Abstract
A minimum of 12 harvested lymph nodes (hLNs) are recommended in colorectal cancer. However, a paucity of hLNs is frequently presented after preoperative chemoradiation (pCRT) in rectal cancer and the significance of this is still uncertain. The aim of this study is to analyze the impact of hLNs on long-term oncologic outcomes.
A total of 302 patients with locally advanced rectal cancer who underwent pCRT and curative resection between 1989 and 2009 were reviewed. Patients were categorized into 2 groups according to the number of hLNs: <12 versus ≥12 LN. The 2 groups were compared with respect to 5-year disease-free and overall survival. The optimal number or ratio of hLNs was investigated in subgroup analysis according to LN status.
The median follow-up was 57 months. Patient characteristics other than age did not differ between the 2 groups. The group with <12 LNs had more favorable ypTNM and ypN stage than those with ≥12 LNs. However, the long-term oncologic outcomes were not significantly different between the 2 groups. In subgroup analysis of ypN(−), the group with <5 hLNs had the most favorable oncologic outcomes. In ypN(+) cases, a higher LN ratio tended to be associated with poorer 5-year overall survival.
The paucity of hLNs in locally advanced rectal cancer after chemoradiation did not imply poor oncologic outcomes in this study. In addition, <5 hLNs in ypN(−) patients could reflect a good tumor response rather than suboptimal radicality.
INTRODUCTION
Colorectal cancer is the third most common malignant tumor in South Korea and rectal cancer accounts for approximately 40% of these cases. In Korea, approximately 56.1 patients per 100,000 were newly diagnosed with rectal cancer in 2011 and the number of patients is expected to increase further.1
During the past 2 decades, treatment modalities have improved and many randomized controlled trials have proved that total mesorectal excision (TME) after chemoradiation improves long-term oncologic outcomes in locally advanced rectal cancer.2–12
As previously reported, positive lymph nodes are one of the most powerful risk factors for recurrence and survival in colorectal cancer.13,14 Retrieval of fewer nodes during surgery is associated with an increased chance of stage migration.15 To avoid understaging and stage migration, the American Joint Committee on Cancer (AJCC) recommends harvesting a minimum of 12 lymph nodes in the surgical colorectal cancer specimens to ensure adequacy of surgery.
However, the number of harvested LNs varies across individuals and is influenced by several factors including age, tumor location, and status of preoperative chemoradiation (pCRT). Mean LN number decreases with increasing age and with progression from the proximal to the distal colon/rectum. Shen et al demonstrated that the mean number of LNs harvested was significantly lower in patients aged 60 years or younger compared with those older than 60 years (P = 0.002) and in the sigmoid colon/rectum compared with the cecum/ascending colon (P = 0.001).15,16 In addition, <12 lymph nodes could be expected in a surgical specimen after high-dose pCRT for rectal cancer. Govindarajan et al demonstrated a marked left shift toward fewer nodes, with 63% of patients assessed in the neoadjuvant therapy group having <12 nodes. The mean number of nodes retrieved in the surgery group was 15.5 (median, 13) compared with 10.8 nodes (median, 10; P < 0.001) in the neoadjuvant group.17 However, some authors reported that the absence of lymph nodes was associated with favorable pathologic features and oncologic outcomes and might reflect improved response to pCRT rather than suboptimal oncologic radicality.18,19 Habr-Gama et al reported 281 patients who underwent CRT prior to surgery for rectal cancer. Patients were grouped as having no LNs (ypNx, n = 32, 11%), negative LNs (ypN0, n = 171, 61%), and positive LNs (ypN+, n = 82, 28%). The ypNx patients in this study were found to have better 5-year disease-free survival (DFS) than patients with ypN0 and ypN+ (74% vs 59% vs 30%, P < 0.001).18
Considering the variability across individuals, the lymph node ratio (LNR, the number of LN involved to the number of LN examined), which was first proposed for colorectal cancer by Berger et al20 and subsequently adopted by others,21–25 could be useful for evaluating oncologic outcomes in a LN-positive group. The exact figures for the LNR are still a subject of debate, but the data demonstrate that a higher LNR is associated with poorer survival. However, few studies have been performed on the role of the LNR in locally advanced rectal cancer after CRT.
The oncologic impact of <12 LNs in patients receiving pCRT is still uncertain. The initial aim of this study was to analyze the impact of fewer than 12 LNs in terms of long-term oncologic outcomes. We also investigated the number of harvested LNs that would present a good balance between good tumor response and suboptimal radicality in ypN(−) patients. For ypN(+) patients, oncologic outcomes were compared according to the LNR to determine the proper cut-off value.
MATERIALS AND METHODS
A total of 332 consecutive patients who underwent surgical resection after pCRT for rectal cancer at Severance Hospital, Yonsei University College of Medicine, Seoul, Korea, between January 1989 and December 2009 were reviewed. Thirty patients who underwent extended pelvic lymph node dissection were excluded and a total of 302 patients were finally included in this study (Figure 1). Patients were categorized into 2 groups according to the number of harvested LNs: <12 versus ≥12 LNs. The 2 groups were compared with respect to patient demographics, pathologic characteristics, perioperative outcomes, and long-term oncologic outcomes. In addition, patients with negative LNs (ypN(−), n = 206) were categorized into 2 groups according to the cut-off number of harvested LNs and those with positive LNs (ypN(+), n = 96) were sub-grouped into 2 groups according to LNR by receiver-operating characteristic (ROC) curve analysis and analyzed with regard to long-term oncologic outcomes. The study was approved by the institutional review board in Severance Hospital (4–2015–0311).
FIGURE 1.
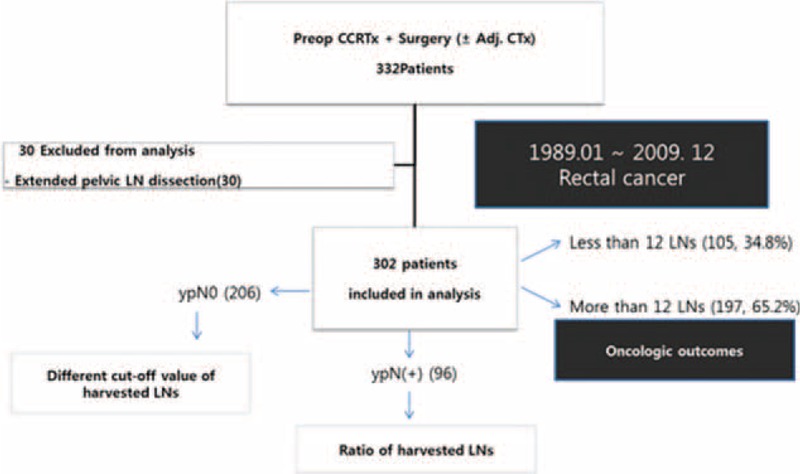
Overall study design and overview of patient population. Adj. CTx = adjuvant chemotherapy, LN = lymph node, Preop CCRTx = preoperative concurrent chemoradiation therapy, ypN0 = pathologic node negative, ypN(+) = pathologic node positive.
Preoperative assessment included clinical examination, blood cell count, serum chemistries, and serum carcinoembryonic antigen (CEA) levels. Preoperative tumor staging was performed by chest radiography and/or chest computed tomography scan (CT scan), abdominal and pelvic CT scan, transrectal ultrasound (TRUS), and/or pelvic magnetic resonance imaging (MRI).
Indications for preoperative chemoradiation included T3, T4, or positive lymph node based on clinical and radiologic examinations. Preoperative CRT consisted of 5-fluorouracil (5-FU)-based chemotherapy and pelvic irradiation (4500–5040 cGy) delivered in 25 fractions of 180 cGy/day over 5 weeks. At our institution, RT was delivered with a 6 MV/10 MV dual photon linear accelerator using the 4-field box technique. The most common type of chemotherapy regimen was given as continuous intravenous infusion of 5-FU at 425 mg/m2/day and leucovorin at 20 mg/m2/day during weeks 1 and 5 of radiotherapy. The remaining patients received oral 5-FU preparation (Xeloda®, Capecitabine; Roche Laboratories Inc, Nutley, NJ), a combination of irinotecan (CPT-11) with 5-FU or TS-1, or a triple combination of 5-FU, leucovorin, and cisplatin (DDP).
In most cases, curative resection was performed 6 to 8 weeks after completion of pCRT and all patients received surgical resection on the basis of TME with preservation of the hypogastric nerve. A diverting loop ileostomy was made to protect the anastomosis in case of a positive air leak test or ultralow anterior resection. Reversal of the ileostomy was carried out after completion of adjuvant chemotherapy.
Adjuvant chemotherapy was applied to almost all patients within 4 to 6 weeks after surgery except in cases of patient refusal of additional therapy or severe chemotoxicity.
The pathologic examination was standardized in our institution. Each pathologic slide was examined by one qualified pathologist who was a specialist in colorectal cancer. Tumors were staged according to the TNM classification (AJCC 7th edition) after the final pathologic report. Specimens were evaluated for tumor differentiation, depth of tumor penetration, lymph node metastasis, circumferential resection margin (CRM), lymphovascular invasion, and tumor regression grade (TRG) as suggested by Mandard et al.26 CRM involvement was defined as the presence of tumor cells from the outermost margin of the lesion to the proper mesorectal fascia or when the maximum distance between the tumor and proper rectal fascia was <1 mm. Postoperative morbidity and mortality were classified based on Clavien-Dindo classification.27
Follow-Up
Clinical evaluation was performed every 3 months during the first year after surgery, every 6 months for the subsequent 2 years, and yearly thereafter for 2 more years. Rectal examination and serum CEA measurement were carried out whenever the patient visited the outpatient clinic. Contrast-enhanced helical CT was performed every 6 months during the follow-up period and MRI was performed if needed. Local recurrence was defined as evidence of tumor within the lesser pelvis or the perineum and distant metastasis was defined as evidence of tumor in any other area. Local and distant recurrences were confirmed radiologically or histologically by qualified radiation oncologists and pathologists.
Statistical Analysis
Data were analyzed using the SPSS statistical program (Statistical Product and Service Solution 20.0 for Windows; SPSS Inc., Chicago, IL). An independent t test was performed for comparison of continuous variables. Chi-squared tests were used to compare proportions. Mann–Whitney tests were used to compare quantitative and ordinal variables. Univariate analysis of survival was carried out by the Kaplan–Meier method and comparison between 2 groups was performed with the log-rank test. Multivariate analysis of recurrence and survival was performed by the Cox proportional hazard model. The benchmark of harvested LNs was regarded as the lowest point of recurrence by analysis of oncologic outcomes according to the different cut-off values of harvested LNs. The cut-off points for LNR were chosen by means of ROC curve. A 2-sided P value of ≤0.05 was considered to indicate statistical significance.
RESULTS
Patient Characteristics
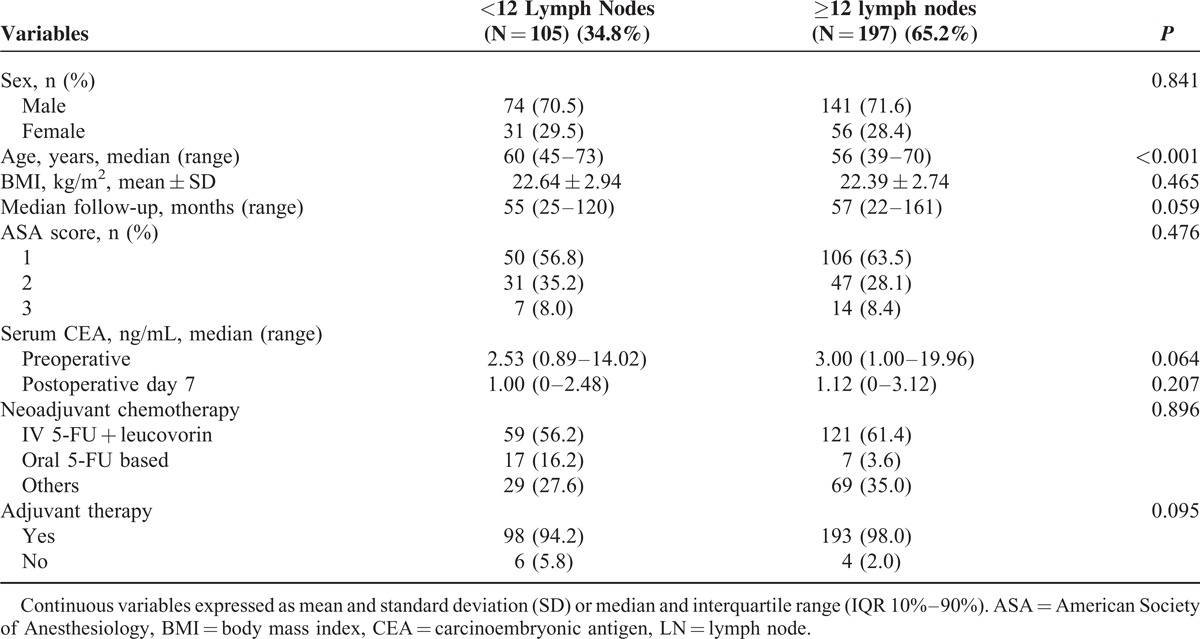
A total of 302 consecutive patients were enrolled in this study. Of these, 105 (34.8%) patients had fewer than 12 LNs and 197 (65.2%) had 12 or more LNs (Table 1). Median follow up was 57 months (range 22–147). No significant differences were observed regarding median follow-up, sex, preoperative body mass index (BMI), and serum CEA level between the 2 groups, but patient age was significantly older in the <12 LN group (60 years, range 45–73) than in the ≥12 LN group (56 years, range 39–70, P < 0.001). The type of preoperative chemotherapy and the implementation of adjuvant chemotherapy were not markedly different between the 2 groups (Table 1).
TABLE 1.
Patient Demographics
Perioperative Surgical Outcomes
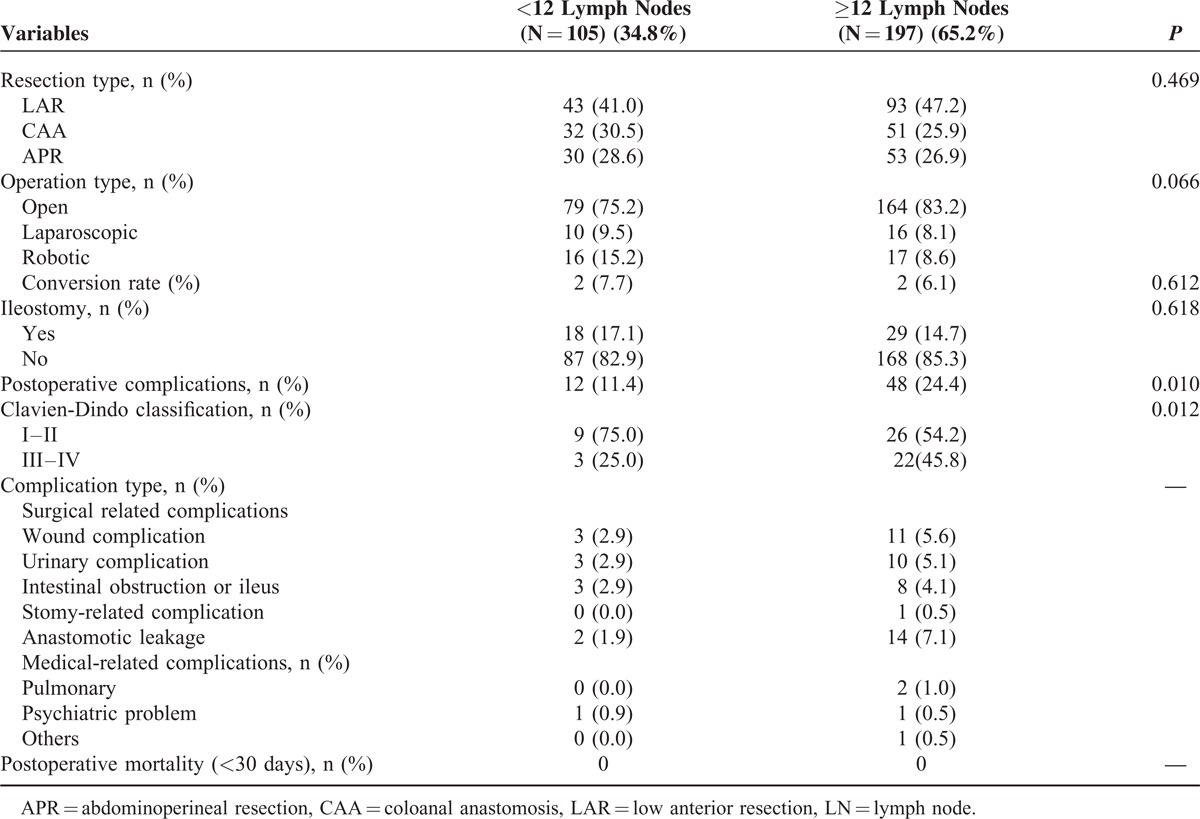
Patients assigned to the <12 LN group had fewer perioperative complications than those assigned to the ≥12 LN group, as shown in Table 2 (11.4% vs 24.4%, P = 0.010). However, the incidence of high-grade (grade III-IV) postoperative complications according to Clavien-Dindo classification was not statistically different between the 2 groups. Anastomotic leakage rate was 1.9% in the <12 LN group and 7.1% in the ≥12 LN group. All other surgical characteristics, such as resection type, operation type, conversion rate, and ileostomy, were not statistically different between the groups and are summarized in Table 2.
TABLE 2.
Surgical Methods and Perioperative Outcomes in the Patients With Surgery After Chemoradiation
Tumor Characteristics
The clinicopathologic stage and tumor characteristics are shown in Table 3. The median number of harvested LNs was 14. Tumor size was smaller and more patients with low rectal cancer were included in the <12 LN group compared with the ≥12 LN group. Although the pCR rate was not statistically different between the 2 groups (17.1% vs 11.7%, P = 0.217), ypTNM stage (P = 0.047), and nodal status (P = 0.044) were more favorable in the <12 LN group than the ≥12 LN group. Other characteristics, including tumor differentiation, involvement of CRM, and distal resection margin, were not markedly different between the groups. Although a complete dataset for TRG could not be obtained because of missing data, good tumor responders tended to be included in the <12 LN group compared with the ≥12 LN group (TRG 1–3, 83.8% vs 71.8%, P = 0.072) (Table 4).
TABLE 3.
Tumor Characteristics in the Patients With Surgery After Chemoradiation
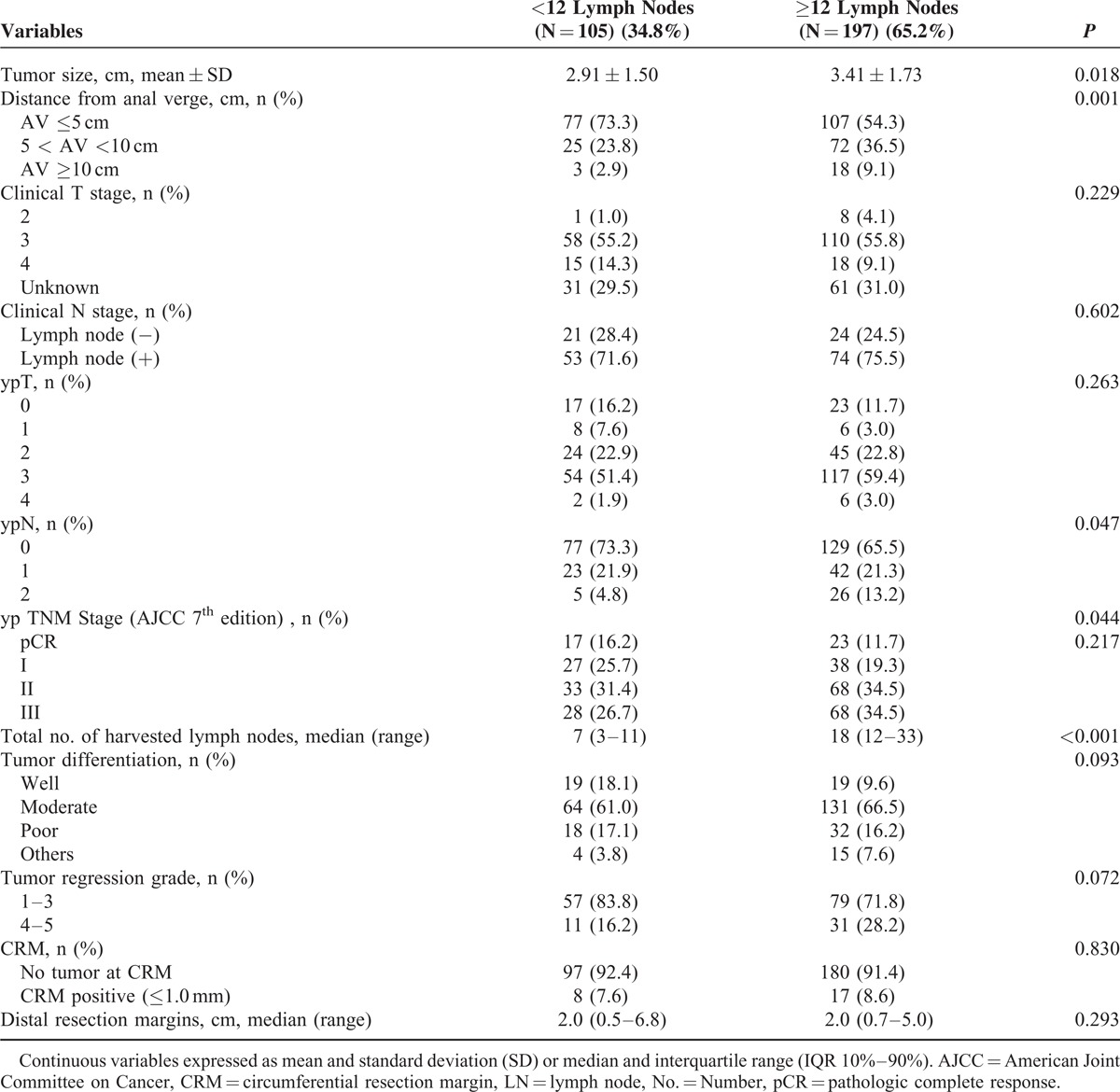
TABLE 4.
Recurrence Pattern in the Patients With Surgery After Chemoradiation
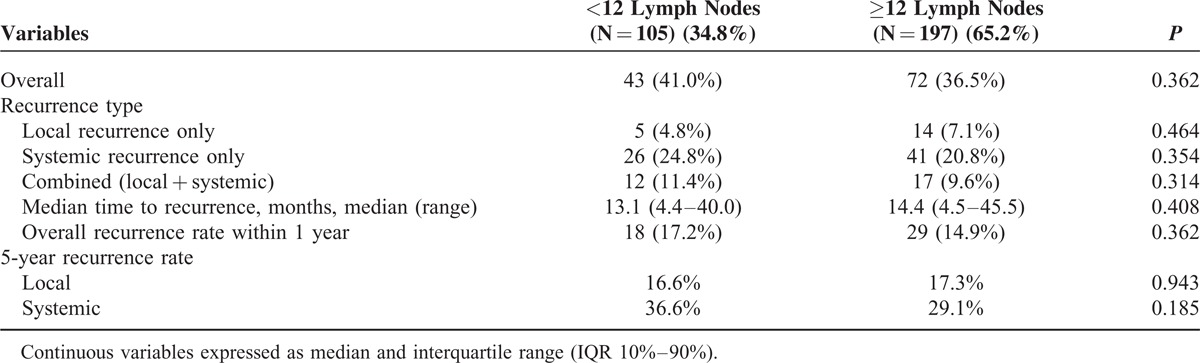
Long-Term Oncologic Outcomes
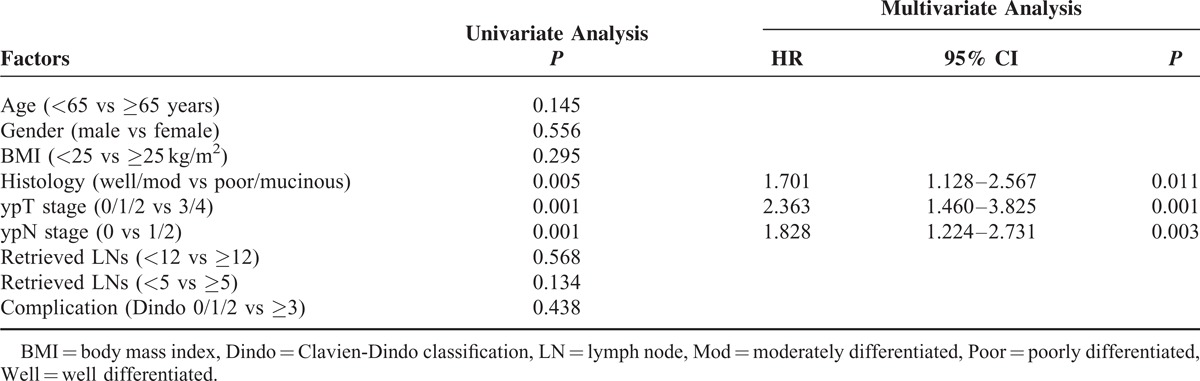
The median follow-up times were 55 months for the <12 LN group and 57 months for the ≥12 LN group. As shown in Figure 2, 5-year local (LR) and systemic recurrence (SR) rates were not different between the 2 groups. (LR, 16.6% vs 17.3%, P = 0.94; SR, 36.7% vs 29.1%, P = 0.19). The estimated 5-year OS were 72.0% in the <12 LN group and 71.2% in the ≥12 LN group (P = 0.63) and the estimated 5-year DFS were not statistically different between the 2 groups (59.7% vs 64.7%, P = 0.36). As reported in Tables 5 and 6, the Cox regression analysis showed that histology and ypTNM stage were significant contributors to the model, but the number of harvested LNs were not associated with disease progression and survival.
FIGURE 2.
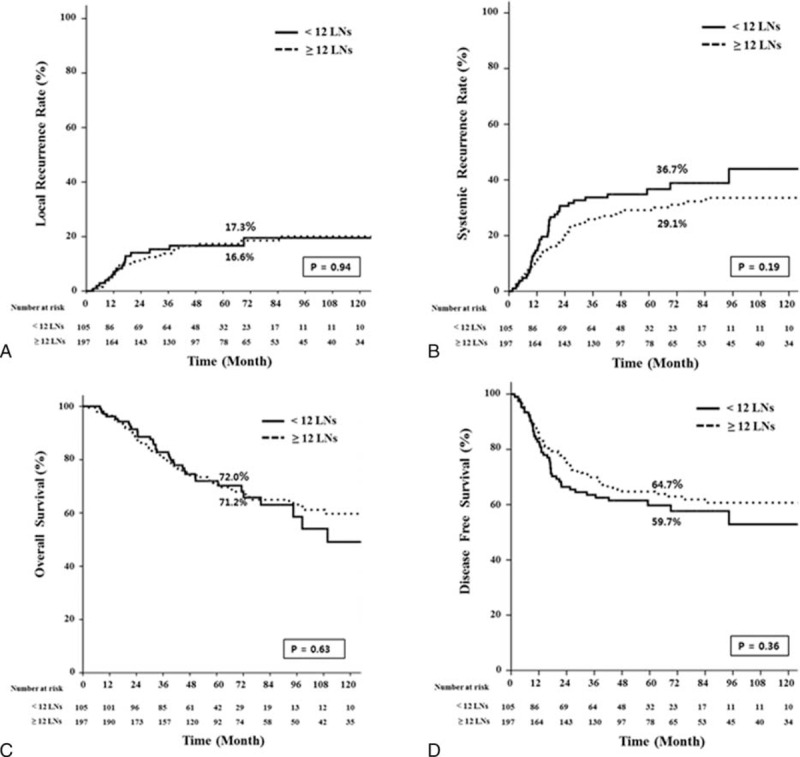
Kaplan–Meier estimates and log-rank test for oncologic outcomes between the LN <12 and LN ≥12 group. LN = lymph node.
TABLE 5.
Uni- and Multivariate Analysis of Recurrence Prediction of Total Patients (N = 302)
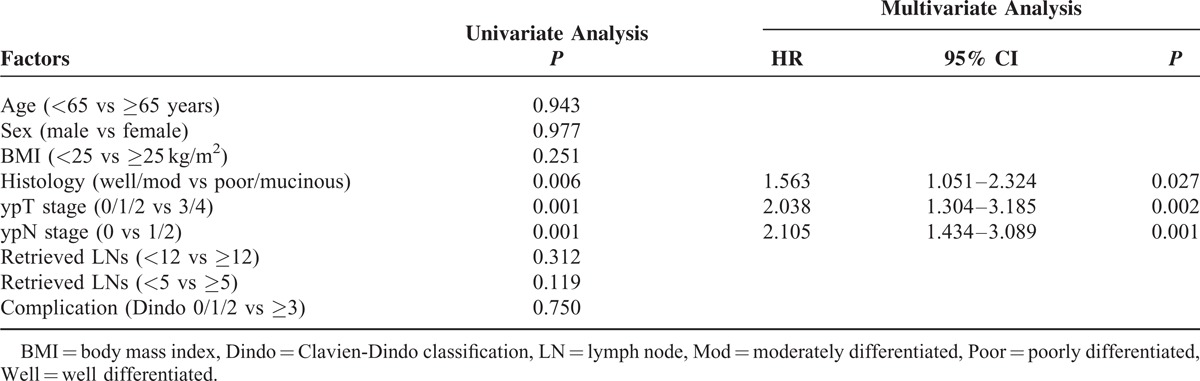
TABLE 6.
Uni- and Multivariate Analysis of Prognosis Prediction of Total Patients (N = 302)
The lack of a statistical difference in long-term oncologic outcomes between the <12 LN and the ≥12 LN groups and previous reports that fewer nodes were retrieved in pCRT patients with rectal cancer17 encouraged us to investigate a more appropriate cut-off number of harvested LNs. Based on previous reports,20–25 the cut-off number was evaluated by subcategorizing ypN(−) patients into 2 groups according to different cut-off values of harvested LNs, and a meaningful cut-off for LNR was analyzed in ypN(+) patients.
In subgroup analysis of ypN(−) patients (n = 206), 5-year oncologic outcomes were not different between the <12 LN and the ≥12 LN groups (Figure 3). When the ypN(−) patients were classified into 2 groups according to different cut-off values of harvested LNs and compared with respect to 5-year oncologic outcomes (Table 7), the group with <5 harvested LNs had more favorable oncologic results than the other groups. Even though those results were not statistically significant because of the small number of patients included, 5-year recurrence and OS rates tended to decrease below the cut-off value of 5 harvested LNs, which could therefore be regarded as a benchmark value for harvested LNs (Figure 4). Moreover, only 3 patients had recurrences in the <5 harvested LN group and one of these recurrences might have occurred because of inadequate distal resection margin rather than inadequate harvested LNs (Table 8). Among the 96 ypN(+) patients, Kaplan–Meier survival curves demonstrated that the 5-year OS was clearly dependent on the LNR. As shown in Figure 5, a higher LNR is associated with worse survival (5-year OS, 66.8% for low LNR vs 36.6% for high LNR, P = 0.008).
FIGURE 3.
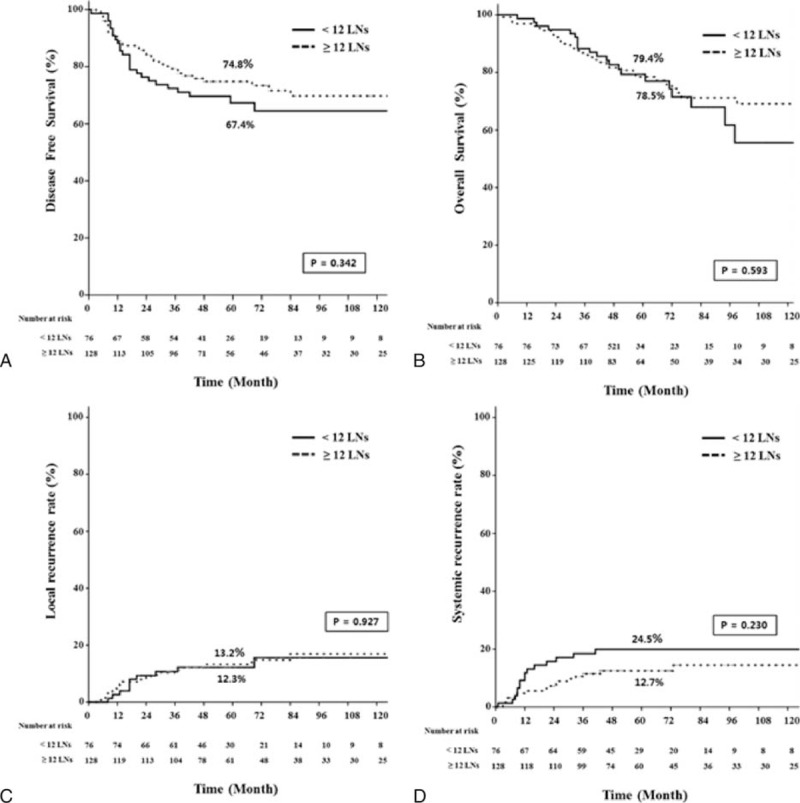
Kaplan–Meier estimates and log-rank test for oncologic outcomes between the LN <12 and LN ≥12 group in the ypN(−) patients with surgery after chemoradiation. LN = lymph node, ypN(−) = pathologic negative lymph node.
TABLE 7.
Comparison of 5-Year DFS, LR, SR, and OS According to the Adjusted Cut-Off Value of Harvested Lymph Nodes in ypN(−) Patients
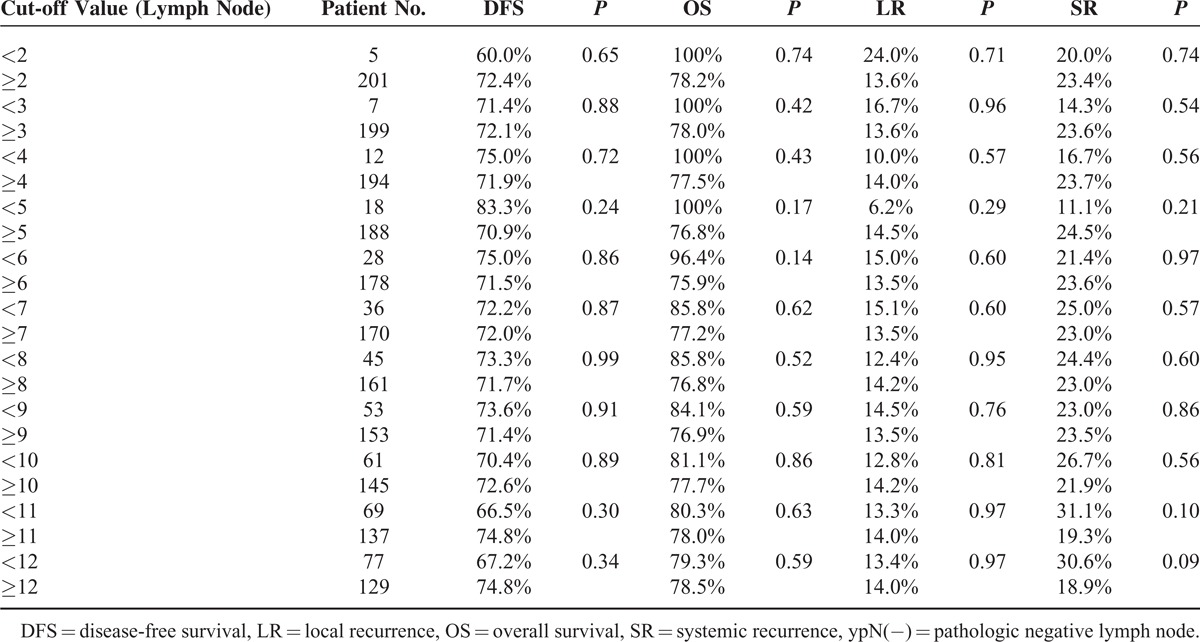
FIGURE 4.
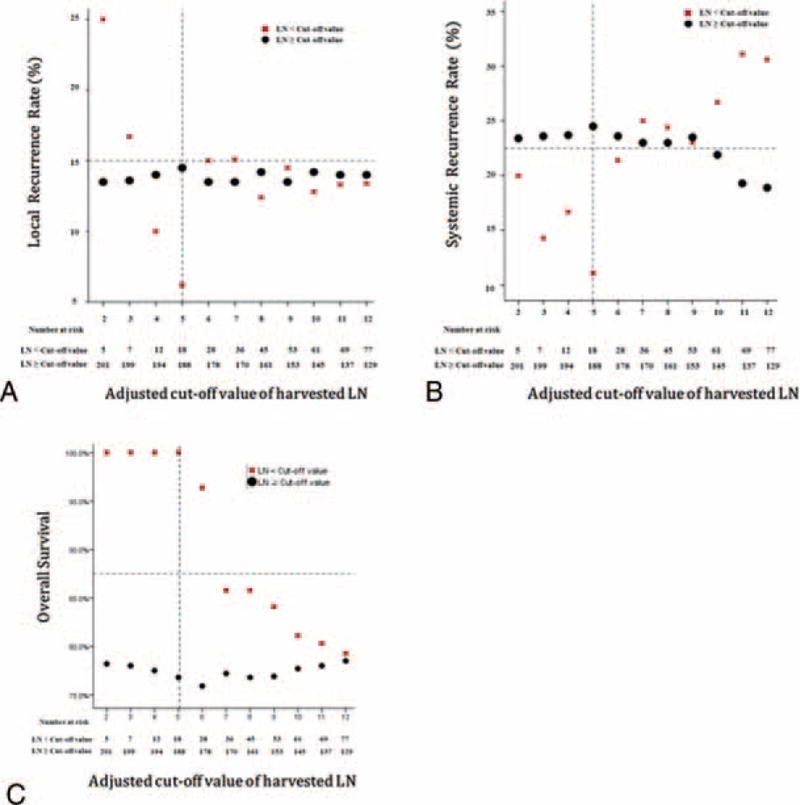
Comparison of 5-year local recurrence, systemic recurrence, and overall survival according to the adjusted cut-off value of harvested lymph nodes in ypN(−) patients. ypN(−) = pathologic negative lymph node.
TABLE 8.
Patient Characteristics of Recurrence in <5 Harvested LNs Group With ypN (−)
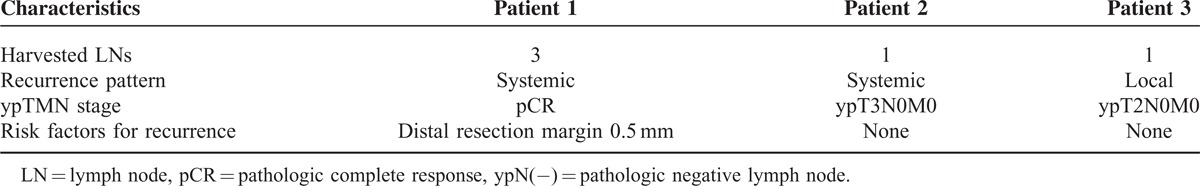
FIGURE 5.
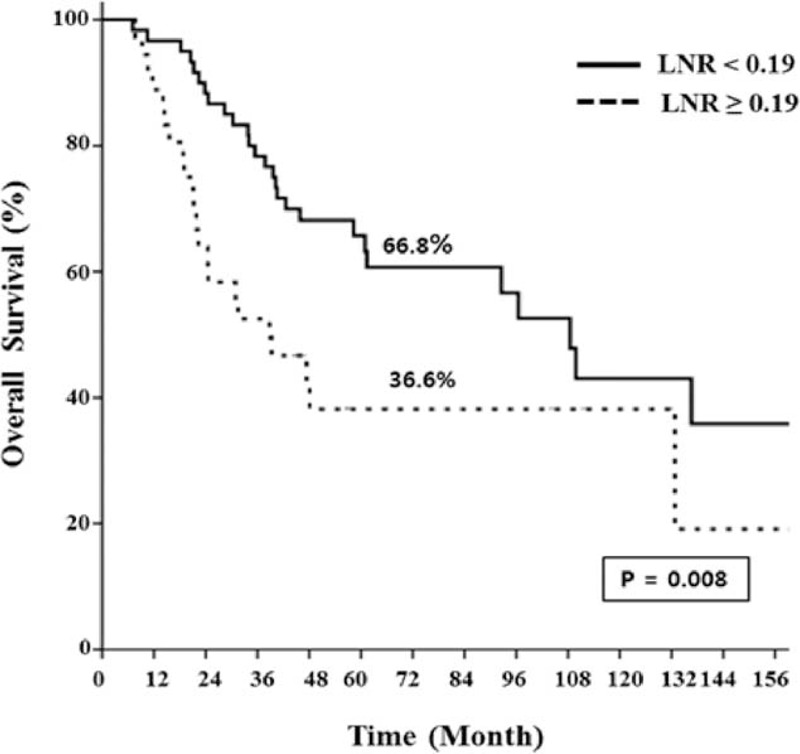
Kaplan–Meier estimates and log-rank test for oncologic outcomes in ypN(+) patients classified into subgroups according to the LNR (no. of positive LNs/total no. of harvested LNs). LNR = lymph node ratio, No = number, ypN(+) = pathologic positive lymph node.
DISCUSSION
The number of lymph nodes harvested varies across individuals and is affected by various factors such as age, sex, tumor location, and the status of preoperative chemoradiation.15–17 The phenomenon of fewer harvested lymph nodes after chemoradiation was explained by radiation-induced lymphocyte depletion and replacement with stromal fibrosis, which might render lymph nodes difficult to detect.18,19,28,29 However, the retrieval of fewer nodes after chemoradiation had clinical significance. Habr-Gama et al18 reported that the absence of harvested LNs was not associated with poor oncologic outcomes. In fact, they demonstrated that the 5-year DFS in the group without retrieved LNs was not only superior compared with the ypN+ group (74% vs 30%, P < 0.001) but was also not statistically different from that of the ypN0 group (74% vs 59%, P = 0.2). By extension, Campos et al30 proposed that retrieval of <12 LNs after chemoradiation might be a marker of tumor response and consequently improved prognosis. They reported that the local recurrence rate was significantly different between the <12 and ≥12 LN groups (0% vs 11%, P = 0.004). However, the fact that there was not a single recurrence in patients with fewer than 12 LNs reflects the limitation of their retrospective study. Govindarajan et al17 reported that a higher number of retrieved LNs tended to be associated with higher node positivity, but the number of retrieved LNs was not a risk factor for disease-specific survival in multivariate analysis (hazard ratio 0.94; 95% CI, 0.88–1.01; P = 0.09)
In this study, the older age of the <12 LN group appeared to result in retrieval of fewer nodes (Table 3). The fact that the tumor characteristic of distal rectal cancer, which has a tendency to have more nodes retrieved, was more frequent in the <12 LN group might be associated with suboptimal radicality. Considering that there were no statistical differences between the <12 LN and the ≥12 LN groups in terms of 5-year OS and DFS, patients who had suboptimal radicality and patients with more favorable ypN and ypTNM stage, which indicate a good tumor response, might be included together in the <12 LN group. Among ypN(−) patients, the <5 LN group tended to have more favorable oncologic outcomes because patients in this group had better tumor responses than those in other groups (ypT 0/1/2, 77.8% vs. 48.4%, P = 0.025). Even though our study failed to demonstrate statistically better oncologic outcomes for fewer than 5 LNs retrieved because of the small number of patients included, the trend for good oncologic outcomes with <5 LNs could have clinical importance. In other words, <5 LNs retrieved in rectal cancer after chemoradiation might be oncologically safe and suggest better oncologic outcomes.
As our previous study emphasized a clinical importance of LNR in ypN(+) rectal cancer after pCRT,31 patients were subdivided into ypN(+) group for analyzing a cut-off value of LNR in this study. In ypN(+) patients, a higher LNR (0.19–0.92) appeared to be associated with worsening prognosis the same as our previous reports did. (Figure 5)
To our knowledge, this is the first report to suggest a balanced cut-off number of harvested LNs between a good tumor response and suboptimal radicality in ypN(−) patients with locally advanced rectal cancer after chemoradiation. Our finding is clinically important because some oncologists have questioned the routine use of adjuvant chemotherapy for patients with rectal cancer who undergo curative surgery and have been rendered node-negative by CRT. Kiran et al32 reported that the oncologic benefits of adjuvant chemotherapy were especially questionable for patients with complete pathologic response (chemotherapy vs no chemotherapy: LR at 5 years, 0% vs 2.9%, P > 0.99; DFS, 79.1% vs 88%, P = 0.51; and OS 90.9% vs 95.2%, P = 0.41). Considering our current results, <5 harvested LNs might be an additional guideline for adjuvant chemotherapy in pCR patients.
However, the retrospective design of this study has some potential drawbacks. For a clear correlation with good tumor response in the group with fewer than the adjusted cut-off value of harvested LNs, it is necessary to analyze TRG, but the incomplete dataset made this impossible. In addition, the small number of patients included in the group with fewer than the adjusted cut-off value of LNs retrieved failed to present statistical differences in terms of long-term oncologic outcomes. Nevertheless, the data that we present in this study (Figure 4) provide inspiration for further large-scale studies to verify the proper number of harvested LNs in ypN(−) patients who undergo preoperative CRT followed by TME.
CONCLUSION
The paucity of hLNs in locally advanced rectal cancer after chemoradiation did not imply poor oncologic outcomes in this study. In addition, <5 LNs in ypN(−) patients could reflect a good tumor response rather than suboptimal radicality. Considering the limitations of our study, a further large-scale study is needed to verify the appropriate number of harvested LNs in advanced rectal cancer in the era of preoperative chemoradiation.
Footnotes
Abbreviations: 5- FU = 5-fluorouracil, Adj. CTx = Adjuvant chemotherapy, AJCC = the American Joint Committee on Cancer, CEA = carcinoembryonic antigen, CRM = circumferential resection margin, CT scan = computed tomography scan, DFS = disease free survival, hLNs = harvested lymph nodes, LNR = the lymph node ratio, LR = local recurrence, MRI = magnetic resonance imaging, OS = overall survival, pCRT = preoperative chemoradiation, Preop CCRTx = Preoperative concurrent chemoradiation therapy, SR = systemic recurrence, TME = total mesorectal excision, TRG = tumor regression grade, TRUS = transrectal ultrasound, ypN(+) = pathologic node positive, ypN0 = pathologic node negative.
All the authors have no conflicts of interest or financial ties to disclose.
REFERENCES
- 1.Korean Central Cancer Registry KCCR. 2011 National Cancer Registry Statistics. 2013. [Google Scholar]
- 2.Frykholm GJ, Glimelius B, Pahlman L. Preoperative or postoperative irradiation in adenocarcinoma of the rectum: final treatment results of a randomized trial and an evaluation of late secondary effects. Dis Colon Rectum 1993; 36:564–572. [DOI] [PubMed] [Google Scholar]
- 3.MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet 1993; 341:457–460. [DOI] [PubMed] [Google Scholar]
- 4.Martling AL, Holm T, Rutqvist LE, et al. Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Stockholm Colorectal Cancer Study Group, Basingstoke Bowel Cancer Research Project. Lancet 2000; 356:93–96. [DOI] [PubMed] [Google Scholar]
- 5.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001; 345:638–646. [DOI] [PubMed] [Google Scholar]
- 6.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 7.Folkesson J, Birgisson H, Pahlman L, et al. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 2005; 23:5644–5650. [DOI] [PubMed] [Google Scholar]
- 8.Guillem JG, Chessin DB, Cohen AM, et al. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg 2005; 241:829–836.discussion 836–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kao PS, Chang SC, Wang LW, et al. The impact of preoperative chemoradiotherapy on advanced low rectal cancer. J Surg Oncol 2010; 102:771–777. [DOI] [PubMed] [Google Scholar]
- 10.Wong RK, Berry S, Spithoff K, et al. Preoperative or postoperative therapy for stage II or III rectal cancer: an updated practice guideline. Clin Oncol 2010; 22:265–271. [DOI] [PubMed] [Google Scholar]
- 11.van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011; 12:575–582. [DOI] [PubMed] [Google Scholar]
- 12.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012; 30:1926–1933. [DOI] [PubMed] [Google Scholar]
- 13.Kim NK, Kim YW, Min BS, et al. Factors associated with local recurrence after neoadjuvant chemoradiation with total mesorectal excision for rectal cancer. World J Surg 2009; 33:1741–1749. [DOI] [PubMed] [Google Scholar]
- 14.Kim TH, Chang HJ, Kim DY, et al. Pathologic nodal classification is the most discriminating prognostic factor for disease-free survival in rectal cancer patients treated with preoperative chemoradiotherapy and curative resection. Int J Radiation Oncol Biol Phys 2010; 77:1158–1165. [DOI] [PubMed] [Google Scholar]
- 15.Sarli L, Bader G, Iusco D, et al. Number of lymph nodes examined and prognosis of TNM stage II colorectal cancer. Eur J Cancer (Oxford, England: 1990) 2005; 41:272–279. [DOI] [PubMed] [Google Scholar]
- 16.Shen SS, Haupt BX, Ro JY, et al. Number of lymph nodes examined and associated clinicopathologic factors in colorectal carcinoma. Arch Pathol Lab Med 2009; 133:781–786. [DOI] [PubMed] [Google Scholar]
- 17.Govindarajan A, Gonen M, Weiser MR, et al. Challenging the feasibility and clinical significance of current guidelines on lymph node examination in rectal cancer in the era of neoadjuvant therapy. J Clin Oncol 2011; 29:4568–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habr-Gama A, Perez RO, Proscurshim I, et al. Absence of lymph nodes in the resected specimen after radical surgery for distal rectal cancer and neoadjuvant chemoradiation therapy: what does it mean? Dis Colon Rectum 2008; 51:277–283. [DOI] [PubMed] [Google Scholar]
- 19.Lee WS, Lee SH, Baek JH, et al. What does absence of lymph node in resected specimen mean after neoadjuvant chemoradiation for rectal cancer. Radiation Oncol (London, England) 2013; 8:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berger AC, Sigurdson ER, LeVoyer T, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol 2005; 23:8706–8712. [DOI] [PubMed] [Google Scholar]
- 21.De Ridder M, Vinh-Hung V, Van Nieuwenhove Y, et al. Prognostic value of the lymph node ratio in node positive colon cancer. Gut 2006; 55:1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HY, Choi HJ, Park KJ, et al. Prognostic significance of metastatic lymph node ratio in node-positive colon carcinoma. Ann Surg Oncol 2007; 14:1712–1717. [DOI] [PubMed] [Google Scholar]
- 23.Park IJ, Choi GS, Jun SH. Nodal stage of stage III colon cancer: the impact of metastatic lymph node ratio. J Surg Oncol 2009; 100:240–243. [DOI] [PubMed] [Google Scholar]
- 24.Schumacher P, Dineen S, Barnett C, Jr, et al. The metastatic lymph node ratio predicts survival in colon cancer. Am J Surg 2007; 194:827–831.discussion 831–822. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Hassett JM, Dayton MT, et al. Lymph node ratio: role in the staging of node-positive colon cancer. Ann Surg Oncol 2008; 15:1600–1608. [DOI] [PubMed] [Google Scholar]
- 26.Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994; 73:2680–2686. [DOI] [PubMed] [Google Scholar]
- 27.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ha YH, Jeong SY, Lim SB, et al. Influence of preoperative chemoradiotherapy on the number of lymph nodes retrieved in rectal cancer. Ann Surg 2010; 252:336–340. [DOI] [PubMed] [Google Scholar]
- 29.Marks JH, Valsdottir EB, Rather AA, et al. Fewer than 12 lymph nodes can be expected in a surgical specimen after high-dose chemoradiation therapy for rectal cancer. Dis Colon Rectum 2010; 53:1023–1029. [DOI] [PubMed] [Google Scholar]
- 30.de Campos-Lobato LF, Stocchi L, de Sousa JB, et al. Less than 12 nodes in the surgical specimen after total mesorectal excision following neoadjuvant chemoradiation: it means more than you think!. Ann Surg Oncol 2013; 20:3398–3406. [DOI] [PubMed] [Google Scholar]
- 31.Kang J, Hur H, Min BS, et al. Prognostic impact of the lymph node ratio in rectal cancer patients who underwent preoperative chemoradiation. J Surg Oncol 2011; 104:53–58. [DOI] [PubMed] [Google Scholar]
- 32.Kiran RP, Kirat HT, Burgess AN, et al. Is adjuvant chemotherapy really needed after curative surgery for rectal cancer patients who are node-negative after neoadjuvant chemoradiotherapy? Ann Surg Oncol 2012; 19:1206–1212. [DOI] [PubMed] [Google Scholar]


