Supplemental Digital Content is available in the text
Abstract
Decline of semen quality in past decades is suggested to be potentially associated with environmental and sociopsychobehavioral factors, but data from population-based cohort studies is limited.
The male reproductive health in Chongqing College students (MARHCS) study was established in June 2013 as a perspective cohort study that recruited voluntary male healthy college students from 3 universities in Chongqing. The primary objectives of the MARHCS study are to investigate the associations of male reproductive health in young adults with sociopsychobehavioral factors, as well as changes of environmental exposure due to the relocation from rural campus (in University Town) to metro-campus (in central downtown). A 93-item questionnaire was used to collect sociopsychobehavioral information in manner of interviewer–interviewing, and blood, urine and semen samples were collected at the same time.
The study was initiated with 796 healthy young men screened from 872 participants, with a median age of 20. About 81.8% of this population met the WHO 2010 criteria on semen quality given to the 6 routine parameters. Decreases of 12.7%, 19.8%, and 17.0%, and decreases of 7.7%, 17.6%, and 14.7% in total sperm count and sperm concentration, respectively, were found to be associated with the tertiles of accumulated smoking amount. Fried food consumption (1–2 times/wk or ≥3 times/wk vs nonconsumers) was found to be associated with decreased total sperm count (10.2% or 24.5%) and sperm concentration (13.7% or 17.2%), respectively. Coffee consumption was found to be associated with increased progressive and nonprogressive motility of 8.9% or 15.4% for subjects consuming 1–2 cups/wk or ≥3 cups/wk of coffee, respectively. Cola consumption appeared an association with decreased semen volume at 4.1% or 12.5% for 1–2 bottles/wk or ≥3 bottles/wk.
A cohort to investigate the effects of environmental/sociopsychobehavioral factors act on semen quality was successfully set up. We found smoking, coffee/cola/fried foods consumption to be significantly associated with semen quality from the baseline investigation.
INTRODUCTION
Approximately 10% of couples suffer from infertility worldwide,1 and half of them are due to male factors.2 Semen analysis is important for examining male fertility potential.3 Carlsen et al4 summarized semen quality change between 1938 and 1990 and found the dramatic decline in general populations across continents, although the results are being debated afterward.5 Current studies are mostly cross-sectional, some of them mainly recruited participants from infertility clinic patients or sperm bank donators, the season of sampling, methods used in semen analysis, and the participants’ age varied among or within studies, and the results remain controversial.6–18 Our previous study found only 38.9% of healthy male volunteers in Chongqing area met with all 6 semen parameters6 according to World Health Organization (WHO) 1999 criteria,19 although this rate can reach 62.0% as recalculated according to WHO recommendations (5th edition, WHO, Switzerland)20; it still suggests that the current semen quality, at least in Chongqing males, requires higher public health concern. Although some genetic and pathological factors for male infertility have been confirmed,21,22 in general population, accumulating evidence,11,23–28 including our previous studies,29–33 linked semen quality to environmental factors, among which persistent organic pollutants/endocrine disrupters, and electronic magnetic field exposures are extensively reported25,29,32,34–44; sociopsychobehavioral factors were also suggested to be associated with semen parameters.18,30,31,40,45–47 Moreover, age is extensively associated with semen quality,48,49 other factors such as season, geographic variation, lifestyles, methods used in semen analysis, may also modify the results. A general population-based longitudinal cohort study is necessary for illustrating the roles of environmental pollutants exposure and sociopsychobehavioral factors in semen quality alternation. Based on this, we carried out a cohort study (Figure 1) on male reproductive health in Chongqing College students with environmental exposure change on purpose of comparing semen parameters before and after the campuses relocation; assessing the effects of sociopsychobehavioral factors on semen quality; evaluating the effects of environmental pollutants, mainly polyaromatic hydrocarbons (PAHs) and phthalic acid esters (PAEs), on semen quality.
FIGURE 1.
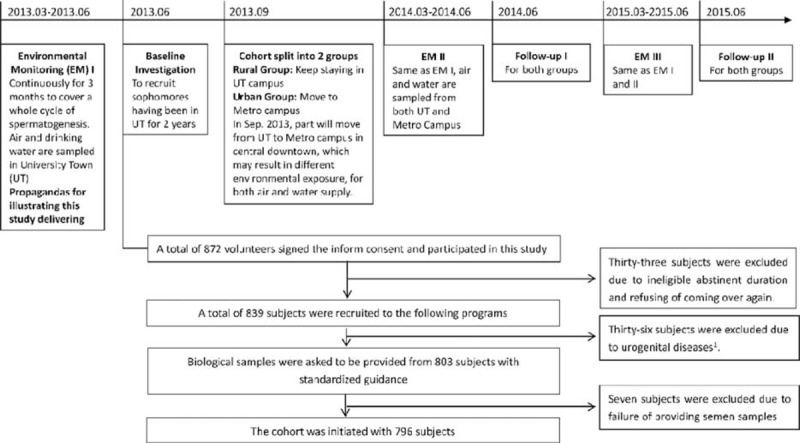
Flow chart of the design for the whole study and including/excluding process. The overall design and including/excluding process of the whole cohort study. Seventy-six patients were excluded because of the disease history of the following: 4 of urinary disease, 3 of incomplete orchiocatabasis, 2 of diabetes, 4 of tuberculosis, 4 of inguinal hernia, 2 of testicular injury, and 17 of inflammation in urogenital system.
METHODS
Study Design
Chinese universities/colleges are encountering space shortage due to fast development; local government usually assign specific lands in suburban or rural areas to set up new campuses.50 Chongqing city set up its new University Town in Huxi town, a rural area far away from downtown. Better air quality index and deep processed water can be found in the University Town when compared with the urban area.51
Most specialties in Chongqing's universities/colleges split their courses into elementary and professional phases, deploy the elementary courses in University Town during the first 2 years, and the professionals in urban old campuses during the last 2 years, leading to the students relocation from rural to urban campus to finish all courses, whereas the students from other specialties, such as Computer Science, Art, etc., will stay in the University Town till graduation. Relocated students will consequently experience environmental exposure changes naturally. This particular environmental exposure change due to campus relocation offered us an opportunity to carry out this study.
The baseline investigation was carried out in June 2013, at the end of August 2013, the fall semester began in universities/colleges, most of the participants moved to urban campuses in Shapingba district whereas the rest remained in the University Town, no more relocation would happen to either group before graduation. All eligible volunteers, moved or not, were invited to the baseline investigation. This study was approved by the Ethics Committees of Third Military Medical University, and signed informed consent was obtained from each participant.
Taking seasonal affection and change of environmental exposures into consideration, the first follow-up was performed in June 2014 with a 10-month interval (September 2013 to June 2014) covering 3 whole cycles of spermatogenesis, which may reduce possible delayed effects of environmental exposures, as well as the seasonal differences. The second follow-up is scheduled to be carried out in June 2015.
Study Population
According to the semen parameters in Chongqing general population from our previous study,6 and on the consideration of perhaps 20% of failure in follow-up and another 20% of failure in semen sample collection, we calculated the sample size with NCSS PASS 2008, suggesting that a sample size of 646 would enable explanation of biological differences and their associations to environmental or sociopsychobehavioral factors with a statistical power of 0.9 and significant level of 0.05.
Since April 2013, all male sophomores in University Town received our propaganda on illustrating this study. Online registration was opened to all male sophomores till the number of registered volunteers reached the expected sample size. In June 2013, those who met all the 3 criteria were included: >18 years old; 2 to 7 days of abstinent; and second-grade college students studying in University Town. An individual would be excluded if he met any of the following criteria: history of urologist diagnosed inflammation of urogenital system; history of epididymitis; history of testicular injury; treatment history of varicocele; history of incomplete orchiocatabasis; or any of the following was detected by the urologist at the physical examination stage during the investigation: absence of prominentia laryngea; absence of pubes; abnormal breast; abnormal penis; absence of testis; epididymal knob, or varicocele.
Environmental Contaminants Collection
Environmental exposures, including PAHs from PM2.5 and PAEs from water, were monitored for 90 days to cover one whole spermatogenesis cycle prior to sample collection. For collecting PM2.5 from ambient air in both the University Town and urban campus, impactor method was deployed and the volume of flow was set to 1 m3/min on a High Volume Ambient Air Sampler (TE-6001; Tisch Environmental, Cleves, OH) close to the students’ dormitory. PM2.5 was collected for continuously 24 hours every 2 days, and its amount was determined by weighing. From each campus in University Town and urban area, approximately 200 mL of tap water was collected into a clean, hexane-washed glass bottle, and 1 bottle of commercial bottled water (18.9 L/package) was collected in its commercial package. Water samples were collected every 4 weeks. The types and concentration of PAHs (ng/g) or PAEs (ng/μL) in air or water were sent to the Department of Biology, National Institute of Measurement and Testing Technology (Chengdu, Sichuan, China) for analyzing with gas chromatography-mass spectrometry.
Questionnaires
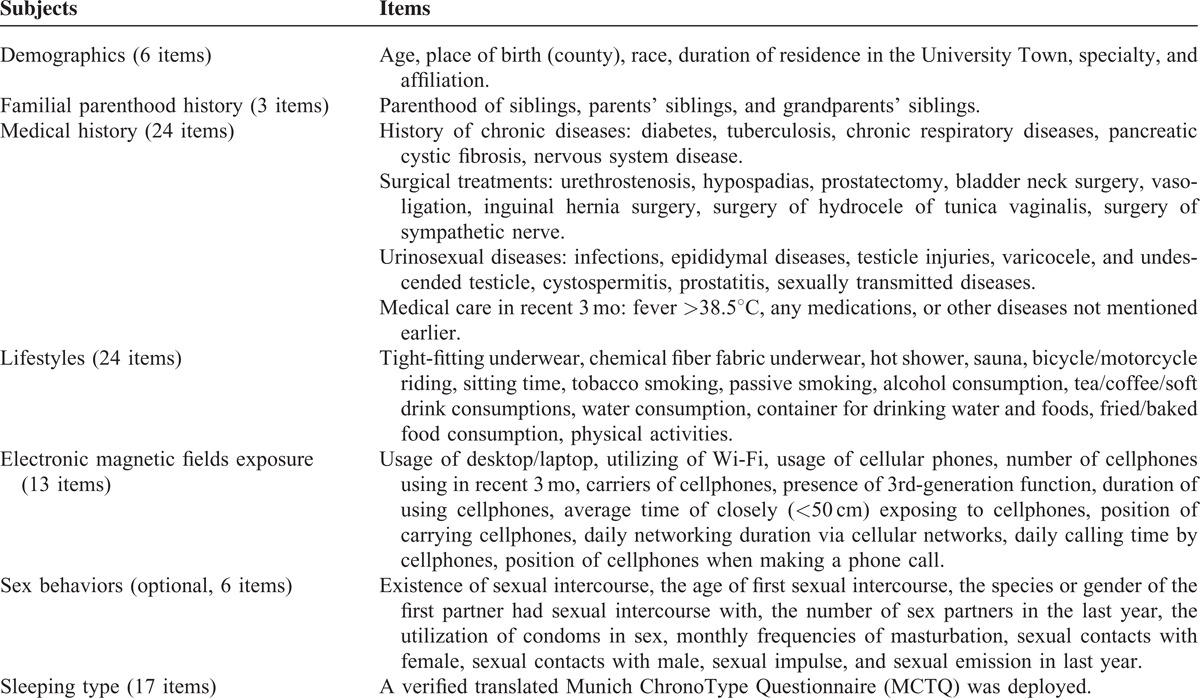
For interviewer–interviewing, the questionnaires (Table 1) consist of demographic information, a standard self-rating depression scale, an optional sexual behavioral questionnaire, a lifestyle–behavioral questionnaire (modified from our previously validated one),6 a translated Munich Chronotype Questionnaire,52 and history of diseases. For tobacco smoking, pack-year was calculated and the population was divided into tertile according to the pack-year amount. Alcohol use was defined according to the recommendation from the China Health Association.53 A bottle of cola was defined as the 550 mL commercial package on Chinese market, whereas a cup of coffee was defined as the commercial sold unit on Chinese market.
TABLE 1.
Subjects and Items Included in the Lifestyle-Behavioral Questionnaire
Physical Examination
Physical examination, performed by an experienced urologist with license, included secondary sexual characteristics (prominentia laryngea, pubes, breast, and penis), current status of pudendum (the status of foreskin, presence and volume of testis, status of epididymis, presence of varicocele, and anogenital distance), and basic biological information such as height and weight.
Biological Samples Collection and Analysis
Semen, blood, and urine were collected. For semen collection, exact abstinent duration (in days) was documented, private room was provided, and the ejaculate was collected into an ID-marked, sterile, preweighed, wide-mouth plastic container by masturbation according to WHO 2010 recommendations (5th edition, WHO, Switzerland).20 Semen sample was immediately delivered to the laboratory on the same floor and incubated in a 37°C incubator, the duration of completely liquefying (<1 hour) was documented, or until reached 1 hour. Semen quality was evaluated according to WHO 2010 guidelines20 by experienced urologist. Semen appearance was recorded by observation; semen volume was measured by weighing, assuming 1 g of weight equals to 1 mL of volume; sperm morphology was identified from semen smears prepared with 10 μL of well-mixed semen; sperm concentration and sperm motility were assessed with computer-aided sperm analysis (SCA CASA System; Microptic S.L., Barcelona, Spain). Sperm motility parameters, including curvilinear velocity (μm/s), straight-line velocity (μm/s), average path velocity (μm/s), amplitude of lateral head displacement (μm), beat-cross frequency (Hz), linearity, wobble, and straightness were recorded/calculated by CASA. Sperm motility was graded into progressive (PR) motility, nonprogressive (NP) motility, and immotility (IM).
For each subject, a total of 50 mL of medistream urine was collected into clean, hexane-washed glass tubes, and a total of 20 mL of venous blood was collected into EDTA-anticoagulant and nonanticoagulant tubes.
Serum sex hormones (testosterone, estrogen, progesterone, follicle-stimulating hormone, luteinizing hormone, and prolactin) were determined by a Beckman Unicel DXI 800.
Statistical Analysis
Demographic data was determined by direct counting. Semen parameters were presented in median and percentiles as semen parameters always follow nonnormal distributions. Percentages coincident with the criteria of WHO (2010) were also calculated. Nonparametric methods were chosen to compare distributions between groups given to lifestyles; Mann–Whitney U test or Kruskal–Wallis test of variance was deployed according to the number of groups. Jonckheere–terpstra test was used when comparing ordered groups. χ2 test was applied to compare frequencies between or among groups. Confounders were adjusted with linear regression model to analyze single lifestyles act on semen parameters. Analysis of covariance (ANCOVA) with Sidak correction was used to compare the log-transformed data for means with adjustment for confounders. Individuals with missing data were excluded in analysis among corresponding variables. All analyses were completed with IBM SPSS 22 (IBM Corp., Armonk, NY).
RESULTS
Population Characteristics
Of the 872 registers, 796 eligible subjects finished all investigations in the baseline stage. Seventy-six were excluded due to ineligible abstinent duration (N = 33), urogenital diseases (N = 36), or fail of providing semen sample (N = 7), as shown in Figure 1.
About one-fifth subjects were current smokers; almost half (47.2%) of the subjects were current drinkers. Considering all kinds of tea together, including green tea, red tea, black tea, and other tea, the tea users added up to one fifth of all subjects. More than 60% subjects consumed cola (all brands of cola were calculated together), only one-fourth subjects consumed coffee regularly (Table 2 ).
TABLE 2.
Basic Characteristics and Univariate Correlations Between Semen Parameters and Lifestyles
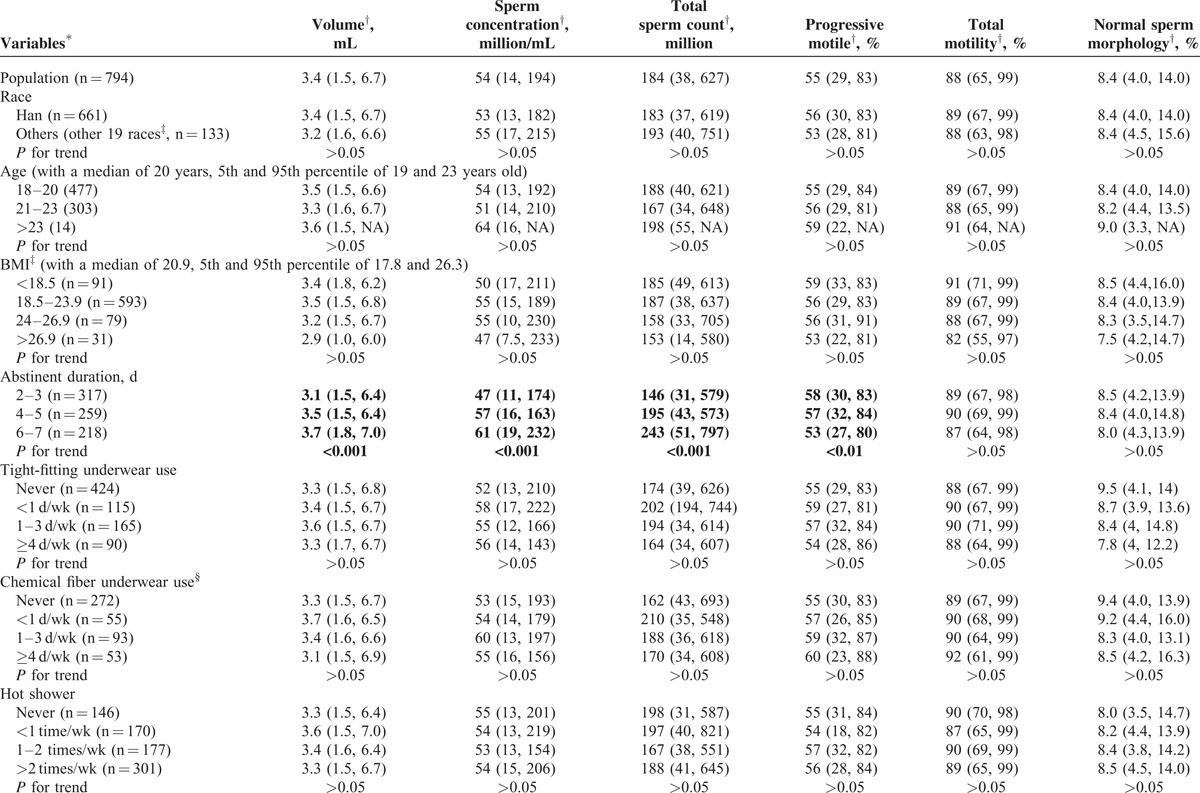
TABLE 2 (Continued).
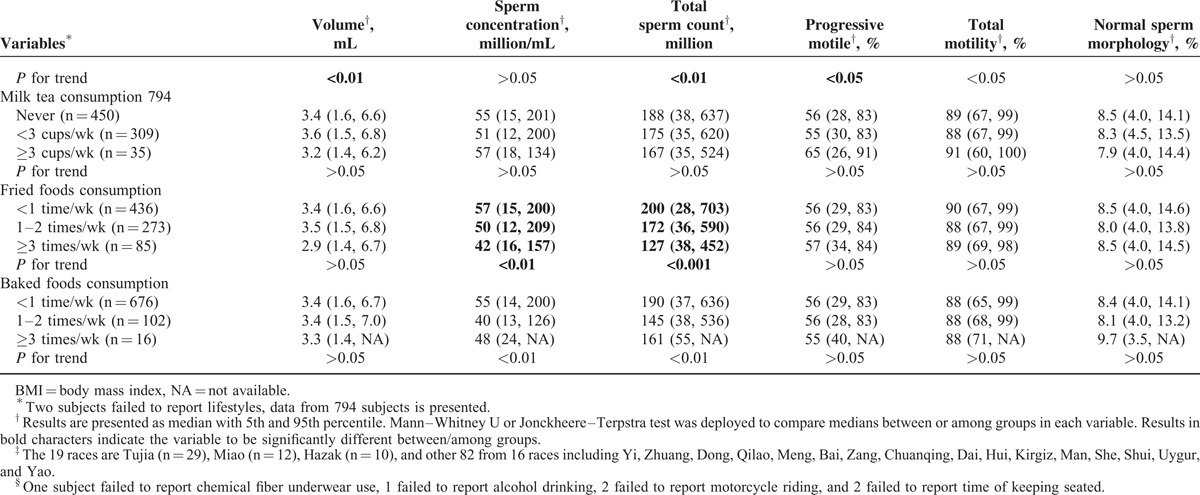
Basic Characteristics and Univariate Correlations Between Semen Parameters and Lifestyles
In August 2013, about 70.3% of the subjects (N = 454) moved to urban campuses and the rest (N = 192, 29.7%) remained in University Town, and both were followed as exposing group and control group, respectively. Subjects failed to be followed up were interviewed by phone call to determine the reason.
Semen Parameters
About 83.7% (N = 666) of the 796 college students met with the WHO 2010 criteria for all the 6 recommended parameters, which is higher than that of Chongqing general population from our previous study (62.0%).6 For each single parameter, the normal rates of semen volume, sperm concentration, total sperm number, PR, total motility (PR+NP), and normal sperm morphology were 96.4%, 94.8%, 94.8%, 92.8%, 99.9% (Figure 2), and 97.9% in college students, respectively, whereas these rates in the above-mentioned Chongqing general population6 were 82.7%, 96.8%, 91.9%, 87.0%, 90.0%, and 85.7%, respectively.
FIGURE 2.
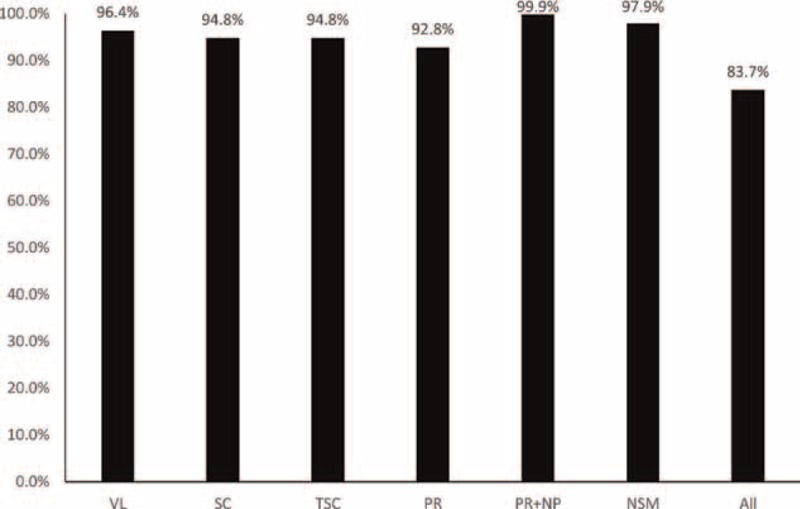
Proportions of patients with semen parameters meet with WHO 2010 criteria. The bar chart shows the rate of the college students met with each of the 6 semen parameters, as well as those met with all the 6 semen parameters.
Lifestyles Act on Semen Parameters
College students live, repast and study together, but their lifestyles varied (Table 2 ). Both univariate and multivariate analyses were deployed for evaluating the associations between lifestyles and semen parameters. In univariate analyses, a slightly decline, though nonsignificant, of semen quality in the current study was observed in the highest overweight group (body mass index, BMI, >26.9) categorized according to recommended reference for Chinese adults54; however, other studies, including our previous study,6 suggested a positive association between semen quality and obesity (BMI > 40) as systematically reviewed.18 Abstinent duration, sauna experience, smoking, coffee/cola drinking, and fried/baked foods consuming showed significant association with semen parameters (Table 2 ): first, longer abstinent duration was found to be positively associated with semen volume, sperm concentration, and total sperm count (P < 0.001), and negatively associated with PR (P < 0.01). Second, sauna experience was the only lifestyle that was associated with normal sperm morphology rate, a significantly higher normal sperm morphology rate (9.9%) was found in subjects with sauna experience when compared to that (8.3%) in those without it (P < 0.05); however, the low frequencies (usually 1 time in 3 months) may reduce the effects of high temperature on semen parameters, thus this result may be a coincidence. Third, smoking was found to be associated with decreased total sperm count (P < 0.05). Fourth, both coffee and cola drinking were found to be associated with increased PR (P < 0.01, P < 0.05, respectively); coffee drinking was also associated with increased PR+NP (P < 0.001) but cola drinking was associated with decreased semen volume and total sperm count (both P < 0.01). Fifth, both fried foods and baked foods consumption were observed to have negative associations with sperm concentration and total sperm count (all P < 0.01).
Variables that were found to be associated with semen parameters were included in the multivariate analyses, after adjusting for age, tobacco and alcohol consumption, duration of abstinent, BMI, coffee/cola/fried food/baked foods consumption with a linear regression model, we still observed smoking, coffee/cola drinking, fried foods consumption to be associated with semen parameters: first, decreases of 12.7%, 19.8%, and 17.0%, and decreases of 7.7%, 17.6%, and 14.7% in total sperm count and sperm concentration, respectively, were found to be associated with the tertiles of accumulated smoking amount (Ptrend = 0.012, 0.023, respectively; Figure 3A and B). Second, for frequencies of 1 to 2 times/wk or ≥3 times/wk, fried food consumption was found to be associated with decreased total sperm count (10.2% or 24.5%) and sperm concentration (13.7% or 17.2%), respectively (Ptrend = 0.005, 0.008, respectively; Figure 3C and D). Third, coffee consumption was found to be positively associated with PR+NP, increase of 8.9% or 15.4% for subjects consuming 1 to 2 cups/wk or ≥3 cups/wk of coffee were found, respectively (Ptrend < 0.001, Figure 3E). Suggesting caffeine may be associated only with improved sperm motility, which is not accordant to a study carried out in western countries,55,56 the much lower level of coffee intake in the current population may partially explain the difference. Fourth, cola consumption appeared an association with decreased semen volume at 4.1% or 12.5% for 1 to 2 bottles/wk or ≥3 bottles/wk (Ptrend = 0.024, Figure 3F).
FIGURE 3.
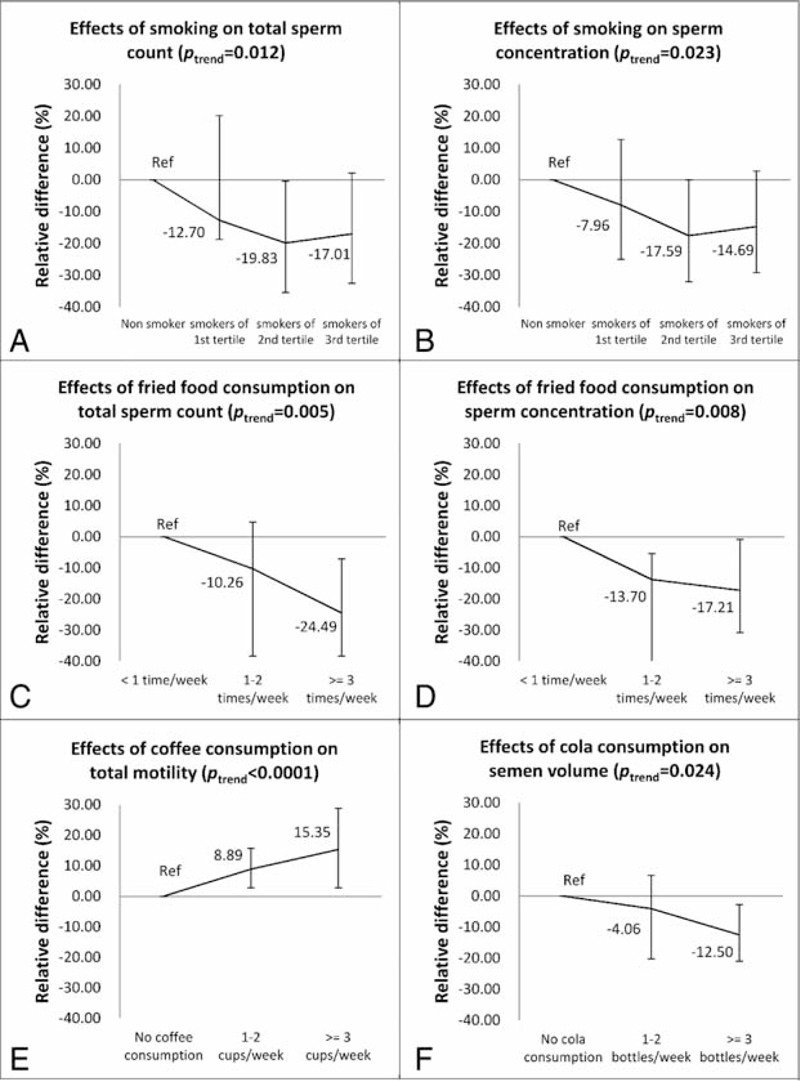
Multivariate analysis of effects of potential risks on semen quality. The 6 figures show the adjusted relative difference calculated by using a linear regression model, for each figure, nonconsumers tobacco coffee or cola, respectively, or lest consumer of fried foods, were set as reference group. (A) Adjusted for alcohol consumption, duration of abstinent, BMI, sauna experience, cola consumption, coffee consumption, and fried/baked foods consumption, Ptrend = 0.012. (B) Adjusted for alcohol consumption, duration of abstinent, BMI, sauna experience, cola consumption, coffee consumption, and fried/baked foods consumption, Ptrend = 0.023. (C) Adjusted for tobacco and alcohol consumption, duration of abstinent, BMI, sauna experience, cola consumption, coffee consumption and baked foods consumption, Ptrend = 0.005. (D) Adjusted for tobacco and alcohol consumption, duration of abstinent, BMI, sauna experience, cola consumption, coffee consumption, and baked foods consumption, Ptrend = 0.008. (E) Adjusted for tobacco and alcohol consumption, duration of abstinent, BMI, sauna experience, cola consumption, and fried/baked foods consumption, Ptrend < 0.0001. (F) Adjusted for tobacco and alcohol consumption, duration of abstinent, BMI, sauna experience, cola consumption, and fried/baked foods consumption, Ptrend = 0.024.
Including age, tobacco and alcohol consumption, duration of abstinent, BMI, and coffee/cola/fried food/baked foods consumption as covariates in an ANCOVA model, coffee consumption was found to be significantly associated with total motility. Compared with nonconsumers, consumers with <3 cups/wk showed an average of 1.09% increase in the mean of total motility, whereas for consumers with ≥3 cups/wk, that was 1.15% (P < 0.05, respectively). Consuming >3 times/wk of fried foods was found to be associated with decreased total sperm count at an average of 1.29 million (P < 0.05). Although the rest lifestyles did not show any significant difference in ANCONVA model, data was shown in Supplementary Table, http://links.lww.com/MD/A334.
DISCUSSION
Semen quality is of great concern and is debated a lot since the publication from Calsen et al.4,5 It is clear that regional decline in male fertility is happening,11 whereas the reason is not that clear. Environmental pollutions,11,23,25,33,57 as well as the change in lifestyles23,25,30,31,40,58 are considered to be the most valuable factors contributing to the decline in semen quality. To elucidate this, comparing semen quality from populations exposing to different levels of environmental pollutants may be important. However, semen quality varies a lot among populations, districts,10,59 and ages.48 These inherent factors bring confounders that are hard to be adjusted when comparing studies carried out in different populations or districts. Moreover, unlike other human samples, semen is more difficult to obtain, and men suspecting themselves to have a fertility problem might be more interested to become volunteers to participate in this kind of research. So it is suggested to sample semen from young men before they were attempting to have offspring may overcome this problem. To avoid problems raised by populations and ages, longitudinal studies may help. Longitudinal studies carried out in Czech and Denmark have been looking into this60,61; however, control group was absent in these studies. Thus, we designed this cohort study to investigate the relationship between environmental exposure and semen quality. To our knowledge, this study is the first cohort study to assess the effects of environmental exposure change on male reproductive health.
In this baseline investigation, we recruited 872 volunteers and 796 of them were eligible for this study, most excluded volunteers were due to the inappropriate abstinent time or had medications affecting semen parameters in the recent half a year. This indicates the average genital physical status of these volunteers may be relatively good when comparing to our previous study.6 We also found the average semen quality in college students to be better than that in the previous studied population,6 the differences between ages of the 2 studies may be contributed to this result. There were fewer volunteers addicted to tobacco or alcohol consumption when compared with the general population in Chongqing (nonsmokers: 74.7% vs 38.9%, nondrinkers: 51.4% vs 36.6%) from our previous study. When comparing with the general college students in western China, both the rates for smoking and drinking were close (students from our study vs college students in western area of China, nonsmokers: 74.7% vs 70.2%, nondrinkers: 51.4% vs 49.2%, but the latter study counted female into the rate, too, which may lower the rates for nonsmokers and nondrinkers as only 6.1% or 26.9% females consume tobacco or alcohol across the whole population in their study).62 This indicates the bias for lifestyle may not be significant in our study. Given to smoking, our results showed a significant decline in total sperm count but not in semen volume or sperm concentration. This is similar to another study,40 but the total sperm count was not calculated when comparing among smoker, exsmokers, and nonsmokers in that study.
Coffee consumption was found to be associated with better sperm motility in this study, which is accordant to the study by Jurewicz et al,40 but higher neck abnormalities was also found in that study, which was not observed in this study. But in other studies, decreased semen quality or its tendency was also observed.55,56,63 Similar result was found for cola consumption in this study, but lower semen volume and lower total sperm count were found in cola consumers. Researchers suggest that higher caffeine consumption usually show up with other problems in lifestyles, such as the less-healthy diet.55 Thus, the differences between this study and others toward to the effects of caffeine consumption on semen quality could be contributed to the fairly lower consumption of coffee and cola in the population from our study, as few students consumed coffee or cola excessively when compared with subjects from studies mentioned above, which means the observation of altered semen quality in cola and coffee consumption might be induced by the accompanied unhealthy lifestyles as we found that coffee and cola consumption were correlated to baked and fried foods consumption (Spearman correlation, P < 0.01 for all tests, data not shown). However, the reason why coffee did not show adverse effect in this population require more investigation, less consumption might be one of the reasons. Moreover, due to the nature of observational study, the possibility of reverse causation could not be excluded, nor could the casual association between these mentioned lifestyles and semen quality be established. Further researches are needed to confirm the findings.
By searching from PubMed, we found 10 studies carried out in populations with comparable age to our study, with semen parameters reported.9,12,14–17,64–67 Among these, 2 were carried out in Japan, Asia; 1 was carried out in the United States, and the rest were all investigated in Europeans. The age (median) varied from 18 to 24 years across all studies. Selected parameters are shown in Table 3. The data indicates regional differences among continents (Asia, America, and Europe), especially for sperm concentration and total motility (%). Studies from Europe generally reported lower sperm concentrations and total motility than those reported from Asia and America. Halling et al14 reported the lowest sperm concentration in Faroese men, and contributed this to environmental persistent organic pollutants exposures. Mendiola et al16 reported a decreasing in sperm counts in Southern Spain, the lowest percent of motile sperm was also reported by this study among the 10, yet the reason has not been revealed.
TABLE 2 (Continued).
Basic Characteristics and Univariate Correlations Between Semen Parameters and Lifestyles
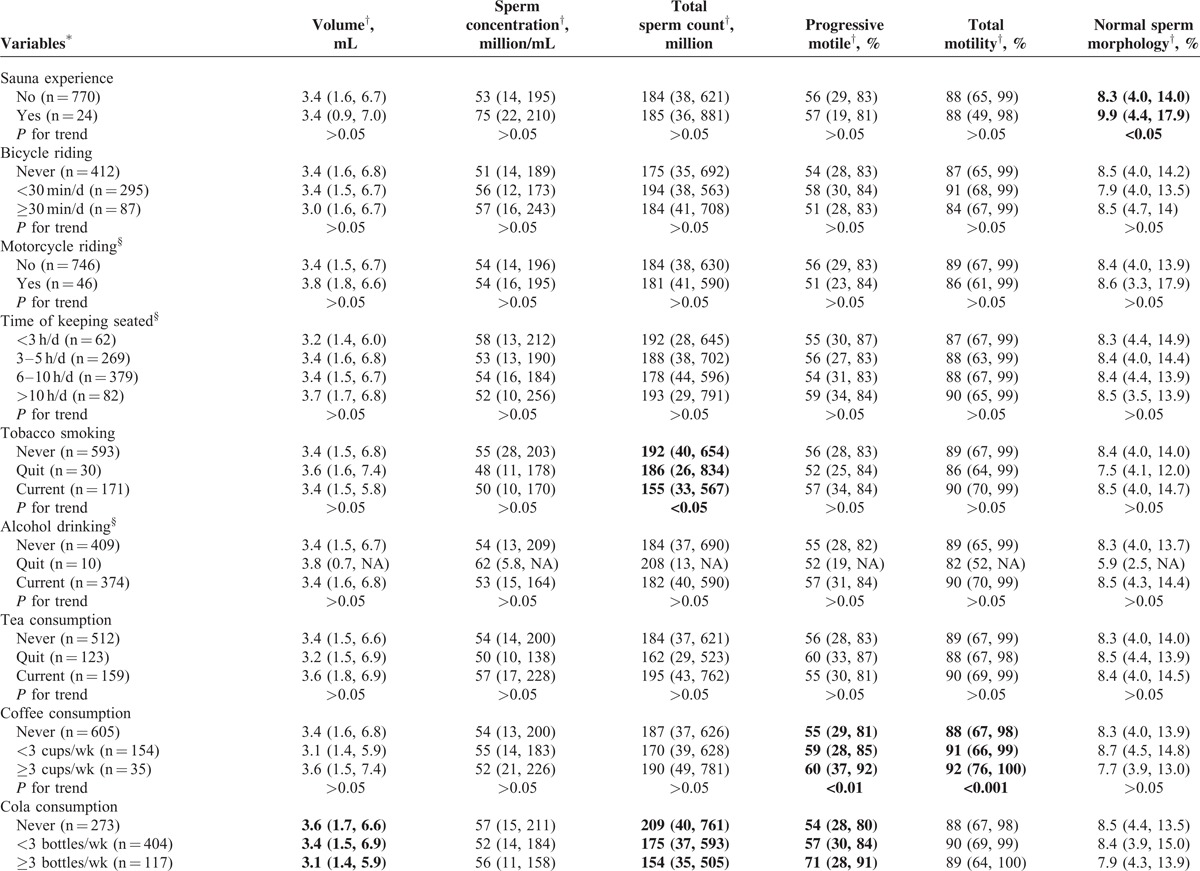
TABLE 3.
Comparison of Semen Parameters Among Populations With Considerable Age Published Between 2010 and 2014
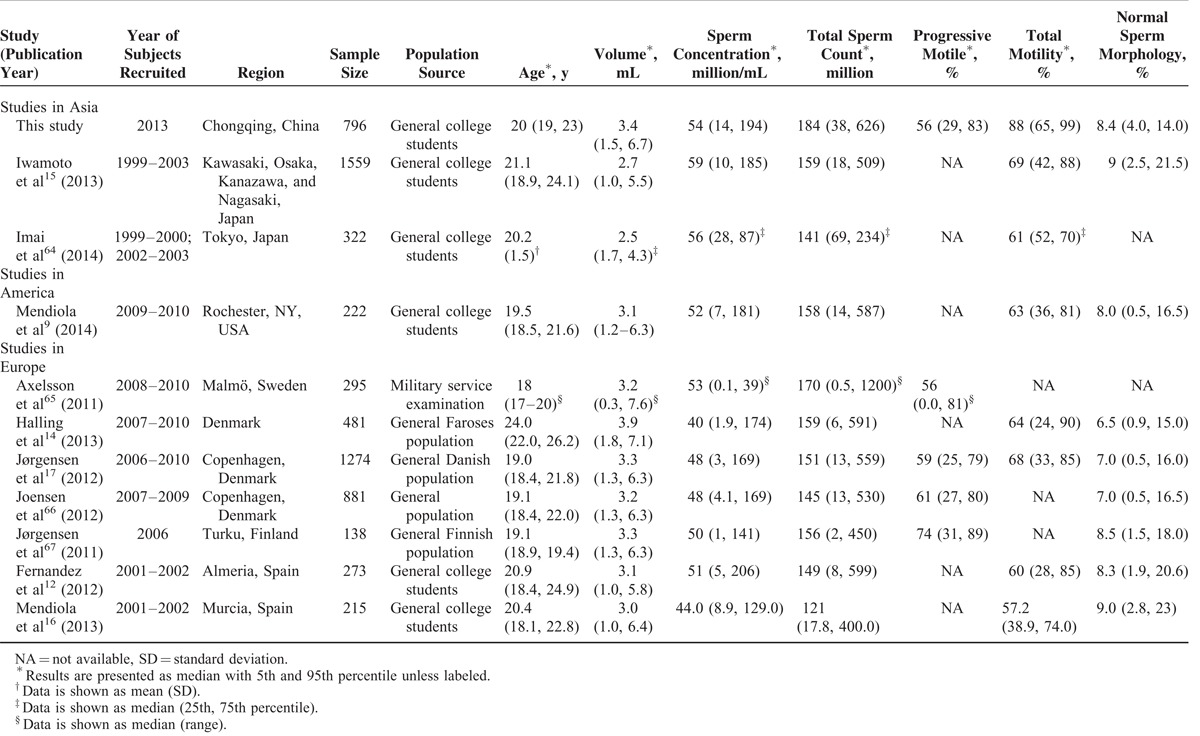
Comparing within Asians, we reported better semen volume, total sperm count and total motility, but worse sperm concentration and normal sperm morphology than the other 2 studies. Further studies looking for the reasons leading to this are warranted.
The main strength of this cohort study are first, a proportion of the studied population experienced environmental exposure change naturally due to campus relocation, leading to the before–after study in the cohort to be performed effectively. Second, the environmental exposure surveillance covered a whole cycle of spermatogenesis, and the outer and inner exposures to PAHs and PAEs were evaluated in all participants, the connections between the evaluated exposures and outcomes should be more reasonable. Third, the larger sample size, healthy population, narrowed age,49 and the same season of sampling can greatly reduce confounders. Fourth, participants were young adults prior to childbearing, which may avoid the reverse causality due to psychological stress from infertility,68 as psychological stress may result in declined semen quality. Fifth, the advanced methods for determining environmental and inner exposures should give out relatively accurate results, and the semen quality evaluation methods used as WHO recommended makes the results more comparable.
The weakness we found in this study should be stated as follows: first, the results from a population of narrowed age may lead to the explanation to be restricted in populations with comparable age. Second, only PAHs and PAEs were determined for outer and inner exposure so far due to a limited financial budget; other environmental pollutants may be potential confounders to our results. Third, no replicated sampling was deployed for each subject in each investigation, which may introduce intraindividual bias to this study. Fourth, the population mainly consisted of Chinese Han race (83.0%). The possible genetic differences among races may generate potential differences in semen parameters, but the limited population of minorities did not allow subgroup analyses. Fifth, casual associations cannot be established by this study, further researches are still needed. Sixth, during the recruitment of participants, no genetic nor chromosomal examination was applied to detect those with substantial deficit such as karyotype or chromosomal anomalies. This may lead to nondifferential misclassification, and thereby the underestimation of association investigated, although the prevalence of genetic deficit was low in general population.
CONCLUSION
The current cohort study initiated 769 healthy college male students and the baseline data showed relatively good semen parameters. Abstinent duration affected semen quality a lot. Lifestyles, such as coffee/cola drinking and fried/baked foods consumption, may be associated with semen quality. The results are similar to other studies carried out in Asians and Americans at the same age stage, but better than that was found in our previous study carried out in general population in Chongqing area. The study has several strengths and can be future developed, such as genetic association study between clock gene and semen quality, metabolizing enzymes and inner biomarkers indicating exposure to environmental pollutants, such as PAHs and PAEs. Moreover, more data are still being assessed. The follow-up investigation will provide more information on environmental exposures, and finally the relationship between the exposure and semen quality should be assessed.
Footnotes
Abbreviations: ANCOVA = analysis of covariance, BMI = body mass index, CASA = computer-aided sperm analysis, IM = immotility, MARHCS = male reproductive health in Chongqing College students, NP = nonprogressive, PAEs = phthalic acid esters, PAHs = polyaromatic hydrocarbons, PR = progressive, WHO = World Health Organization.
HuanY and QC contributed equally to this work.
This work was supported by the State Key Project 81130051 supported by the National Natural Science Foundation of China, the National Scientific and Technological Support Program (2013B112B02), and the Youth Project 81202276 supported by the National Natural Science Foundation of China.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Nachtigall RD. International disparities in access to infertility services. Fertil Steril 2006; 85:871–875. [DOI] [PubMed] [Google Scholar]
- 2.Smith RP, Coward RM, Lipshultz LI. The office visit. Urol Clin North Am 2014; 41:19–37. [DOI] [PubMed] [Google Scholar]
- 3.Centola GM. Semen assessment. Urol Clin North Am 2014; 41:163–167. [DOI] [PubMed] [Google Scholar]
- 4.Carlsen E, Giwercman A, Keiding N, et al. Evidence for decreasing quality of semen during past 50 years. BMJ 1992; 305:609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisch H. Declining worldwide sperm counts: disproving a myth. Urol Clin North Am 2008; 35:137–146. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Lin H, Ma M, et al. Semen quality of 1346 healthy men, results from the Chongqing area of southwest China. Hum Reprod Oxf Engl 2009; 24:459–469. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Y, Bonde JP, Ernst E, et al. Is semen quality related to the year of birth among Danish infertility clients? Int J Epidemiol 1997; 26:1289–1297. [DOI] [PubMed] [Google Scholar]
- 8.Zou Z, Hu H, Song M, et al. Semen quality analysis of military personnel from six geographical areas of the People's Republic of China. Fertil Steril 2011; 95:2018–2023. [DOI] [PubMed] [Google Scholar]
- 9.Mendiola J, Jørgensen N, Andersson A-M, et al. Reproductive parameters in young men living in Rochester, New York. Fertil Steril 2014; 101:1064–1071. [DOI] [PubMed] [Google Scholar]
- 10.Jørgensen N, Andersen AG, Eustache F, et al. Regional differences in semen quality in Europe. Hum Reprod Oxf Engl 2001; 16:1012–1019. [DOI] [PubMed] [Google Scholar]
- 11.Nordkap L, Joensen UN, Blomberg Jensen M, et al. Regional differences and temporal trends in male reproductive health disorders: semen quality may be a sensitive marker of environmental exposures. Mol Cell Endocrinol 2012; 355:221–230. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez MF, Duran I, Olea N, et al. Semen quality and reproductive hormone levels in men from Southern Spain. Int J Androl 2012; 35:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Afeiche M, Williams PL, Mendiola J, et al. Dairy food intake in relation to semen quality and reproductive hormone levels among physically active young men. Hum Reprod Oxf Engl 2013; 28:2265–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halling J, Petersen MS, Jørgensen N, et al. Semen quality and reproductive hormones in Faroese men: a cross-sectional population-based study of 481 men. BMJ Open 2013; 3:e001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwamoto T, Nozawa S, Mieno MN, et al. Semen quality of 1559 young men from four cities in Japan: a cross-sectional population-based study. BMJ Open 2013; 3:e002222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendiola J, Jørgensen N, Mínguez-Alarcón L, et al. Sperm counts may have declined in young university students in Southern Spain. Andrology 2013; 1:408–413. [DOI] [PubMed] [Google Scholar]
- 17.Jørgensen N, Joensen UN, Jensen TK, et al. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open 2012; 2:e000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sermondade N, Faure C, Fezeu L, et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update 2013; 19:221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Department of Reproductive Health and Research WHO. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. Fourth edition; 1999. [Google Scholar]
- 20.Department of Reproductive Health and Research WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen. Fifth edition; 2010. [Google Scholar]
- 21.Krausz C. Male infertility: pathogenesis and clinical diagnosis. Best Pract Res Clin Endocrinol Metab 2011; 25:271–285. [DOI] [PubMed] [Google Scholar]
- 22.Lotti F, Maggi M. Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update 2015; 21:56–83. [DOI] [PubMed] [Google Scholar]
- 23.Pacey AA. Environmental and lifestyle factors associated with sperm DNA damage. Hum Fertil Camb Engl 2010; 13:189–193. [DOI] [PubMed] [Google Scholar]
- 24.Pant N, Pant A, Shukla M, et al. Environmental and experimental exposure of phthalate esters: the toxicological consequence on human sperm. Hum Exp Toxicol 2011; 30:507–514. [DOI] [PubMed] [Google Scholar]
- 25.Barazani Y, Katz BF, Nagler HM, et al. Lifestyle, environment, and male reproductive health. Urol Clin North Am 2014; 41:55–66. [DOI] [PubMed] [Google Scholar]
- 26.Balabanič D, Rupnik M, Klemenčič AK. Negative impact of endocrine-disrupting compounds on human reproductive health. Reprod Fertil Dev 2011; 23:403–416. [DOI] [PubMed] [Google Scholar]
- 27.Giwercman A, Carlsen E, Keiding N, et al. Evidence for increasing incidence of abnormalities of the human testis: a review. Environ Health Perspect 1993; 101 (suppl 2):65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonde JP, Kolstad H. Fertility of Danish battery workers exposed to lead. Int J Epidemiol 1997; 26:1281–1288. [DOI] [PubMed] [Google Scholar]
- 29.Han X, Zhou N, Cui Z, et al. Association between urinary polycyclic aromatic hydrocarbon metabolites and sperm DNA damage: a population study in Chongqing, China. Environ Health Perspect 2011; 119:652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Lin H, Li Y, et al. Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil Steril 2011; 95:116–123. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Li Y, Zhou N, et al. Socio-psycho-behavioural factors associated with male semen quality in China: results from 1346 healthy men in Chongqing. J Fam Plan Reprod Health Care Fac Fam Plan Reprod Health Care 2013; 39:102–110. [DOI] [PubMed] [Google Scholar]
- 32.Han X, Cui Z, Zhou N, et al. Urinary phthalate metabolites and male reproductive function parameters in Chongqing general population, China. Int J Hyg Environ Health 2014; 217:271–278. [DOI] [PubMed] [Google Scholar]
- 33.Zhou N, Cui Z, Yang S, et al. Air pollution and decreased semen quality: a comparative study of Chongqing urban and rural areas. Environ Pollut Barking Essex 1987 2014; 187C:145–152. [DOI] [PubMed] [Google Scholar]
- 34.Sorgucu U, Develi I. Measurement and analysis of electromagnetic pollution generated by GSM-900 mobile phone networks in Erciyes University, Turkey. Electromagn Biol Med 2012; 31:404–415. [DOI] [PubMed] [Google Scholar]
- 35.Song XF, Chen ZY, Zang ZJ, et al. Investigation of polycyclic aromatic hydrocarbon level in blood and semen quality for residents in Pearl River Delta Region in China. Environ Int 2013; 60:97–105. [DOI] [PubMed] [Google Scholar]
- 36.Jeng HA, Pan C-H, Lin W-Y, et al. Biomonitoring of polycyclic aromatic hydrocarbons from coke oven emissions and reproductive toxicity in nonsmoking workers. J Hazard Mater 2013; 244-245:436–443. [DOI] [PubMed] [Google Scholar]
- 37.Pant N, Shukla M, Kumar Patel D, et al. Correlation of phthalate exposures with semen quality. Toxicol Appl Pharmacol 2008; 231:112–116. [DOI] [PubMed] [Google Scholar]
- 38.Jurewicz J, Radwan M, Sobala W, et al. Human urinary phthalate metabolites level and main semen parameters, sperm chromatin structure, sperm aneuploidy and reproductive hormones. Reprod Toxicol Elmsford N 2013; 42:232–241. [DOI] [PubMed] [Google Scholar]
- 39.Dama MS, Bhat MN. Mobile phones affect multiple sperm quality traits: a meta-analysis. F1000Research 2013; 2:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jurewicz J, Radwan M, Sobala W, et al. Lifestyle and semen quality: role of modifiable risk factors. Syst Biol Reprod Med 2014; 60:43–51. [DOI] [PubMed] [Google Scholar]
- 41.Rago R, Salacone P, Caponecchia L, et al. The semen quality of the mobile phone users. J Endocrinol Invest 2013; 36:970–974. [DOI] [PubMed] [Google Scholar]
- 42.Gutschi T, Mohamad Al-Ali B, Shamloul R, et al. Impact of cell phone use on men's semen parameters. Andrologia 2011; 43:312–316. [DOI] [PubMed] [Google Scholar]
- 43.Davar R, Sekhavat L, Naserzadeh N. Semen parameters of non-infertile smoker and non-smoker men. J Med Life 2012; 5:465–468. [PMC free article] [PubMed] [Google Scholar]
- 44.Povey AC, Clyma J-A, McNamee R, et al. Modifiable and non-modifiable risk factors for poor semen quality: a case-referent study. Hum Reprod Oxf Engl 2012; 27:2799–2806. [DOI] [PubMed] [Google Scholar]
- 45.Akl EA, Gaddam S, Gunukula SK, et al. The effects of waterpipe tobacco smoking on health outcomes: a systematic review. Int J Epidemiol 2010; 39:834–857. [DOI] [PubMed] [Google Scholar]
- 46.Gollenberg AL, Liu F, Brazil C, et al. Semen quality in fertile men in relation to psychosocial stress. Fertil Steril 2010; 93:1104–1111. [DOI] [PubMed] [Google Scholar]
- 47.Lotti F, Corona G, Vitale P, et al. Current smoking is associated with lower seminal vesicles and ejaculate volume, despite higher testosterone levels, in male subjects of infertile couples. Hum Reprod 2015; 30:590–602. [DOI] [PubMed] [Google Scholar]
- 48.Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril 2001; 75:237–248. [DOI] [PubMed] [Google Scholar]
- 49.Zhu Q-X, Meads C, Lu M-L, et al. Turning point of age for semen quality: a population-based study in Chinese men. Fertil Steril 2011; 96:572–576. [DOI] [PubMed] [Google Scholar]
- 50.Yang T, Chen X. On the Concept of University City. J Beijing Polytech Univ Soc Sci Ed 2003; 3:86–89. [Google Scholar]
- 51.Chongqing Environmental Science Research Institute. The Announcement of Daily Air Quality Index of Chongqing. Chongqing Environmental Science Research Institute. http://www.cepb.gov.cn/kq/?index=2 Accessed on March 8th, 2012 and updated on January 16th, 2013. [Google Scholar]
- 52.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms 2003; 18:80–90. [DOI] [PubMed] [Google Scholar]
- 53.Healthy Drinking Habits Survey in 25 Provinces over China, 2007. China Health Care Association; 2008. [Google Scholar]
- 54.Bei-Fan Z. Cooperative Meta-Analysis Group of Working Group on Obesity in China Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults: study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Asia Pac J Clin Nutr 2002; 11 (suppl 8):S685–S693. [PubMed] [Google Scholar]
- 55.Jensen TK, Swan SH, Skakkebaek NE, et al. Caffeine intake and semen quality in a population of 2,554 young Danish men. Am J Epidemiol 2010; 171:883–891. [DOI] [PubMed] [Google Scholar]
- 56.Sadeu JC, Hughes CL, Agarwal S, et al. Alcohol, drugs, caffeine, tobacco, and environmental contaminant exposure: reproductive health consequences and clinical implications. Crit Rev Toxicol 2010; 40:633–652. [DOI] [PubMed] [Google Scholar]
- 57.Jurewicz J, Hanke W, Radwan M, et al. Environmental factors and semen quality. Int J Occup Med Environ Health 2009; 22:305–329. [DOI] [PubMed] [Google Scholar]
- 58.Sermondade N, Faure C, Fezeu L, et al. Obesity-Fertility Collaborative Group Obesity and increased risk for oligozoospermia and azoospermia. Arch Intern Med 2012; 172:440–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swan SH, Brazil C, Drobnis EZ, et al. Geographic differences in semen quality of fertile U.S. males. Environ Health Perspect 2003; 111:414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Selevan SG, Borkovec L, Slott VL, et al. Semen quality and reproductive health of young Czech men exposed to seasonal air pollution. Environ Health Perspect 2000; 108:887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carlsen E, Swan SH, Petersen JH, et al. Longitudinal changes in semen parameters in young Danish men from the Copenhagen area. Hum Reprod Oxf Engl 2005; 20:942–949. [DOI] [PubMed] [Google Scholar]
- 62.Guo S, Yu X, Zhang X, et al. Cluster analysis of smoking, alcohol drinking and other health risk behaviors in undergraduate students. Beijing Da Xue Xue Bao 2013; 45:382–386. [PubMed] [Google Scholar]
- 63.Wogatzky J, Wirleitner B, Stecher A, et al. The combination matters—distinct impact of lifestyle factors on sperm quality: a study on semen analysis of 1683 patients according to MSOME criteria. Reprod Biol Endocrinol RBE 2012; 10:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Imai K, Yoshinaga J, Yoshikane M, et al. Pyrethroid insecticide exposure and semen quality of young Japanese men. Reprod Toxicol Elmsford N 2014; 43:38–44. [DOI] [PubMed] [Google Scholar]
- 65.Axelsson J, Rylander L, Rignell-Hydbom A, et al. No secular trend over the last decade in sperm counts among Swedish men from the general population. Hum Reprod Oxf Engl 2011; 26:1012–1016. [DOI] [PubMed] [Google Scholar]
- 66.Joensen UN, Frederiksen H, Jensen MB, et al. Phthalate excretion pattern and testicular function: a study of 881 healthy Danish men. Environ Health Perspect 2012; 120:1397–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jorgensen N, Vierula M, Jacobsen R, et al. Recent adverse trends in semen quality and testis cancer incidence among Finnish men. Int J Androl 2011; 34:e37–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jensen TK, Andersson A-M, Skakkebæk NE, et al. Association of sleep disturbances with reduced semen quality: a cross-sectional study among 953 healthy young Danish men. Am J Epidemiol 2013; 177:1027–1037. [DOI] [PubMed] [Google Scholar]


