Lipid phosphate phosphatases (LPPs, encoded by the PPAP2 genes) are broad specificity enzymes that can dephosphorylate lipid phosphate esters such as phosphatidic acid (PA), lysophosphatidic acid (LPA), and sphingosine- 1- phosphate (S1P) that serve as critical intermediates in intracellular pathways of lipid metabolism and signaling (Fig. 1) (1). Of these substrates, LPA and S1P are also bioactive mediators that can be released or generated extracellularly and/or act on cell surface receptors to initiate a broad range of cellular responses (2). LPPs can localize to the cell surface with their active site facing the extracellular space allowing them to function as ecto enzymes (3, 4). Accordingly, it has been attractive to speculate that one function of these enzymes is to terminate the receptor-mediated signaling actions of LPA and S1P by dephosphorylating them to generate the corresponding alcohols, which are not receptor-active. In support of this concept, overexpression of LPP1 or LPP3 increases the LPP activity of several intact cells, and this activity is decreased by silencing or genetic inactivation of the corresponding PPAP2A and PPAP2B genes (5–7). These manipulations are broadly associated with reciprocal effects on LPA responsiveness of stereotypic cellular responses to this stimulus, particularly cell growth and migration. Mice that are genetically hypomorphic for the PPAP2A gene encoding LPP1 exhibit increased circulating LPA levels (8) whereas inducible postnatal and/or tissue-restricted inactivation of the PPAP2B gene encoding LPP3 alters S1P gradients that control lymphocyte egress from the lymphatic system to the peripheral circulation (9) and renders vascular smooth muscle cells more responsive to LPA (6).
Fig. 1.
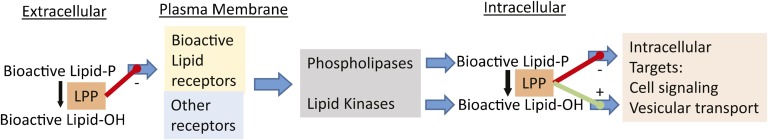
Regulation of extra- and intra-cellular lipid metabolism and signaling by LPPs. Cell surface localized LPPs can act on extracellular or plasma membrane-associated substrates to limit their actions on cell surface receptors. LPPs can also act intracellularly to alter the balance between bioactive lipid phosphate mediators and their bioactive dephosphorylation products; for example, phosphatidic acid and diacylglycerol.
In Drosophila, LPP homologs encoded by the Wunen genes regulate the migration of germ cells in the developing embryo (10). Ectopic expression of these genes in somatic tissues that are normally permissive for germ cell migration results in repulsion of the migrating germ cells. This nonautonomous function of the Wunen genes is consistent with the idea that a diffusible Wunen substrate regulates germ cell migration (11). However, Wunens are also expressed in the germ cells themselves from maternally inherited mRNA and inactivation of Wunen in the female germline also decreases germ cell migration identifying a cell autonomous function for these genes (12).
LPPs also localize to intracellular membranes. Manipulations of LPP expression have also been shown to alter levels of cell-associated LPP substrates and products including PA and its dephosphorylation product diacylglycerol (DG) that are well established to regulate intracellular signaling pathways. These include pathways mediated by the Raf and mTOR protein kinases and conventional protein kinases C (13, 14). Evidence that LPP3 has an intracellular role as a regulator of vesicular transport between the endoplasmic reticulum and Golgi apparatus has also been reported (15). It is also important to note that not all studies of the effects of LPP overexpression have revealed phenotypes in cell and animals that can be explained simply by attenuation of LPA signaling (16). Accordingly, although there is a broad consensus that manipulating LPP expression can alter cellular signaling responses to bioactive lipid phosphate mediators in some systems and often in a manner that depends on catalytic activity of the enzymes, these effects could be mediated by directly limiting the agonist actions of the mediators by degradation of extracellular or cell-surface associated lipids, by postreceptor effects on downstream lipid signaling pathways, or a combination of both (Fig. 1).
In the current issue of the Journal of Lipid Research, Tang et al. (17) generated breast cancer cell lines with inducible expression of wild-type and catalytically inactive LPP1. Increased ecto LPA and S1P phosphatase activity in these cells was associated with decreased growth and migration in response to LPA and serum (a rich source of LPA). Migration responses of these cells to LPA but not epidermal growth factor (EGF) were selectively attenuated by increased LPP1 expression, and these effects are likely mediated, at least in part, by decreased activation of Rho family guanosine triphosphate hydrolases (GTPases). Phosphatase resistant LPA analogs with agonist activities at LPA receptors (phosphonates and thiophosphates) offer an elegant approach to determine the extent to which observed decreases in responses to bioactive lipid phosphate agonists in LPP-overexpressing cells can be explained by increased dephosphorylation and consequent inactivation of these mediators (5–7). Tang et al. found that mobilization of intracellular Ca2+ in response to LPA receptor activation by a metabolically stable LPA analog or to an agonist acting at protease activated receptor 1 was preserved in LPP1-overexpressing cells but still decreased in comparison to the response observed in cells overexpressing a catalytically inactive LPP1 variant. While consistent with observations that LPP-resistant LPA analogs can circumvent decreases in LPA signaling responses observed in LPP-overexpressing cells, these observations provide further evidence that overexpression of LPP1 has postreceptor effects on signaling responses to both LPA and a nonlipid agonist, at least on the Ca2+ mobilization response studied. These authors reported that overexpression of LPP1 dramatically attenuates metastasis and tumor growth when these cells were studied in a mouse xenograft model, providing further evidence that targeting LPA metabolism and signaling could be a beneficial strategy for breast cancer therapy (18).
Understanding how LPPs regulate lipid metabolism and signaling has broad relevance to human disease because a common variant of the PPAP2B gene encoding LPP3 that appears to predict lower expression of the enzyme is associated with increased risk of coronary artery disease (19). LPA is present in oxidized low density lipoprotein and elicits responses that are broadly inflammatory and pro-atherogenic in multiple blood and vascular cell types (20, 21). While PPAP2B is essential for embryonic development (22) (and must therefore function nonredundantly with the other PPAP2 genes during this process), mice with restricted or inducible postnatal inactivation of LPP3 in vascular smooth muscle cells and vascular endothelial cells exhibit increased injury-induced intimal hyperplasia and vascular permeability, respectively (6, 23). Because LPA receptor deficient mice are protected in models of aortic atherosclerosis, the most parsimonious explanation of these data would be that LPP3 normally suppresses atherosclerosis by opposing LPA signaling. However, this idea needs to be tested experimentally. Taken together with previous reports, this new study by Tang et al. focuses attention on the possibility that LPPs can also regulate signaling responses to LPA and potentially other agonists through postreceptor effects on intracellular signaling pathways. These observations suggest that the range and impact of alterations in LPP expression extend beyond inactivation of bioactive lipids at the cell surface or in the extra-cellular space. Postreceptor effects of LPP expression could be associated with human heritable cardiovascular disease risk and with breast and ovarian cancers.
REFERENCES
- 1.Morris A. J., Smyth S. S., Salous A. K., Renault A. D. 2013. Lipid phosphate phosphatases: recent progress and assay methods. In Lysophospholipid Receptors. John Wiley & Sons, Inc. Hoboken, NJ. 229–263. [Google Scholar]
- 2.Choi J. W., Herr D. R., Noguchi K., Yung Y. C., Lee C. W., Mutoh T., Lin M. E., Teo S. T., Park K. E., Mosley A. N., et al. 2010. LPA receptors: subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 50: 157–186. [DOI] [PubMed] [Google Scholar]
- 3.Roberts R., Sciorra V. A., Morris A. J. 1998. Human type 2 phosphatidic acid phosphohydrolases. Substrate specificity of the type 2a, 2b, and 2c enzymes and cell surface activity of the 2a isoform. J. Biol. Chem. 273: 22059–22067. [DOI] [PubMed] [Google Scholar]
- 4.Jasinska R., Zhang Q. X., Pilquil C., Singh I., Xu J., Dewald J., Dillon D. A., Berthiaume L. G., Carman G. M., Waggoner D. W., et al. 1999. Lipid phosphate phosphohydrolase-1 degrades exogenous glycerolipid and sphingolipid phosphate esters. Biochem. J. 340: 677–686. [PMC free article] [PubMed] [Google Scholar]
- 5.Tanyi J. L., Morris A. J., Wolf J. K., Fang X., Hasegawa Y., Lapushin R., Auersperg N., Sigal Y. J., Newman R. A., Felix E. A., et al. 2003. The human lipid phosphate phosphatase-3 decreases the growth, survival, and tumorigenesis of ovarian cancer cells: validation of the lysophosphatidic acid signaling cascade as a target for therapy in ovarian cancer. Cancer Res. 63: 1073–1082. [PubMed] [Google Scholar]
- 6.Panchatcharam M., Miriyala S., Salous A., Wheeler J., Dong A., Mueller P., Sunkara M., Escalante-Alcalde D., Morris A. J., Smyth S. S. 2013. Lipid phosphate phosphatase 3 negatively regulates smooth muscle cell phenotypic modulation to limit intimal hyperplasia. Arterioscler. Thromb. Vasc. Biol. 33: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hooks S. B., Santos W. L., Im D. S., Heise C. E., Macdonald T. L., Lynch K. R. 2001. Lysophosphatidic acid-induced mitogenesis is regulated by lipid phosphate phosphatases and is Edg-receptor independent. J. Biol. Chem. 276: 4611–4621. [DOI] [PubMed] [Google Scholar]
- 8.Tomsig J. L., Snyder A. H., Berdyshev E. V., Skobeleva A., Mataya C., Natarajan V., Brindley D. N., Lynch K. R. 2009. Lipid phosphate phosphohydrolase type 1 (LPP1) degrades extracellular lysophosphatidic acid in vivo. Biochem. J. 419: 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bréart B., Ramos-Perez W. D., Mendoza A., Salous A. K., Gobert M., Huang Y., Adams R. H., Lafaille J. J., Escalante-Alcalde D., Morris A. J., et al. 2011. Lipid phosphate phosphatase 3 enables efficient thymic egress. J. Exp. Med. 208: 1267–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang N., Zhang J., Purcell K. J., Cheng Y., Howard K. 1997. The Drosophila protein Wunen repels migrating germ cells. Nature. 385: 64–67. [DOI] [PubMed] [Google Scholar]
- 11.Starz-Gaiano M., Cho N. K., Forbes A., Lehmann R. 2001. Spatially restricted activity of a Drosophila lipid phosphatase guides migrating germ cells. Development. 128: 983–991. [DOI] [PubMed] [Google Scholar]
- 12.Renault A. D., Sigal Y. J., Morris A. J., Lehmann R. 2004. Soma-germ line competition for lipid phosphate uptake regulates germ cell migration and survival. Science. 305: 1963–1966. [DOI] [PubMed] [Google Scholar]
- 13.Sciorra V. A., Morris A. J. 1999. Sequential actions of phospholipase D and phosphatidic acid phosphohydrolase 2b generate diglyceride in mammalian cells. Mol. Biol. Cell. 10: 3863–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alderton F., Darroch P., Sambi B., McKie A., Ahmed I. S., Pyne N., Pyne S. 2001. G-protein-coupled receptor stimulation of the p42/p44 mitogen-activated protein kinase pathway is attenuated by lipid phosphate phosphatases 1, 1a, and 2 in human embryonic kidney 293 cells. J. Biol. Chem. 276: 13452–13460. [DOI] [PubMed] [Google Scholar]
- 15.Gutiérrez-Martinez E., Fernandez-Ulibarri I., Lazaro-Dieguez F., Johannes L., Pyne S., Sarri E., Egea G. 2013. Lipid phosphate phosphatase 3 participates in transport carrier formation and protein trafficking in the early secretory pathway. J. Cell Sci. 126: 2641–2655. [DOI] [PubMed] [Google Scholar]
- 16.Yue J., Yokoyama K., Balazs L., Baker D. L., Smalley D., Pilquil C., Brindley D. N., Tigyi G. 2004. Mice with transgenic overexpression of lipid phosphate phosphatase-1 display multiple organotypic deficits without alteration in circulating lysophosphatidate level. Cell. Signal. 16: 385–399. [DOI] [PubMed] [Google Scholar]
- 17.Tang X., Benesch M. G. K., Dewald J., Zhao Y. Y., Patwardhan N., Santos W. L., Curtis J. M., McMullen T. P. W., Brindley D. N. 2014. Lipid phosphate phosphatase-1 expression in cancer cells attenuates tumor growth and metastasis in mice. J. Lipid Res. 55: 2389–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brindley D. N., Lin F. T., Tigyi G. J. 2013. Role of the autotaxin-lysophosphatidate axis in cancer resistance to chemotherapy and radiotherapy. Biochim. Biophys. Acta. 1831: 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schunkert H., Konig I. R., Kathiresan S., Reilly M. P., Assimes T. L., Holm H., Preuss M., Stewart A. F., Barbalic M., Gieger C., et al. 2011. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 43: 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Z., Subramanian P., Sevilmis G., Globke B., Soehnlein O., Karshovska E., Megens R., Heyll K., Chun J., Saulnier-Blache J. S., et al. 2011. Lipoprotein-derived lysophosphatidic acid promotes atherosclerosis by releasing CXCL1 from the endothelium. Cell Metab. 13: 592–600. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C., Baker D. L., Yasuda S., Makarova N., Balazs L., Johnson L. R., Marathe G. K., McIntyre T. M., Xu Y., Prestwich G. D., et al. 2004. Lysophosphatidic acid induces neointima formation through PPARgamma activation. J. Exp. Med. 199: 763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escalante-Alcalde D., Hernandez L., Le Stunff H., Maeda R., Lee H. S., Jr Gang C., Sciorra V. A., Daar I., Spiegel S., Morris A. J., et al. 2003. The lipid phosphatase LPP3 regulates extra-embryonic vasculogenesis and axis patterning. Development. 130: 4623–4637. [DOI] [PubMed] [Google Scholar]
- 23.Panchatcharam M., Salous A. K., Brandon J., Miriyala S., Wheeler J., Patil P., Sunkara M., Morris A. J., Escalante-Alcalde D., Smyth S. S. 2014. Mice With Targeted Inactivation of Ppap2b in Endothelial and Hematopoietic Cells Display Enhanced Vascular Inflammation and Permeability. Arterioscler. Thromb. Vasc. Biol. 34: 837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]


