8.Benz MR, Tchekmedyian N, Eilber FC, et al. Utilization of positron emission tomography in the management of patients with sarcoma. Curr Opin Oncol 2009; 21:345–351. [DOI] [PubMed] [Google Scholar]
- 9.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer 2002; 2:683–693. [DOI] [PubMed] [Google Scholar]
- 10.Eary JF, O'Sullivan F, O'Sullivan J, et al. Spatial heterogeneity in sarcoma 18F-FDG uptake as a predictor of patient outcome. J Nucl Med 2008; 49:1973–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenner W, Conrad EU, Eary JF. FDG PET imaging for grading and prediction of outcome in chondrosarcoma patients. Eur J Nucl Med Mol Imaging 2004; 31:189–195. [DOI] [PubMed] [Google Scholar]
- 12.Choi ES, Ha SG, Kim HS, et al. Total lesion glycolysis by 18F-FDG PET/CT is a reliable predictor of prognosis in soft-tissue sarcoma. Eur J Nucl Med Mol Imaging 2013; 40:1836–1842. [DOI] [PubMed] [Google Scholar]
- 13.Costelloe CM, Macapinlac HA, Madewell JE, et al. 18F-FDG PET/CT as an indicator of progression-free and overall survival in osteosarcoma. J Nucl Med 2009; 50:340–347. [DOI] [PubMed] [Google Scholar]
- 14.Eary JF, O'Sullivan F, Powitan Y, et al. Sarcoma tumor FDG uptake measured by PET and patient outcome: a retrospective analysis. Eur J Nucl Med Mol Imaging 2002; 29:1149–1154. [DOI] [PubMed] [Google Scholar]
- 15.Fuglo HM, Jorgensen SM, Loft A, et al. The diagnostic and prognostic value of (1)(8)F-FDG PET/CT in the initial assessment of high-grade bone and soft tissue sarcoma. A retrospective study of 89 patients. Eur J Nucl Med Mol Imaging 2012; 39:1416–1424. [DOI] [PubMed] [Google Scholar]
- 16.Hong SP, Lee SE, Choi YL, et al. Prognostic value of 18F-FDG PET/CT in patients with soft tissue sarcoma: comparisons between metabolic parameters. Skeletal Radiol 2014; 43:641–648. [DOI] [PubMed] [Google Scholar]
- 17.Schuetze SM, Rubin BP, Vernon C, et al. Use of positron emission tomography in localized extremity soft tissue sarcoma treated with neoadjuvant chemotherapy. Cancer 2005; 103:339–348. [DOI] [PubMed] [Google Scholar]
- 18.Schwarzbach MH, Hinz U, Dimitrakopoulou-Strauss A, et al. Prognostic significance of preoperative [18-F] fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging in patients with resectable soft tissue sarcomas. Ann Surg 2005; 241:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skamene SR, Rakheja R, Dalhstrom KR, et al. Metabolic activity measured on PET/CT correlates with clinical outcomes in patients with limb and girdle sarcomas. J Surg Oncol 2014; 109:410–414. [DOI] [PubMed] [Google Scholar]
- 20.Casali PG, Blay JY. Soft tissue sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010; 21 (Suppl 5):v198–v203. [DOI] [PubMed] [Google Scholar]
- 21.Hogendoorn PC, Athanasou N, Bielack S, et al. Bone sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010; 21 (Suppl 5):v204–v213. [DOI] [PubMed] [Google Scholar]
- 22.Guillou L, Coindre JM, Bonichon F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol 1997; 15:350–362. [DOI] [PubMed] [Google Scholar]
- 23.Rubin BP, Antonescu CR, Gannon FH, et al. Protocol for the examination of specimens from patients with tumors of bone. Arch Pathol Lab Med 2010; 134:e1–e7. [DOI] [PubMed] [Google Scholar]
- 24.Boellaard R, O’Doherty MJ, Weber WA, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging 2010; 37:181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berthelsen AK, Holm S, Loft A, et al. PET/CT with intravenous contrast can be used for PET attenuation correction in cancer patients. Eur J Nucl Med Mol Imaging 2005; 32:1167–1175. [DOI] [PubMed] [Google Scholar]
- 26.Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009; 50 (Suppl 1):122S–150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams MC, Turkington TG, Wilson JM, et al. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am J Roentgenol 2010; 195:310–320. [DOI] [PubMed] [Google Scholar]
- 28.Larson SM, Erdi Y, Akhurst T, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging 1999; 2:159–171. [DOI] [PubMed] [Google Scholar]
- 29.Lee JW, Kang CM, Choi HJ, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with pancreatic cancer. J Nucl Med 2014; 55:898–904. [DOI] [PubMed] [Google Scholar]
- 30.Liao S, Penney BC, Wroblewski K, et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2012; 39:27–38. [DOI] [PubMed] [Google Scholar]
- 31.Pak K, Cheon GJ, Nam HY, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med 2014; 55:884–890. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Wroblewski K, Liao S, et al. Prognostic value of metabolic tumor burden from (18)F-FDG PET in surgical patients with non-small-cell lung cancer. Acad Radiol 2013; 20:32–40. [DOI] [PubMed] [Google Scholar]
- 33.Hofheinz F, Lougovski A, Zophel K, et al. Increased evidence for the prognostic value of primary tumor asphericity in pretherapeutic FDG PET for risk stratification in patients with head and neck cancer. Eur J Nucl Med Mol Imaging 2015; 42:429–437. [DOI] [PubMed] [Google Scholar]
- 34.Schulte M, Brecht-Krauss D, Heymer B, et al. Fluorodeoxyglucose positron emission tomography of soft tissue tumours: is a non-invasive determination of biological activity possible? Eur J Nucl Med 1999; 26:599–605. [DOI] [PubMed] [Google Scholar]
- 35.Schulte M, Brecht-Krauss D, Heymer B, et al. Grading of tumors and tumorlike lesions of bone: evaluation by FDG PET. J Nucl Med 2000; 41:1695–1701. [PubMed] [Google Scholar]
- 36.Soret M, Riddell C, Hapdey S, et al. Biases affecting the measurements of tumor-to-background activity ratio in PET. IEEE Trans Nucl Sci 2002; 49:2112–2118. [Google Scholar]


