Abstract
Current guidelines established in the USA and Europe for coronary artery bypass graft (CABG) suggest that patients ≥65 and ≥70 years of age, or with certain atherosclerotic-risk factors, should be screened preoperatively for extracranial carotid artery stenosis (CAS) to assess their risk of perioperative stoke. We sought factors that should be taken into consideration when treating Chinese CABG patients using CABG guidelines based on an analysis of CAS in a large cohort of Chinese CABG patients.
We analyzed data for 1558 Chinese CABG patients who were screened preoperatively for CAS using duplex ultrasonography at a single institution. We defined significant and severe CAS as ≥50% and ≥70% stenosis, respectively, in one or more common or internal carotid arteries. We investigated the prevalence of CAS, the incidence of perioperative stroke, and the risk factors for CAS in the CABG cohort.
The prevalence of CAS in the CABG cohort was 21.2%. Multivariate stepwise logistic regression analysis showed that an age ≥50 years and a history of smoking (odds ratios = 8.36 and 1.83, respectively) were independent risk factors for CAS (P < 0.05 for both). The incidence of perioperative stroke among CABG patients with significant or severe CAS was significantly higher (2.4% and 2.9%, respectively) than in CABG patients with <50% stenosis (0.5%; P = 0.004 and 0.029, respectively).
Chinese CABG patients with a history of smoking or ≥50 years of age should undergo preoperative screening for CAS to assess their risk of perioperative stroke.
INTRODUCTION
Cardiovascular disease accounts for approximately 30% of all deaths worldwide, and cerebrovascular disease causes roughly 10% of all deaths.1 The risk factors are smoking, elevated levels of triglycerides and cholesterol in the blood, high blood pressure, and diabetes, but recent research has revealed that miRNAs play a role in the initiation and progression of cardiovascular disease2 as well as in their treatment.3 Extracranial carotid artery stenosis (CAS) is a risk factor for perioperative stroke in patients undergoing coronary artery bypass graft (CABG) surgery.4 In previous studies of CABG patients with 50% to 80% stenosis of the carotid arteries, the incidence of stroke ranged from 3% to 10%, and approached 22% in patients with >80% stenosis.5,6 A recent meta-analysis of American and European studies of CAS found that the risk of perioperative stroke following cardiac surgery ranged from 3.8% to 7.4% in patients with ≥50% stenosis, increasing to 2% to 9.1% in those with ≥70% stenosis.7 Mortality rates of 3.6% to 15.8% have also been reported in CABG patients with CAS.6,8,9 The economic burden of diagnosing and treating CAS has contributed significantly to the growing overall cost of CABG procedures, which further adds to the necessity of optimizing CABG treatment strategies.10,11
According to guidelines established in the USA in 2011, CABG patients older than 65 years or with certain risk factors, such as left main CAS, hypertension, peripheral artery disease (PAD), smoking, and diabetes, should be screened preoperatively for CAS using duplex ultrasonography (DU) to determine their risk of perioperative major cardiovascular events, as recommendations for tandem CABG and CAS treatments are dependent upon the severity of stenosis (≥50%).12,13 The 2011 European PAD guidelines extend the recommendation for preoperative DU screening for CAS in CABG patients ≥70 years of age or the presence of carotid bruit, multivessel coronary artery disease (CAD), or class I PAD (level of evidence B), or a history of cerebrovascular disease.12,14
Although multiple studies in the USA and Europe have shown that patients who underwent CABG because of severe CAD had a high prevalence of CAS, the results of these studies differed because of differences in the inclusion criteria and the definition of clinically significant CAS. In addition, a previous study in Canada showed that differences in atherosclerosis and cardiovascular-risk factors exist between different ethnic groups, including differences between various Asian ethnicities and non-Asians.15 Large-scale clinical studies and meta-analyses of CAS in Asian CABG patients have not yet been performed. Therefore, whether the American or European guidelines are optimal for Chinese CABG patients is unclear. We aimed to find factors that should be taken into consideration when treating Chinese CABG patients according to the CABG guidelines, based on an analysis of CAS in a large cohort of Chinese CABG patients.
METHODS
Patient Selection
We retrospectively analyzed data from 1558 patients who underwent CABG in the Department of Cardiology at Beijing Anzhen Hospital between January 1 and December 31, 2012. Written informed consent was obtained from each patient included in our study. Patients undergoing emergency CABG or cardiac valve procedures were excluded from our study.
CAS Screening
All of the patients were examined using bilateral DU at our institution within 7 days preceding the CABG procedure to quantify the level of stenosis of the common and internal carotid arteries. A diagnosis of significant CAS was defined as ≥50% stenosis in one or more of the left or right common and internal carotid arteries, with a systolic velocity > 140 cm/s, a diastolic velocity < 110 cm/s, and the presence of carotid plaque. Severe CAS was defined as ≥70% stenosis, with a systolic velocity > 140 cm/s, a diastolic velocity > 110 cm/s, and the presence of carotid plaque.16 The carotid vessels were considered completely occluded (occlusion CAS) when no patent lumen or blood flow could be detected.
Study Variables
The study variables analyzed are listed in Table 1. Hypertension was defined as repeated measurements of systolic pressure ≥140 mm Hg, diastolic pressure ≥90 mm Hg, or the current use of prescribed medication for hypertension. Type II diabetes mellitus (T2DM) was defined as repeated measurements of a fasting plasma glucose level >126 mg/dL or the current use of prescribed medication for T2DM. Hyperlipidemia was defined as a total serum cholesterol (TCho) level ≥220 mg/dL, low-density lipoprotein cholesterol (LDL-C) ≥140 mg/dL, high-density lipoprotein cholesterol (HDL-C) ≥40 mg/dL, serum triglyceride ≥150 mg/dL, or the current use of lipid-lowering medications. A family history of CAD was defined as a diagnosis of CAD in any first-degree relative younger than 60 years of age. The patients were followed for 24 months after CABG to evaluate the incidence of perioperative morbidity and mortality.
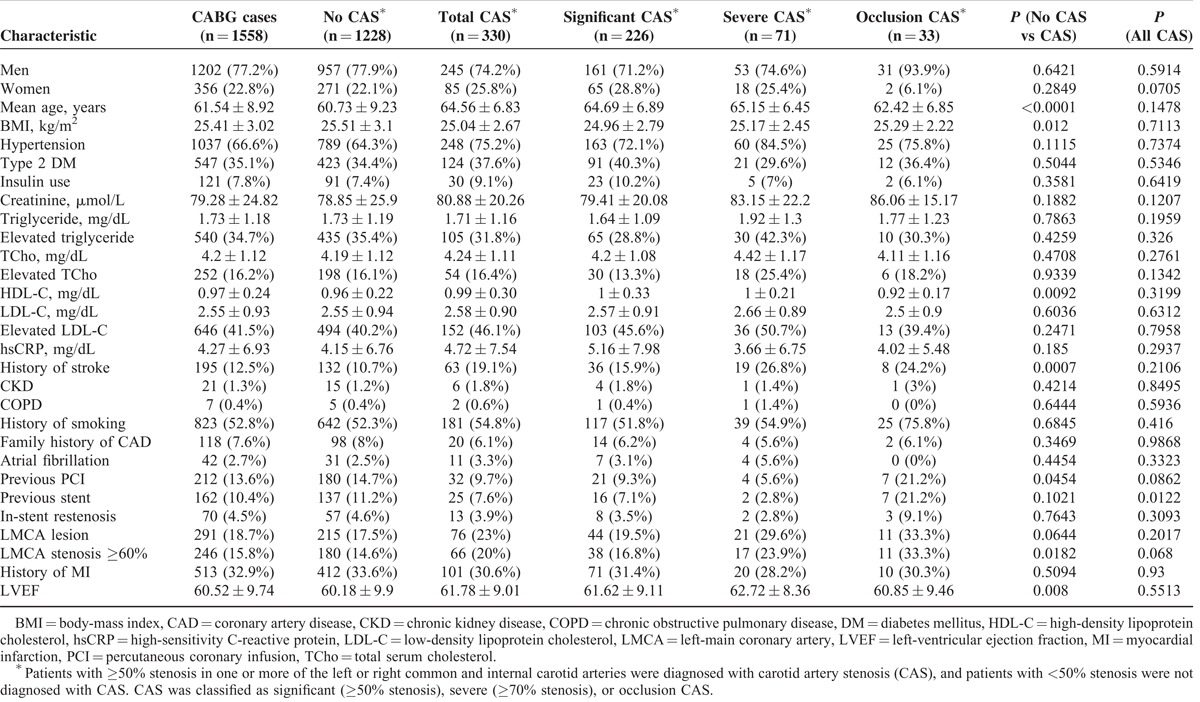
TABLE 1.
Baseline Characteristics of Coronary Artery Bypass Graft (CABG) Patients
Statistical Analysis
Data analysis was performed using the SPSS, version 19.0 (IBM, Armonk, NY). Continuous data are expressed as the mean ± standard deviation. Continuous variables were evaluated using 2-sided unpaired t-tests, and categorical variables were evaluated using a Chi-squared test or the Fisher exact test. A univariate analysis was performed to identify the study variables that were significantly associated with CAS (≥50% stenosis) in CABG patients, and independent predictors of clinically significant CAS were identified using a multivariate stepwise logistic regression analysis of the variables identified in the univariate analysis, based on the odds ratio (OR) and 95% confidence interval (CI). The level of statistical significance was set at P < 0.05.
RESULTS
Demographic and Clinical Characteristics
The demographic and clinical characteristics of the CABG patients are shown in Table 1. Of the 1558 CABG patients included in our study, 1202 (77.2%) were men. The CABG patients ranged in age from 30 to 88 years, and had a mean age of 61.3 ± 9.1 years (mean ± SD). Highly prevalent noteworthy clinical characteristics included hypertension (n = 1037; 66.6%), a history of smoking (n = 823; 52.8%), elevated LDL-C (n = 646; 41.5%), T2DM (n = 547; 35.1%), elevated triglyceride (n = 540; 34.7%), a history of myocardial infarction (n = 513; 32.9%), and elevated TCho (n = 252; 16.2%).
CAS in the CABG Cohort
The results of the preoperative DU screening are shown in Table 1. A total of 330 (21.2%) of the CABG patients were diagnosed with CAS. In the CABG cohort, 226 (14.5%) of the patients had significant CAS. Seventy-one (4.6%) of the CABG patients had severe CAS, and 33 (2.1%) were diagnosed with occlusion CAS, which contributed to a combined prevalence of 6.7% (104/1558) for ≥70% stenosis. The patients with CAS were significantly older (64.6 ± 6.8 years) than those without CAS (60.7 ± 9.2 years, P < 0.0001), and a significantly greater proportion of patients with CAS had a history of stroke (19.1%), compared with the patients without CAS (10.7%, P = 0.0007).
The left-ventricular ejection fraction (61.8 ± 9.0) of the patients with CAS was significantly higher than that of the patients without CAS (60.2 ± 9.9, P = 0.008). We also found that a significantly greater proportion of patients with occlusion CAS (21.2%) had previously undergone a stenting procedure, compared to patients with significant or severe CAS (7.1%, 2.8%, P = 0.012, respectively). By contrast, the mean body mass index (BMI) of patients with CAS was significantly lower (25.0 ± 2.7 kg/m2) than that of the patients without CAS (25.5 ± 3.1 kg/m2, P = 0.012). In addition, the HDL-C levels (0.99 ± 0.30 mg/dL) of patients with CAS were significantly higher than that of the patients without CAS (0.96 ± 0.22 mg/dL, P = 0.009). Furthermore, the proportion of patients with CAS who had previously undergone a percutaneous coronary intervention was significantly lower (9.7%) than that of the patients without CAS (14.7%, P = 0.045) (Table 1).
Contribution of Age to CAS in CABG Patients
To understand better the relationship between age and the onset of CAS in Chinese CABG patients, we performed a stratified analysis of the prevalence of CAS based on age. The proportion of CABG patients in each age group was stratified based on the diagnosis of CAS. From 1558 CABG patients, 20 (1.3%) were aged 30 to 39-years, 147 (9.4%) were 40 to 49-years, 447 (28.7%) were 50 to 59-years, 641 (41.1%) were 60 to 69-years, 293 (18.8%) were 70 to 79-years, and 10 (0.64%) were 80 to 88-years old. As shown in Figure 1, significant CAS was diagnosed more frequently in patients >50 years of age and all of the patients diagnosed with severe CAS were older than 50 years. The 60 to 69-year age group contained the largest proportion of patients diagnosed with significant CAS (49.6%, 112/226), severe CAS (53.5%, 38/71), and occlusion CAS (51.5%, 17/33). However, in the 50 to 59 compared to the 40 to 49-year old group, the prevalence of significant (11.4% and 3.4%) and severe (2.0% and 0%) CAS was notably higher (P < 0.001 and 0.015). These results suggest that the prevalence of ≥50% stenosis in the Chinese CABG patients increased markedly after 50 years of age (Figure 1).
FIGURE 1.
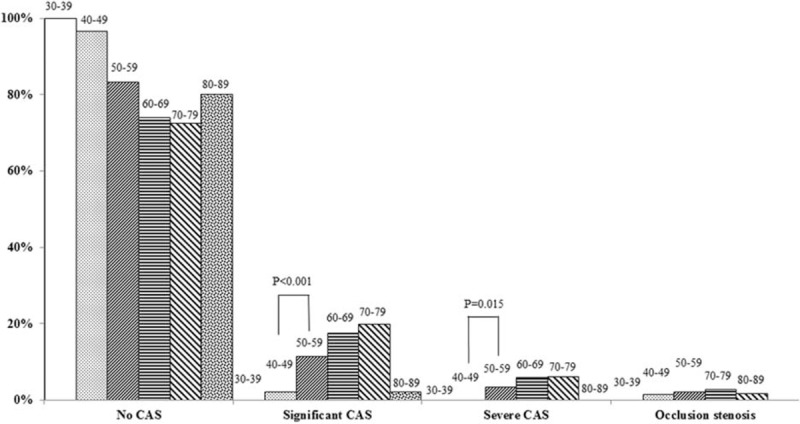
Carotid artery stenosis (CAS) severity distribution in the indicated age groups.
Incidence of Perioperative Stroke in CABG Patients
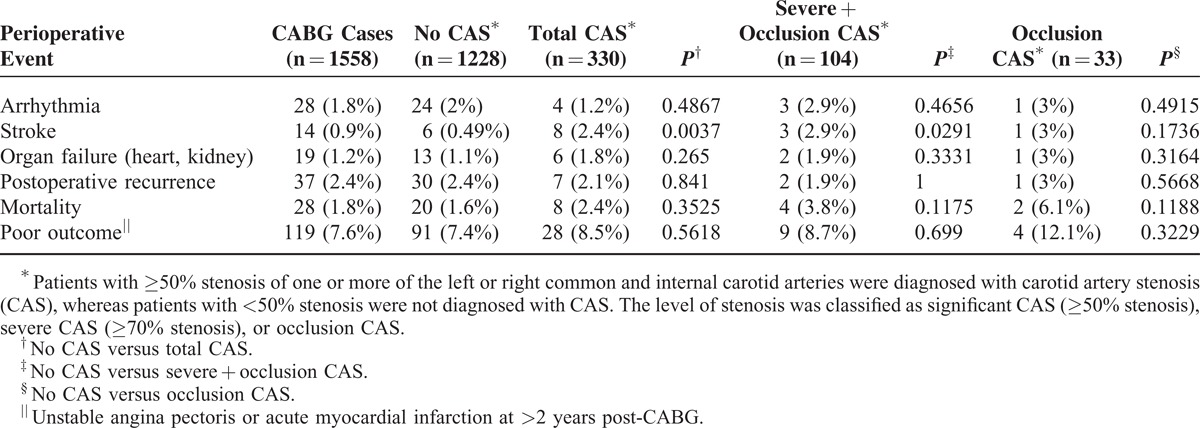
As CAS is a known risk factor for perioperative stroke in CABG patients, we investigated the incidence of perioperative morbidity and mortality in our CABG cohort. As shown in Table 2, the incidence of perioperative stroke in the patients with ≥50% and ≥70% stenosis were approximately 5-fold (2.4%) and 6-fold (2.9%) higher, respectively, than in patients with <50% stenosis (0.49%), indicating that a greater degree of stenosis was related to a higher incidence of perioperative stroke.
TABLE 2.
Incidence of Perioperative Morbidity and Mortality in Coronary Artery Bypass Graft (CABG) Patients
Risk Factors for CAS in CABG Patients
We used logistic regression to evaluate the risk of CAS associated with gender, age, BMI, hypertension, T2DM, insulin use, creatinine, triglyceride and elevated triglyceride levels, TCho and elevated TCho, HDL-C, LDL-C and elevated LDL-C, high-sensitivity C-reactive protein, history of stroke, chronic kidney disease, chronic obstructive pulmonary disease, history of smoking, family history of CAD, atrial fibrillation, previous percutaneous coronary infusion, previous stent, in-stent restenosis, left-main coronary artery lesion, left-main coronary artery stenosis ≥60%, history of myocardial infarction, and left-ventricular ejection fraction in our CABG cohort. Among the variables that exhibited significant differences between the CAS and non-CAS groups, an age ≥50 years was associated with an approximate 8-fold increase in the risk of CAS (OR = 8.358; 95% CI: 2.576–27.12; P < 0.001), and a history of smoking was associated with an approximate 2-fold increase in the risk of CAS (OR = 1.829; 95% CI: 1.174–2.850; P = 0.008). These results showed that an age ≥50 years and a history of smoking are independent risk factors for CAS (≥50% stenosis) in Chinese CABG patients.
DISCUSSION
The prevalence of CAS (≥50% stenosis) in CABG patients in Western countries has ranged from 12.8% to 22%17–19 and the prevalence of ≥70% stenosis from 7% to 11%.20 The prevalence of CAS with ≥50% and ≥70% stenosis in our Chinese CABG cohort was 21.2% and 6.7%, respectively, findings consistent with the results of previous studies in Western countries. Rates of 1% to 1.5% have been reported for carotid occlusion in CABG patients in Western countries.21,22 We observed a similar prevalence of carotid occlusion (2.1%) in our Chinese CABG cohort. A recent meta-analysis of American and European studies of the relationship between CAS and stroke following cardiac surgery found that the risk of perioperative stroke ranged from 2.0% to 7.4%, depending on the pattern and severity of carotid stenosis and the revascularization methods used.7 Therefore, the incidence of perioperative stroke in our Chinese CABG patients with ≥50% and ≥70% stenosis (2.4% and 2.9%, respectively) are consistent with the findings of previous studies of CAS in CABG patients in the USA and Europe.
Relatively few studies of CAS in Asian CABG patients have been performed. Tanimoto et al (2005)23 reported that the prevalence of ≥50% stenosis was 25.4% in Japanese CAD patients and Yoon et al (2001)24 reported that the prevalence of CAS in a Korean CABG cohort was 23.9%. In a previous study in China, Chen et al reported that the prevalence of ≥50% stenosis of the internal and common carotid arteries was 11% and 2%, respectively, in CAD patients. The prevalence of ≥50% stenosis observed in our Chinese cohort (21.2%) was higher than that reported by Chen et al (1998).25 However, Chen et al (1998) examined CAS in a cohort of CAD patients, whereas our cohort was composed entirely of patients who underwent CABG. It is possible that patients with mild CAD were less likely to be included in our CABG cohort. Therefore, the prevalence of ≥50% stenosis in our Chinese cohort (21.2%) is consistent with that reported in American, European, and Asian studies of CAS in CABG patients.
In a study performed in the USA in 1990, Faggioli et al (1990)26 concluded that the prevalence of CAS significantly increased after 60 years of age. In contrast, Fukuda et al27 recommended that all Japanese CABG patients should undergo preoperative CAS screening, regardless of age. Current American and European CABG guidelines recommend CAS screening for patients ≥65 and ≥70 years of age, respectively.13,14 In our Chinese CABG cohort, the prevalence of significant CAS (≥50% stenosis) and severe CAS (≥70% stenosis) in patients 50 to 59-years old were notably higher than in patients aged 40 to 49 years (11.4% versus 2.0% and 3.4% versus 0%, respectively). These results suggest that the prevalence of significant and severe CAS in our Chinese cohort increased markedly after 50 years of age. Given that 28.7% and 41.1% of the CABG cohort was comprised of patients aged 50 to 59 and 60 to 69 years, respectively, it seems unlikely that the number of CAS patients in the 50 to 59-year age group was biased by the age distribution of the CABG cohort. Our multivariate analysis showed that an age ≥50 years was associated with an approximate 8-fold increase in the risk of CAS. Therefore, we propose that Chinese CABG patients aged 50 years or older should undergo preoperative screening for CAS.
Because the watershed age for CAS screening may vary between different CABG populations, it may be equally important to consider the presence of other risk factors for CAS. Previous studies in Europe and the USA have shown that hypertension,28 male sex,29,30 smoking,28,30 T2DM,30 and a history of stroke are associated with CAS. With the exception of our occlusion CAS patients, no notable difference in the proportion of men was observed between the CAS and non-CAS groups, but a history of stroke was found to be associated with CAS in the Chinese CABG cohort. However, multivariate analysis showed that of these potential risk factors only a history of smoking was a significant risk factor for CAS in our Chinese CABG patients. Differences between the present findings and those of previous studies of CAS in CABG patients in European countries, the USA, and Japan might be due to differences in life style, patient characteristics, surgical methods, or biases in analyzing risk factors.31 A nationwide survey in China in 2002 showed that only 30% of people with hypertension were aware of their condition, and that only 6% of them managed their hypertension effectively.32 In the USA and Europe, patients diagnosed with hypertension are most often treated with lipid-lowering medication for long periods before their cardiovascular disease progresses to an extent requiring CABG. In China, such medication is prescribed less often, and cardiovascular surgeons may elect to perform CABG in some cases with no prerequisite medical treatment. These factors may contribute to a higher rate of CABG in younger patients in China, compared with the ages of CABG patients in Europe and North America.
Limitations of the present study were the retrospective analysis and that the data were obtained from a single center. In addition, other risk factors for CAS eg, lower limb artery disease were not included.
In conclusion, the prevalence of CAS (≥50% stenosis) in our Chinese CABG cohort was similar to that reported for studies of CAS in CABG patients in the USA, Europe, and other Asian countries. A history of stroke was associated with CAS in our Chinese CABG cohort, and a history of smoking was found to be an independent risk factor for CAS. However, in contrast to the current recommendation of the American and European CABG guidelines to perform preoperative CAS screening in patients’ ≥65 and ≥70 years of age, respectively, our findings indicate that the prevalence of CAS in Chinese CABG patients markedly increases after 50 years of age. Therefore, we propose that Chinese CABG patients with a history of smoking or ≥50 years of age should be screened preoperatively for CAS.
Acknowledgments
None.
Footnotes
Abbreviations: CABG = coronary artery bypass graft, CAD = coronary artery disease, CAS = carotid artery stenosis, CI = confidence interval, DU = duplex ultrasonography, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, OR = odds ratio, PAD = peripheral artery disease, T2DM = type 2 diabetes mellitus, TCho = total serum cholesterol.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Santulli G. Epidemiology of cardiovascular disease in the 21st century: updated numbers and updated facts. J Cardiovasc Dis 2013; 1:1–2. [Google Scholar]
- 2.Wronska A, Kurkowska-Jastrzebska I, Santulli G. Application of microRNAs in diagnosis and treatment of cardiovascular disease. Acta Physiol (Oxf) 2015; 213:60–83. [DOI] [PubMed] [Google Scholar]
- 3.Santulli G, Wronska A, Uryu K, et al. A selective microRNA-based strategy inhibits restenosis while preserving endothelial function. J Clin Invest 2014; 124:4102–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roffi M, Ribichini F, Castriota F, et al. Management of combined severe carotid and coronary artery disease. Curr Cardiol Rep 2012; 14:125–134. [DOI] [PubMed] [Google Scholar]
- 5.da Rosa MP, Schwendler R, Lopes R, et al. Carotid artery stenosis associated with increased mortality in patients who underwent coronary artery bypass grafting: a single center experience. Open Cardiovasc Med J 2013; 7:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgazli KM, Bilgin M, Kavukcu E, et al. Which is a better treatment for carotid artery stenosis: stenting or endarterectomy? Eur Rev Med Pharmacol Sci 2013; 17:1025–1032. [PubMed] [Google Scholar]
- 7.Naylor AR, Bown MJ. Stroke after cardiac surgery and its association with asymptomatic carotid disease: an updated systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2011; 41:607–624. [DOI] [PubMed] [Google Scholar]
- 8.Kassaian SE, Abbasi K, Hakki Kazazi E, et al. Staged carotid artery stenting and coronary artery bypass surgery versus isolated coronary artery bypass surgery in concomitant coronary and carotid disease. J Invasive Cardiol 2013; 25:8–12. [PubMed] [Google Scholar]
- 9.Dworschak M, Czerny M, Grimm M, et al. The impact of asymptomatic carotid artery disease on the intraoperative course of coronary artery bypass surgery. Perfusion 2003; 18:15–18. [DOI] [PubMed] [Google Scholar]
- 10.Shenoy AU, Aljutaili M, Stollenwerk B. Limited economic evidence of carotid artery stenosis diagnosis and treatment: a systematic review. Eur J Vasc Endovasc Surg 2012; 44:505–513. [DOI] [PubMed] [Google Scholar]
- 11.Hudorovic N. Reduction in hospitalisation rates following simultaneous carotid endarterectomy and coronary artery bypass grafting; experience from a single centre. Interact Cardiovasc Thorac Surg 2006; 5:367–372. [DOI] [PubMed] [Google Scholar]
- 12.Augoustides JG. Advances in the management of carotid artery disease: focus on recent evidence and guidelines. J Cardiothorac Vasc Anesth 2012; 26:166–171. [DOI] [PubMed] [Google Scholar]
- 13.Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation 2011; 124:e54–e130. [DOI] [PubMed] [Google Scholar]
- 14.European Stroke O, Tendera M, Aboyans V, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J 2011; 32:2851–2906. [DOI] [PubMed] [Google Scholar]
- 15.Anand SS, Yusuf S, Vuksan V, et al. Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE). Lancet 2000; 356:279–284. [DOI] [PubMed] [Google Scholar]
- 16.Schminke U, Motsch L, Lien LM, et al. Screening for high-grade carotid stenosis using a portable ultrasonography instrument. J Neuroimaging 2006; 16:252–259. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz LB, Bridgman AH, Kieffer RW, et al. Asymptomatic carotid artery stenosis and stroke in patients undergoing cardiopulmonary bypass. J Vasc Surg 1995; 21:146–153. [DOI] [PubMed] [Google Scholar]
- 18.Ascher E, Hingorani A, Yorkovich W, et al. Routine preoperative carotid duplex scanning in patients undergoing open heart surgery: is it worthwhile? Ann Vasc Surg 2001; 15:669–678. [DOI] [PubMed] [Google Scholar]
- 19.Steinvil A, Sadeh B, Arbel Y, et al. Prevalence and predictors of concomitant carotid and coronary artery atherosclerotic disease. J Am Coll Cardiol 2011; 57:779–783. [DOI] [PubMed] [Google Scholar]
- 20.Venkatachalam S, Shishehbor MH. Management of carotid disease in patients undergoing coronary artery bypass surgery: is it time to change our approach? Curr Opin Cardiol 2011; 26:480–487. [DOI] [PubMed] [Google Scholar]
- 21.Naylor AR, Mehta Z, Rothwell PM, et al. Carotid artery disease and stroke during coronary artery bypass: a critical review of the literature. Eur J Vasc Endovasc Surg 2002; 23:283–294. [DOI] [PubMed] [Google Scholar]
- 22.Brener BJ, Brief DK, Alpert J, et al. A four-year experience with preoperative noninvasive carotid evaluation of two thousand twenty-six patients undergoing cardiac surgery. J Vasc Surg 1984; 1:326–338. [PubMed] [Google Scholar]
- 23.Tanimoto S, Ikari Y, Tanabe K, et al. Prevalence of carotid artery stenosis in patients with coronary artery disease in Japanese population. Stroke 2005; 36:2094–2098. [DOI] [PubMed] [Google Scholar]
- 24.Yoon BW, Bae HJ, Kang DW, et al. Intracranial cerebral artery disease as a risk factor for central nervous system complications of coronary artery bypass graft surgery. Stroke 2001; 32:94–99. [DOI] [PubMed] [Google Scholar]
- 25.Chen WH, Ho DS, Ho SL, et al. Prevalence of extracranial carotid and vertebral artery disease in Chinese patients with coronary artery disease. Stroke 1998; 29:631–634. [DOI] [PubMed] [Google Scholar]
- 26.Faggioli GL, Curl GR, Ricotta JJ. The role of carotid screening before coronary artery bypass. J Vasc Surg 1990; 12:724–729.discussion 729–731. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda I, Gomi S, Watanabe K, et al. Carotid and aortic screening for coronary artery bypass grafting. Ann Thorac Surg 2000; 70:2034–2039. [DOI] [PubMed] [Google Scholar]
- 28.Crouse JR, Toole JF, McKinney WM, et al. Risk factors for extracranial carotid artery atherosclerosis. Stroke 1987; 18:990–996. [DOI] [PubMed] [Google Scholar]
- 29.de Weerd M, Greving JP, de Jong AW, et al. Prevalence of asymptomatic carotid artery stenosis according to age and sex: systematic review and metaregression analysis. Stroke 2009; 40:1105–1113. [DOI] [PubMed] [Google Scholar]
- 30.Fabris F, Zanocchi M, Bo M, et al. Carotid plaque, aging, and risk factors. A study of 457 subjects. Stroke 1994; 25:1133–1140. [DOI] [PubMed] [Google Scholar]
- 31.Santulli G. Coronary heart disease risk factors and mortality. JAMA 2012; 307:1137.author reply 1138. [DOI] [PubMed] [Google Scholar]
- 32.Yang G, Kong L, Zhao W, et al. Emergence of chronic non-communicable diseases in China. Lancet 2008; 372:1697–1705. [DOI] [PubMed] [Google Scholar]


