Abstract
Lysosomes play a vital role in the maintenance of cellular homeostasis through the recycling of cell constituents, a key metabolic function which is highly dependent on the correct function of the lysosomal hydrolases and membrane proteins, as well as correct membrane lipid stoichiometry and composition. The critical role of lysosomal functionality is evident from the severity of the diseases in which the primary lesion is a genetically defined loss-of-function of lysosomal hydrolases or membrane proteins. This group of diseases, known as lysosomal storage diseases (LSDs), number more than 50 and are associated with severe neurodegeneration, systemic disease, and early death, with only a handful of the diseases having a therapeutic option. Another key homeostatic system is the metabolic stress response or heat shock response (HSR), which is induced in response to a number of physiological and pathological stresses, such as protein misfolding and aggregation, endoplasmic reticulum stress, oxidative stress, nutrient deprivation, elevated temperature, viral infections, and various acute traumas. Importantly, the HSR and its cardinal members of the heat shock protein 70 family has been shown to protect against a number of degenerative diseases, including severe diseases of the nervous system. The cytoprotective actions of the HSR also include processes involving the lysosomal system, such as cell death, autophagy, and protection against lysosomal membrane permeabilization, and have shown promise in a number of LSDs. This review seeks to describe the emerging understanding of the interplay between these two essential metabolic systems, the lysosomes and the HSR, with a particular focus on their potential as a therapeutic target for LSDs.
Keywords: sphingolipids, glycosphingolipids, heat shock proteins, molecular chaperones, stress response
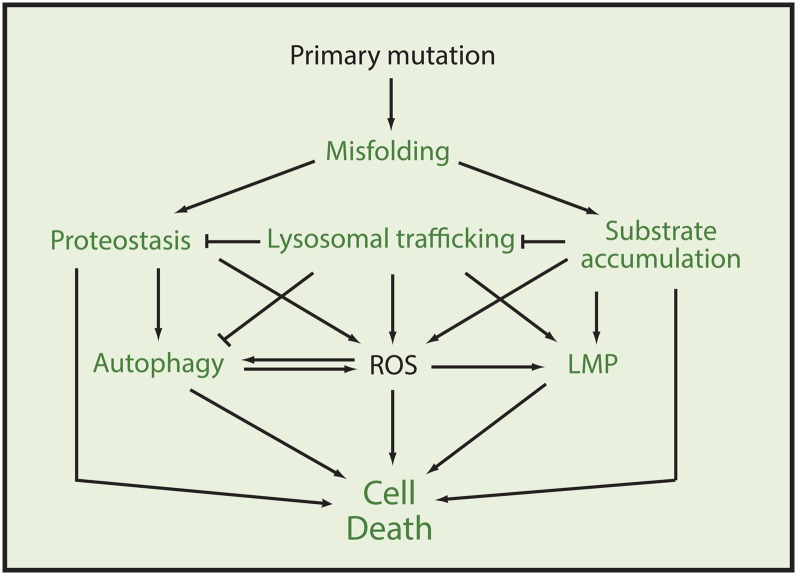
The lysosomal storage diseases (LSDs) describe a heterogenous family of rare inherited diseases caused by mutations in lysosomal proteins and are characterized by accumulation of macromolecules or monomeric compounds inside organelles of the endo-lysosomal system (1–3). The LSDs present complex disease phenotypes with the mechanisms leading to pathology being poorly understood, as the disease and extent of pathology seem to depend on the spatiotemporal accumulation of substrates which have a variety of downstream effects depending on their cellular and physiological context (Table 1). It is thus difficult to generalize for the LSDs, but as the origin of the diseases in all cases is a genetic lesion, which most often causes a misfolded dysfunctional protein, the cellular processes and responses related to misfolded proteins have to be considered as an integral part of the etiology of any of the diseases. Thus, the shared mechanistic principle of disturbed protein homeostasis and the fact that many LSDs also show an overlap of potentially toxic storage compounds might explain why the LSDs, despite their various monogenetic origins, share a number of cellular and clinical manifestations including perturbed lysosomal trafficking and autophagy, increased oxidative stress, impaired calcium homeostasis, loss of lysosomal stability, increased endoplasmic reticulum (ER)-stress responses, and cell death (Fig. 1) (4–7).
TABLE 1.
Common metabolites accumulating in various LSDs and their potential cellular impact
| Storage | Cellular Impact | Reference |
| GT1b | Neurotoxicity (mediated by Akt/GSK-3/tau) | (136) |
| Neuronal cell death (in vivo) | (137) | |
| Regulation of myelin | (138) | |
| Apoptosis | (139) | |
| GD1b | Anti-apoptotic | (140) |
| GD1a | Regulation of myelin | (138) |
| Enhance GM-CSF induced proliferation of monocytes | (141) | |
| GM1a/GM1b | UPR | (142) |
| MMP, apoptosis, and ROS generation | (143) | |
| Inhibits SERCA activity | (144, 145) | |
| Stimulates neurite outgrowth | (146) | |
| Regulates nuclear calcium and displays a cytoprotective role | (147) | |
| Positive role in induction of long term potentiation | (148) | |
| GM2 | Inhibits calcium uptake via SERCA, alters calcium homeostasis | (119, 149) |
| Apoptosis | (139) | |
| Survival of motor neurons | (150, 151) | |
| ROS generation | (78) | |
| GM3 | Neuronal cell death and ROS generation | (152) |
| Apoptosis | (139) | |
| Anti-apoptotic effect on cortical neurons | (153) | |
| Enhance neurite outgrowth | (154, 155) | |
| GD2 | Alters calcium homeostasis of neurons | (149) |
| GD3 | Cell death and ROS generation | (156, 157) |
| Apoptosis in oligodendrocytes | (158) | |
| MMP and neuronal apoptosis | (159) | |
| Enhance neurite outgrowth | (154) | |
| GQ1b | Calcium influx | (145) |
| Enhance neurite outgrowth | (154) | |
| Positive role in induction of long term potentiation | (148) | |
| LacCer | Inhibition of cholesterol efflux | (160) |
| SM | Prevents phago-lysosomal fusion | (161) |
| LMP | (57, 59) | |
| Sphingosine | Stimulates calcium release | (162) |
| LMP and cell death | (84) | |
| Ceramide | Lipid raft integrity | (163) |
| Inflammation | (163) | |
| Stimulates NO synthase in neural astrocytes | (164) | |
| Apoptosis | (165) | |
| Autophagy | (166) | |
| Programmed cell death at the plasma membrane | (167) | |
| Increases membrane stability | (168) | |
| Sulfatide | Myelination | (169–171) |
| GalCer | Activator of the immune system, anti-tumorigenic | (172) |
| Gb3 | Apoptosis | (173, 174) |
| Increases ROS | (75) | |
| Lyso-Gb3 | Correlates with disease severity | (175) |
| Inhibits α-Gal A activity | (176) | |
| Promotes proliferation | (176) | |
| Gb4 | Protects from LPS toxicity | (177) |
| Cholesterol | Mitochondrial membrane potential and ATP synthesis | (178) |
| Protect from apoptosis | (83) | |
| Neurodegeneration | (179) | |
| Apoptosis neuronal cells | (180) | |
| Psychosine | Demyelination | (53) |
| Generates ROS and induces cell death | (107) | |
| Stimulates calcium release via RyaR | (162) | |
| Apoptosis | (104) |
Please refer to the text for details. MMP, mitochondrial membrane permeabilization.
Fig. 1.
Potential cellular pathological events in LSDs and their convergence with the protective capacity of the HSR and HSP70. The figure illustrates a hypothetical interplay of events ultimately leading to the degenerative pathology associated with LSDs. In case of events where the HSR and/or HSP70 has been shown to have a cell-guarding impact the fonts are in bold green. The illustrated processes have been observed in a number of LSDs but are not necessarily a common denominator to all. Please refer to the text for details.
The key functions of the heat shock response (HSR), and in particular the heat shock protein (HSP)70 family, include assisting in the sorting and folding of newly synthesized and damaged proteins, binding of nonnative proteins to prevent protein aggregation, and targeting of severely damaged proteins for degradation (8–10). In close collaboration with the ubiquitin proteasome system, chaperone-mediated autophagy (CMA), and other processes for proper handling of misfolded proteins, the HSP system constitutes the central element of cellular protein quality control and homeostasis (proteostasis). Furthermore, HSP70 has, on its own, been shown to promote cell survival by inhibiting lysosomal membrane permeabilization (LMP), increasing lysosomal catabolism, and preventing cell death and a number of other events associated with stress-induced cell death (11–14). As such, many of the primary and secondary cellular processes of LSDs are potentially influenced by the HSR and the molecular chaperones of the HSP70 family, providing a remarkable convergence between the pathogenic cascades of the LSDs and the well-characterized multifaceted cytoprotective actions of the HSR (Fig. 1).
The following sections will aim to break down these cellular events into their major constituents with a focus on processes where the HSR and HSP70 are considered to have a potential therapeutic relevance, including the initial events of enzyme misfolding, ER stress, and unfolded protein responses (UPRs), as well as the processes of substrate accumulation, LMP, increased oxidative stress, and cell death (Fig. 1).
CELLULAR PROCESSES IN LSDs
Proteostasis
As missense mutations are the most common type of mutations found in LSDs, the salvage of misfolded enzymes from the ER has been a therapeutic target for intervention in LSDs for more than a decade (6); although the understanding of how the cellular response to the misfolded lysosomal proteins plays into the molecular pathology of the diseases is only beginning to emerge (7, 10, 15, 16). Also, despite the fundamental role for molecular chaperones in the quality control of misfolded enzymes, studies implicating and addressing the potential therapeutic role of the HSR and HSP70 have only recently started to be performed within the LSDs; although this potential has been extensively investigated and is an established concept for a number of neurodegenerative diseases with a number of ongoing clinical trials (17–19).
The main biological response to misfolded proteins is induction of molecular chaperones, a process commonly referred to as the HSR, as the best characterized trigger of misfolded proteins and the associated proteotoxic stress historically has been thermal stress. However, cells respond in a similar way to most stress as exemplified by the marked HSR elicited by the covalent modification of proteins by reactive oxygen species (ROS) (20). A molecular chaperone is commonly defined as any protein that interacts with, stabilizes, or helps a nonnative protein to acquire its native conformation, but is not present in the final functional structure (21, 22). Members of the molecular chaperone families are known as HSPs and are classified according to their molecular weight (HSP40, HSP60, HSP70, HSP90, HSP100, and the small HSPs). The chaperones most extensively involved in de novo protein folding and refolding, such as HSP70, HSP90, and HSP60 (chaperonins), are critical to the proper cellular response to misfolded proteins. The systems are sequentially linked with HSP70 acting upstream of HSP60 and HSP90. HSP70 together with heat shock cognate 70 (HSC70) (the constitutively expressed member of the HSP70 family) are central players in protein folding and proteostasis control, and thus constitute the first cellular line of defense against misfolded proteins. In an ATP-driven process, which can be augmented by the presence of cochaperones and nucleotide exchange factors, HSP70 aids de novo protein folding and protein refolding through a process in which HSP70 binds to exposed hydrophobic regions of nonnative proteins, thereby transiently blocking aggregation, and through the ATP-triggered release allowing folding to proceed (8). HSP90 functions downstream of HSP70 and is directly linked to HSP70 via the tetratricopeptide repeat protein HOP, which allows for substrate transfer between the two HSPs (23).
The primary objective of the HSR and HSP70 family members is thus to stabilize and refold misfolded proteins. If unable to do so, the misfolded proteins are targeted to the ubiquitin-proteasome system or to the lysosome. In the ER, accumulation of misfolded proteins is recognized by the ER quality machinery, dominated by the evolutionarily conserved ER membrane-bound stress transducer, Ire1, and the HSP70 family member HSPA5/BiP/Grp78, which ensures proper folding and oligomerization of newly translated proteins (24). In addition, HSPA5 is also required for the protein translocation itself and for the degradation of misfolded proteins of the ER lumen (25, 26). Continued accumulation of incorrectly folded proteins leads to ER-associated degradation (ERAD) mediated by the proteasome and triggers the UPR, which can further regulate the folding capacity of the secretory pathway by upregulation of ER chaperones and/or by attenuating protein synthesis through the actions of Ire1 and the stress transducer membrane proteins PERK and ATF6, which in turn are regulated by their binding to HSPA5 (27). The pivotal role of the HSP70 system in recognition of unfolded or misfolded proteins thus not only applies to the cytosol but also applies to the ER, while the HSP70 family also serves crucial proteostatic functions at the plasma membrane, mitochondria, and lysosomes via HSC70, HSPA9/Mortalin/Grp75, and HSP70 (8, 17, 24, 28). It therefore comes as little surprise that the control of the induction the HSR, and in particular HSP70 and HSP90 genes, is a crucial cellular process.
The transcriptional induction of the HSR is a tightly regulated process in which activation of heat shock transcription factors (HSFs) is a crucial step. Four different HSFs (HSF1–4) have been discovered in vertebrates, with HSF1 being considered the master regulator for the stress-induced transcription of HSPs during most stress conditions (29). Under normal physiological conditions, HSF1 is present as a monomer associated with chaperones, which shifts its activation equilibrium away from its active trimeric state. The chaperones are displaced during conditions of stress as the levels of stress-denatured proteins rise, as well as through a number of posttranslational modifications of HSF1, including phosphorylation, acetylation, and sumoylation, which thereby facilitates the trimerization of HSF1 and its activation. Activated trimerized HSF1 binds to the conserved HSP promoter known as the heat shock element (HSE), which promotes its transactivation through further interactions with a number of regulatory factors downstream of the HSE, ultimately leading to the transcription of HSPs (29). These processes hold a number of pharmaceutical targets of which the most explored are induction of the HSR via interference with the proteostatic machinery, interference with the binding of HSF1 to its chaperones, and the stabilization of the activated form of HSF1, e.g., the use of the proteaseome inhibitor MG-132 induces a dramatic HSR due to the severe interruption of the proteostatic machinery (10). The application of geldanamycin and other ansamycins to inhibit HSP90 by blocking its ATP binding site leads to disruption of the HSP90-HSF1 complex and induces HSF1 activation (30–32). Likewise, hydroxylamine derivatives such as BGP-15 and arimoclomol increase the transcription of HSPs through the stabilization of the active form of HSF1 (33), a process which potentially involves cellular membranes as upstream stress sensors (34). Proof-of-principle experiments using these approaches have already demonstrated efficacy across a number of cell and animal models of genetic diseases characterized by protein misfolding and accumulation, such as amyotrophic lateral sclerosis (ALS), Kennedys disease, Duchenne’s muscular dystrophy, homocystinuria, Parkinson’s disease, Alzheimer’s disease, and poly-Q expansion disorders (17, 20, 33, 35–39). Based on these promising experiments, arimoclomol is currently being investigated in two phase II clinical trials in sporadic inclusion body myositis and the superoxide dismutase 1 form of familial ALS (18, 19). Importantly, for the purpose of this review, the potential benefit of modulation of the HSR is also beginning to emerge in the LSDs.
While some LSDs, such as Fabry’s disease, are X-linked, most of the LSDs are autosomal recessive and are primarily characterized by missense mutations. As described above, such mutations result in inappropriate folding and trafficking of the mutated protein, which thereby faces the chaperone-involving processes described above. Indeed, for Gaucher’s disease, it has been suggested that ER retention and degradation of glucosylceramidase (GCase) is the molecular basis underlying disease heterogeneity (40). In accordance with this, if the missense mutation compromises the enzyme to an extent where it cannot be correctly folded through the aid of ER-resident molecular chaperones, this leads to the loss-of-protein function which is characteristic of the LSDs (7) and forms the biological rationale for the enzyme replacement therapies in which the functional output of the defect enzyme is augmented by the addition of exogenously administered recombinant enzyme (41). Apart from the enzyme replacement therapies, other intervention strategies are also being researched at this crucial junction. These strategies seek to augment the refolding capacity of the ER, i.e., increase the levels of molecular chaperones or use chemical entities as so-called chemical chaperones to facilitate folding of the otherwise misfolded enzymes. The former approach relies on the cells’ inherent systems to overcome the ERAD response, whereas the latter relies on a seemingly counter-intuitive mechanism in which the chemical chaperone actually works as an inhibitor, binding to the active site of the enzyme and thereby aiding folding in the ER after which the inhibitor needs to dissociate to make room for the natural substrate once the enzyme/inhibitor complex reaches the lysosome (6, 42).
As mentioned above, the potential benefit of modulation of the HSR is now emerging in the LSD field with the reporting of a number of studies of this approach in genetically distinct diseases such as Gaucher’s disease, Tay-Sach’s disease, Niemann-Pick disease types A and B, Niemann-Pick disease type C, α-mannosidoses, sialidosis, and mucopolysaccharidosis type 3A (7, 43–49). In cellular studies of Gaucher’s and Tay-Sach’s disease for example, the induction of the HSR or UPR with celastrol and MG-132 results in increases of both cytosolic and ER-resident chaperones, of the HSP70 family in particular, in a process dependent on UPR-responsive transcription factors Ire1, ATF6, and PERK. This upregulation of molecular chaperones is critical to the associated increases in mutant enzyme activity, which in both diseases reaches levels of approximately 50% WT activity (7) after administration of the HSR/UPR-inducing compounds. These data on the positive effect of celastrol on both HSP70 induction and increased enzymatic activity of the compromised enzyme, GCase, in Gaucher’s disease is supported by a recent study in which celastrol significantly increased the quantity and catalytic activity of GCase by interrupting its HSP90-mediated degradation while upregulating the molecular chaperone HSP70 and its associated cochaperone BCL2-associated athanogene 3 (BAG3) (48). These data are supported by additional studies in Gaucher’s disease in which reduced ubiquitination and increased stability of mutant GCase was demonstrated after treatment with the HSP70-inducer/HSP90-inhibitors 17-N-allylamino-17-demethoxygeldanamycin, vorinostat, and LB-205 (45, 50). Interestingly, the molecular mechanism of vorinostat and LB-205, which are both histone deacetylase inhibitors (HDACis), was recently clarified, showing that both compounds inhibit the deacetylation of the middle domain of HSP90, resulting in less recognition of the mutant GCase, and hence less degradation, while increasing the amount of chaperones involved in folding, such as HSPA5 (45). The potential, but also the challenges, of using HDACis is further highlighted by a study of a small panel of HDACis in Niemann-Pick disease type C (44). This study showed marked efficacy for some of the HDACis toward reduced cholesterol accumulation and increased cholesterol transport; whereas others had a more limited effect, likely due to the different inhibitory constants toward individual HDACs. Interestingly, the data argue that inhibition of HDAC1 particularly, but also HDAC2 and HDAC3, is the likely cause of the effect, and as recent studies have also documented induction of HSP70 and HSPA5 by inhibition of class I HDACs, the authors suggest that HDACi could increase passage of mutant NPC1 out of the ER via the increased chaperone activity; although further work will be required to establish the relative contributions of these two mechanisms in increasing NPC1 expression in mutant cells (44). Interestingly, a mechanistic link between expression of molecular chaperones of the HSP70 family and HDACs is supported by a recent report which shows that HSF1 specifically interacts with HDAC1 and HDAC2 to regulate gene expression during heat shock (51).
Further evidence for the benefit of manipulating proteostasis and inducing the HSR/UPR in Niemann-Pick type C comes from studies in Niemann-Pick type C patient cells in which the administration of MG-132 increased the expression of mutated NPC1 protein across a panel of NPC1 genotypes. MG-132 treatment further increased the association of NPC1 with the lysosomal/late endosomal compartment and reduced the storage of ganglioside GM1 and un-esterified cholesterol, examples of some of the key metabolites that are accumulated due to the loss of function characterizing the disease (43). MG-132 has furthermore been shown to be effective in primary patient cells from sialidosis (neuraminidase deficiency). Interestingly, a strong synergy was observed in this study when MG-132 was administered together with celastrol, a finding which is suggested to rely on the capacity of MG132 to induce a HSR/UPR on its own while the HSP inductive action of celastrol is significantly enhanced by the proteotoxic stress induced by MG-132 (49).
In addition to the above reports, a study with the calcium-channel blockers verapamil and diltiazem have been shown to restore mutant enzyme homeostasis in cells from Gaucher’s disease, mucopolysaccharidosis IIIA, and α-mannosidosis patients. By investigating the structure-activity relationship of diltiazem, the studies suggest that its Ca2+-channel blocker activity is responsible for the upregulation of a subset of molecular chaperones, including HSPA5 and HSP40, which provides an enhanced capacity of the ER to fold the misfolding-prone proteins. The study thus suggests that inducing members of the HSR through manipulation of calcium homeostasis is a way to restore protein homeostasis in LSDs (15).
From the emerging number of reports investigating the therapeutic potential of induction of molecular chaperones in LSDs, it is clear that modulation of molecular chaperones holds a significant potential. There is a devil in the detail, however, as the mechanism driving many inducers of molecular chaperones lies in their inherently cytotoxic properties. It is no coincidence that HDACis and proteasome inhibitors are often pursued for their cytotoxic effects, rather than their potential cytoprotective effects. Studies with these compounds should therefore rather be viewed as proof-of-concept, as careful considerations and thorough pharmacological and preclinical studies should be undertaken to understand the safety/efficacy window before administering drugs developed for their anti-oncogenic potential to patients suffering from severe metabolic diseases. However, as mentioned above, nontoxic inducers of the HSR also exist and are currently in phase II clinical trials for other chronic genetic diseases characterized by misfolded proteins, as well as in early development for the LSDs (6, 18, 19).
Substrate accumulation
Functional disruption of a lysosomal enzyme often influences the activity of other lysosomal enzymes, as the incomplete catabolism of a substrate can significantly alter the lysosomal environment, e.g., by perturbation of the membrane lipid stoichometry (52). In addition to the primary storage, many LSDs thus show accumulation of secondary metabolites, which on their own may have a number of derived and potentially toxic effects (Table 1). GM2 and GM3 gangliosides, sphingosine, and cholesterol are common secondary storage metabolites in lysosomal diseases (5, 53). Also, more complex gangliosides, globosides, SM, and other sphingolipids have been found to play a role in the molecular pathology of LSDs (54). An example of detrimental substrate accumulation is β-galactosylsphingosine (psychosine), which accumulates in affected tissue of Krabbe’s disease (globoid-cell leukodystrophy). Krabbe’s disease is caused by deficiency in β-galactocerebrosidase and is characterized by extensive demyelination with loss of oligodendroglia and proliferation of astroglia in the brain and spinal cord (55). Psychosine is a highly cytotoxic lipid, capable of inducing cell death in a variety of cell types including oligodendrocytes, and has been suggested as a toxic secondary metabolite in other LSDs as well (53). Similarly, secondary metabolites are considered to play key roles in a number of the LSDs, including Niemann-Pick disease type C (NPC) in which genetic defects of the transmembrane protein NPC1 lead to a wide array of storage metabolites, including gangliosides, SM, and sphingosine, all of which have reported roles in the pathology of the disease (56). The accumulation of SM has been shown to render lysosomes more susceptible to loss of their structural integrity, and subsequently cell death, as well as to impair autophago-lysosomal clearance (57–60). Interestingly, the mitigation of this secondary substrate accumulation in NPC by enhancing acid SMase (ASMase) activity, has been shown to revert the phenotype of NPC cells; arguing that at least for some LSDs, amelioration of secondary events could hold important therapeutic promises, but also suggests that NPC1 might benefit from recombinant HSP70 therapy, based on HSP70’s ability to increase ASMase activity and lysosomal catabolic activity (12, 57, 61, 62).
The accumulation of primary and secondary substrates has a number of derived consequences, apart from causing further lysosomal catabolic dysfunction. The main consequences of the failure to degrade and recirculate important cellular constituents include not only gain-of-function toxicities related to the direct lysosomal environment, such as loss of lysosomal integrity and cell death, but might also lead to loss-of-function effects, as lipids, receptors, and proteins that should have been recycled are trapped in a dysfunctional compartment. The impact of this dysfunction extends well beyond the lysosomes themselves, as their lack of proper function also leads to bottlenecks for interrelated vesicle trafficking events such as autophagy, endosomal transport and recycling, exocytosis, and synaptic transmitter releases, putting yet more stress on the cells to manage (63–65).
The ultimate outcome of these events is cellular degeneration, but the elucidation of the mechanistic principles regarding how the often complex substrate accumulation leads to this fatal end are still not clear. Interestingly, apart from the reasonably well-characterized culprits, such as psychosine, increased amounts of ROS and susceptibility to LMP seems to be a feature of an increasing number of LSDs (57, 66–69). As LMP is a well-described initiator of cell death programs, modifying this phenotype presents an attractive area for therapeutic intervention, not only in LSDs but also in other diseases in which this deleterious process is involved, such as cancer and pancreatitis (13, 58, 70).
LMP
The lysosome contains a high content of hydrolytic enzymes, and loss of lysosomal integrity is potentially lethal to the cell, as evidenced by the involvement of LMP in a number of cell death cascades and pathologies, including a number of the LSDs (13, 57, 66–69, 71).
Whereas regulation of lysosomal stability is not completely understood, it is clear that the lipid composition of the lysosomal membranes is involved, as is the sensitivity to accumulation of ROS within the lysosomes. One of the best studied groups of ROS generators is heavy metal ions, exemplified by redox-active iron (Fe2+). The lysosomal compartment is rich in labile iron due to autophagic degradation of ferritin and mitochondrial metalloproteins. In the lysosomes, Fe2+ is capable of generating ROS through Fenton-type chemical reactions, which can subsequently lead to oxidization of membrane lipids and thereby initiate LMP (72). ROS can also be generated from other chemically reactive metals trapped within the lysosomal lumen, including Cu2+, Zn2+, and Co2+ (73). Importantly, iron chelation has been shown to desensitize cells to oxidative stress and increase lysosomal stability (74). Interestingly, one of the often observed characteristics of cellular pathology in LSDs is the marked elevation of oxidative stress, and ROS have been found elevated in several LSDs, including Fabry disease, microglia of Gaucher disease, GM1 and GM2 gangliosidoses, and Niemann-Pick disease type C, providing another mechanistic clue to the loss of lysosomal integrity and cell death processes occurring in LSDs (75–78).
Importantly, a well-characterized pro-survival effect of the HSR, in particular HSP70, is toward oxidative stresses, ranging from cellular studies of protection against ROS and membrane oxidation to clinical studies of the effects of HSP70 on ischemia-reperfusion injury (12, 13, 79–81)
Not only heavy metals, but also a variety of lipids such as free fatty acids, arachidonic acid, SM, and sphingosine, have been shown to be directly involved in loss of lysosomal integrity; whereas cholesterol seems to serve a more protective role, which is in accordance with its proposed membrane-stabilizing effect (57, 82–86). Also, generation of ceramide alters the biophysical properties of cellular membranes (87). The ultimate outcome of changes in lysosomal membrane lipid stoichiometry is thus hard to predict, but studies have shown that loss of lysosomal integrity is a feature across the various classes of LSDs, as diseases as diverse as Niemann-Pick disease types A and B, Niemann-Pick disease type C, mucopolysaccharidosis type I, mucolipidosis type II, and late-infantile neuronal ceroid lipofuscinosis (CLN2 disease) all have been reported to be susceptible to LMP (57, 66–69).
Adding to this complexity, lysosomes can also undergo LMP in response to a number of stimuli, including death receptor-activation by TNF-a (71). This signaling pathway thus links the pathogenic cascade of some of the LSDs in which immunological responses play a part, such as Niemann-Pick type C, to LMP and its downstream consequences, providing a point of convergence between these disease processes. Importantly, one of the best characterized effects of HSP70 is its ability to mitigate TNF-a-mediated cell death programs, which, together with the previously highlighted cytoprotective actions of the HSR, stresses the therapeutic potential in HSR-based approaches to mitigate the detrimental consequences of these events (12, 88–90).
Furthermore, a common event in LSDs, disruption of lysosomal trafficking (5), has also been shown to increase LMP susceptibility. Mutation of the LYST gene (Chediak-Higashi syndrome) encoding a lysosomal trafficking regulator results in dysfunctional lysosomes (91). Likewise, downregulation of the lysosomal membrane proteins, lysosomal-associated membrane proteins (LAMP)-1 and -2 (disrupted in Danon disease), by transformation sensitizes cells to drug-induced LMP (92).
Ultimately, the outcome on lysosomal stability for any LSD is most likely a result of the sum of the pathological processes and the resulting lipid profile of the lysosomes at any given time, including effects from the increased reactive and oxidative environment often seen due to improper trafficking and recycling of heavy metal ions. However, with the increasing appreciation of the involvement of LMP in LSDs and with the established evidence that mitigation of LMP has significant cellular pro-survival implications, increased understanding of this part of the pathogenic cascade in LSDs might reveal a number of potential therapeutic targets. Importantly for the context of this review, it has been demonstrated that HSP70 plays a crucial role in stabilizing lysosomal membranes in a number of pathologies and has been shown to be able to directly affect lysosomal membrane stability through its interaction with the anionic lysosomal lipid bis(monoacyl)glycerophosphate and subsequent activation of ASMase, rendering the lysosomes of patients with primary ASMase deficiency (Niemann-Pick type A and B) more protected against LMP while also reverting the accumulation of storage lysosomes and SM, which has recently been implicated in loss of lysosomal membrane integrity (13, 57, 59). In addition, one of the immediate consequences of LMP, cell death, has been shown in a large number of studies to be significantly reduced by the induction of the HSR and HSP70, not only by its direct actions on lysosomes but also through its interactions with the downstream cell death cascades (14).
Cell death
As cell death and neuronal loss are key features of many LSDs, a number of cell death pathways are potentially involved in the disease progress, and the available literature describes a number of events, which, not surprisingly, are strongly associated with the lysosomes or organelles and membranes closely linked to the lysosomes (5, 64). However, due to the key role of lysosomes not only in macromolecular catabolism but also in overall cellular homeostasis such as lipid and receptor recycling to the plasma membrane and autophagic clearance of damaged mitochondria and peroxisomes, the network of signaling pathways affected by dysfunctional lysosomes is vast and complex, and a thorough understanding of these intricate relationships is still elusive.
This section will thus focus on the more direct lysosomal cell death pathways and associated classical apoptotic cascades and the influence of the HSR and HSP70 on these pathways. The reader is referred to the many excellent reviews on various cell death programs for further details on these.
As described above, a number of cellular events can lead to LMP and subsequent cell death. Studies that target the lysosomal membrane integrity have convincingly proven that lysosomal permeabilization can result in programmed cell death (12, 93–97). Interestingly, cells depleted of HSP70 become more susceptible to these lysosome disruptive stimuli, whereas cells either overexpressing HSP70 or administered recombinant HSP70 are significantly protected against LMP and subsequent cell death (11–14, 57, 88, 89, 98, 99). Needless to say, the conclusions on these studies point very clearly to an important overlap between lysosomal cell death processes and the cytoprotective mechanisms employed by the HSR and HSP70 (13, 17, 34, 100).
It has been suggested that a quantitative relationship exists between the degree of lysosomal destabilization and the mode of subsequent cell death pathways. In this model, a limited loss of lysosomal membrane integrity would lead to a limited release of lysosomal contents to the cytoplasm, triggering a predominantly apoptotic or apoptosis-like cascade with activation of the proper caspases, while high intensity stresses and significant loss of lysosomal membrane integrity lead to rapid cellular necrosis (82, 101). Accordingly, sphingosine, a common secondary accumulating metabolite in LSDs, has been shown to induce various degrees of LMP. Sphingosine is an acid ceramidase-generated metabolite of ceramide with detergent-like properties at low pH, and has been shown to induce partial LMP and caspase-mediated apoptosis at low concentrations, whereas higher concentrations result in massive LMP and caspase-independent necrotic cell death. In this model, the death triggered by partial LMP can be inhibited by pharmacological inhibitors of cysteine and aspartate cathepsins, and the increase in the cytosolic cathepsin activity precedes the activation of caspases and mitochondrial membrane potential changes suggesting a direct role for cytosolic cathepsins in the death process (82). Interestingly, sphingosine has been suggested to be the earliest accumulating metabolite in Niemann-Pick disease type C, suggesting that sphingosine-driven reactions are among the first molecular pathological events in this disease (102). Supporting a close link between sphingosine accumulation, LMP and cell death in Niemann-Pick disease type C, Amritraj et al. (69) investigated the role of lysosomal proteases cathepsin B and D, the two best characterized cell death triggering enzymes of the lysosome (71), in determining neuronal vulnerability in NPC1-deficient mouse brains over time. The study elegantly demonstrated that lysosomal release of cathepsins occurs coincidentally with the age-dependent degeneration of the most vulnerable brain regions (e.g., the cerebellum) suggesting a role for these enzymes in the initiation of the degeneration of neurons (69).
Psychosine has also been implicated as a potential cell death-triggering species in LSDs, as it is a highly cytotoxic lipid, capable of inducing cell death in a variety of cell types including oligodendrocytes (53, 55). In Krabbe’s disease, psychosine is a primary storage compound and has been reported to be sufficient to activate caspase 3 in motor neuronal cells in vitro in the absence of myelinating glia. Furthermore, peripheral neuropathy in the Twitcher mouse model of Krabbe’s disease has been shown to correlate with increased caspase 3 activation, indicating a molecular mechanism for the psychosine-related toxicity and neuronal pathology of this disease (104). As described above, LMP and apoptotic pathways with caspase activation are intimately linked. It is thus intriguing that recent data indicate that the toxic effect of psychosine is exerted through perturbation of membrane integrity (105). Although this study focused on the plasma membrane, the psychosine-driven membrane perturbations described could very well be envisioned to be similar for lysosomal perimeter membranes, a hypothesis that would bridge this study and the psychosine-driven caspase 3 activation reported in Krabbe’s disease, and could hold implications for the understanding of the cell death processes occurring in LSDs with psychosine accumulation. In support of this, recent studies have shown that psychosine activates secreted phospholipase A2, a potential initiator of LMP and downstream cell death programs through its generation of arachidonic acid and ROS (13, 106, 107).
LMP also participates in the execution of cell death in response to a wide variety of classic apoptotic stimuli, such as immunological reactions driven by activation of death receptors of the TNF-receptor family (108–111). As immunological responses are often a part of LSD pathology, this provides a link between cell death, lysosomes, and an activated immune system in LSDs; although much is still left to learn on the molecular biology and potential clinical relevance of this interplay (4).
Interestingly, TNF-a also stimulates SM breakdown to phosphorylcholine and ceramide by activating neutral SMase at the plasma membrane and ASMase in the lysosomal compartment (112). Both events have been implicated in TNF-a-induced cell death pathways, but so far only neutral SMase has been connected to LMP through the factor associated with neutral SMase (FAN) (113). In addition to increasing the generation of the sphingosine precursor, ceramide, by activating SMases, TNF-a regulates sphingosine levels by cathepsin B-mediated downregulation of sphingosine kinase-1, an enzyme that converts the pro-apoptotic sphingosine to an anti-apoptotic sphingosine-1-phosphate (114). This TNF-a-driven activity of cathepsin B could result in the accumulation of sphingosine in the lysosomes and may thus, at least partially, explain the requirement of cathepsin B for an efficient LMP in TNF-a-treated hepatocytes (115). In addition it has been shown that TNF-a-induced tumor cell death depends on cathepsin B release from lysosomes, an event which also triggers the generation of arachidonic acid and thus intriguingly connects TNF-a signaling and the release of cathepsin B to a number of the molecular processes already described for LSDs (108, 116).
The cytotoxic effects of LMP often rely, at least partially, on the activation of the mitochondrial death pathway. It is, however, considered beyond this review to discuss these interactions and their downstream effects aside from what has already been presented. Suffice to say that the HSR and HSP70 play important roles, and the interested reader is referred to reviews covering these details (13).
Apart from its direct lysosome-protective effects, which we have already covered, the HSR and HSP70 in particular, have arguably the broadest cytoprotective potential of any cellular agents, a potential which has been demonstrated across a number of disease states and against a vast array of toxic stimuli. Thus HSP70 has been shown to be able to prevent not only the toxic effects of ROS and LMP but also protect against caspase-mediated apoptosis, in particular downstream of caspase-3 activation, and have demonstrated a significant protection against death-receptor, in particular TNF-a-induced cell death programs (12–14, 17, 88, 89, 100, 117).
In conclusion, the HSR and HSP70 confer significant cytoprotection against a number of cell death pathways, many of which are also part of the molecular pathology of LSDs. Together with the already covered aspects of HSR/HSP70-conferred cytoprotection, this, of course, strengthens the rationale for the exploration and development of compounds capable of replicating or modulating these effects as potential therapeutic agents for LSDs.
Calcium homeostasis
Altered Ca2+ homeostasis has been observed in a number of LSDs, although the form and extent of alterations seem to be dependent on the given LSD, and many details still need to be understood (55). Briefly, ER, mitochondrial, and lysosomal calcium stores have all been shown to be affected in different lysosomal diseases (4). In Sandhoff disease, GM2 accumulation inhibits calcium uptake via the sarco/ER Ca2+ ATPase (SERCA) which elevates cytosolic calcium levels, a mechanism which is shared by Niemann-Pick disease type A (119, 120). Importantly, elevated cytosolic calcium can activate calcium-dependent calpain, which in turn can activate caspases and result in apoptosis (121). In addition, the activation of calpains can lead to LMP and subsequent neuronal cell death, a mechanism that has been suggested to occur through the direct cleavage of lysosome-associated and -protective HSP70 (121–124).
With the discovery of the lysosomes as bona fide calcium stores (125, 126), deregulation of lysosomal calcium stores has also been reported in LSDs. In Niemann-Pick type C, sphingosine accumulation has been suggested to lead to depletion of lysosomal calcium with subsequent accumulation of glycosphingolipids, SM, and cholesterol (102). SM, the primary storage substrate in Niemann-Pick types A and B, and which also accumulates in Niemann-Pick types C1 and C2, has also been mechanistically linked to the dysregulation of lysosomal calcium stores via inhibition of the mucolipin transient receptor potential channel 1 (TRPML1) (53, 127). TRPML1 is a ubiquitously expressed Fe2+ and Ca2+-dually permeable channel predominantly localized in late endosomes and lysosomes and serves as the primary Ca2+ channel in the lysosomes. This, interestingly, links iron metabolism to the regulation of lysosomal calcium. Mutations in TRPML1 cause the LSD mucolipidosis type IV which exhibits lysosomal defects similar to those seen in cells from Niemann-Pick diseases, such as dysfunctional autolysosome formation/lysosome reformation, lipid trafficking, and Ca2+ and Fe2+ homeostasis (127).
As we have already covered, the function of the ER and the HSR are intimately linked, but more specifically to the ER-calcium axis; HSP70 has been shown to bind and preserve the function of SERCA under cellular stresses (128). Likewise, the induction of HSP70 via the pharmacological HSP70 coinducer, BGP-15, a compound of the hydroxylamine derivative family which is in early clinical development, has been shown to increase the activity of SERCA and slow the progression of Duchenne’s muscular dystrophy in two mouse models of the disease (34, 37).
Autophagy
Autophagy is a conserved mechanism of turnover of long-lived proteins and organelles within the cell, but is also important for energy sensing and involved in the immune system. Autophagy, like the HSR, is induced in response to a variety of cellular stresses, and plays a cytoprotective role, but has also been found to contribute to cell death if excessively induced (64, 129). Three main pathways of autophagy have been described so far: macro-autophagy, micro-autophagy, and CMA. Micro-autophagy has not been investigated for LSDs and will therefore not be addressed in this review. Macro-autophagy is the major pathway of autophagy, which involves the engulfment of ubiquitinylated and aggregated proteins. The process is initiated by recognition of misfolded proteins by molecular chaperone complexes involving HSC70, the constitutive member of the HSP70 family. After recruitment of P62 and other autophagic receptors such as neighbor of BRCA1 gene 1 (NBR1), which tether the substrate to the autophagic machinery on the forming autophagosome, the autophagosomes fuse with lysosomes for degradation of the engulfed proteins (64). The details of the interplay between autophagy and LSDs have only recently begun to be characterized, but it seems that the involvement of autophagy and its contribution to pathology varies depending on the LSD being studied; although much work is still needed to understand the implications of this process in the various LSDs. Completion of autophagy and reformation of lysosomes (autophagic flux), however, seems to be reduced in many LSDs and has also been implicated in a number of other neurodegenerative diseases (64, 130, 131).
As autophagy relies on a functional lysosomal system in order to function properly, the question as to whether modulation of autophagy can potentially provide a clinical benefit to patients is not easily answered. Clearly, induction of autophagy in a disease with a compromised lysosomal system is not necessarily an advantage, but modulation of the whole system, i.e., improving autophagic flux, including augmentation of the lysosomal capacity to address increased autophagy, could absolutely be of relevance.
CMA is a selective autophagic process which relies on the HSP70 famíly member, HSC70, and its ability to recognize and facilitate lysosomal entry of its client proteins through HSC70 binding to the cytoplasmic tail of LAMP-2A on the lysosomal membrane (28). In addition to this very clear overlap between the HSP70 system and autophagy, the cochaperone, BAG3, facilitates autophagic digestion of HSP bound proteins by directing misfolded proteins into aggregate-like structures suited for efficient autophagic digestion (132). BAG3 can be induced by HSF1 (133), the main transcription factor for the HSR, and is found upregulated in mammalian cells under oxidative stress, heat stress, or upon exposure to heavy metals, suggesting that the HSR also serves to enhance autophagic processes needed for effective clearance of misfolded proteins.
Importantly, the regulation of autophagy and the HSR both serve the same cellular end: the efficient handling of dysfunctional cellular constituents. Despite this, only very limited work describes the interaction between these two systems besides the process of CMA. Recent work, however, suggests that the processes are intimately linked as a feedback loop stimulating autophagic processes seems to be apparent in cells depleted of HSF1, whereas overexpression of HSP70 downregulates these (134).
Interestingly, the autophagic receptors P62 and NBR1 were recently reported to associate with intra-neuronal aggregates containing subunit c of mitochondrial ATP synthase (SCMAS) in a mouse model of the LSD classic CLN2 disease. This was consistent with SCMAS being released from lysosomes through LMP, suggesting that LMP is a previously unrecognized pathogenic event in CLN2 disease linking LMP and the autophagic machinery (67). This is supported by studies in human fibroblasts from patients with CLN2 disease showing increased levels of ROS, a well-described inducer of LMP (135).
In summary, the HSR and HSPs participate in several autophagic processes important for binding, targeting, and engulfment of target proteins; and as the HSR and autophagy are thus intimately linked, it is tempting to speculate that modulation of these processes could provide a therapeutic benefit, although it is evident that much still needs to clarified in order to better understand this intricate interplay.
Future directions and opportunities
The cellular response to metabolic stress induced by deficiency of lysosomal proteins can be influenced by molecular chaperones through various mechanisms. In their classical functions, the HSR and its cardinal members of the HSP70 family can enhance folding of mutated proteins, solubilize protein aggregates, and also facilitate enzyme activity and enhance trafficking to the lysosomes. More efficient clearance can be mediated through the UPR, proteasome-ubiquitin pathway, or autophagy where HSP70 family members are intimately involved. Importantly, the HSR and, in particular, HSP70 have also been found to increase lysosomal stability and thereby inhibit pathology caused by increased oxidative stress and loss of lysosomal membrane integrity, processes that are observed in many LSDs. Furthermore, induction of the HSR and HSP70 is perhaps “the” evolutionarily optimized anti-apoptotic mechanism employed by cells to avoid the otherwise detrimental consequences of unmanaged metabolic stress. With this multitude of convergent pathological and protective mechanisms, it is easy to appreciate the potential of therapies aimed at modulating the HSR and HSP70 system for LSDs and other degenerative maladies. Encouragingly, HSR-inducing molecules are already in clinical development for chronic degenerative diseases, although for the LSDs, the clinical potential of modulating the HSR, albeit promising, has yet to be investigated.
Footnotes
Abbreviations:
- ALS
- amyotrophic lateral sclerosis
- ASMase
- acid SMase
- BAG3
- BCL2-associated athanogene 3
- CLN2
- late-infantile neuronal ceroid lipofuscinosis
- CMA
- chaperone-mediated autophagy
- ER
- endoplasmic reticulum
- ERAD
- endoplasmic reticulum-associated degradation
- GCase
- glucosylceramidase
- HDAC
- histone deacetylase
- HDACi
- histone deacetylase inhibitor
- HSC70
- heat shock cognate 70
- HSE
- heat shock element
- HSF
- heat shock transcription factor
- HSP
- heat shock protein
- HSR
- heat shock response
- LAMP
- lysosomal-associated membrane protein
- LMP
- lysosomal membrane permeabilization
- LSD
- lysosomal storage disease
- NPC
- Niemann-Pick disease type C
- ROS
- reactive oxygen species
- SERCA
- sarco/endoplasmic reticulum Ca2+ ATPase
- SOD1
- superoxide dismutase 1
- TRPML1
- transient receptor potential channel 1
- UPR
- unfolded protein response
REFERENCES
- 1.Luzio J. P., Pryor P. R., Bright N. A. 2007. Lysosomes: fusion and function. Nat. Rev. Mol. Cell Biol. 8: 622–632. [DOI] [PubMed] [Google Scholar]
- 2.Saftig P., Klumperman J. 2009. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat. Rev. Mol. Cell Biol. 10: 623–635. [DOI] [PubMed] [Google Scholar]
- 3.De Duve C., Wattiaux R. 1966. Functions of lysosomes. Annu. Rev. Physiol. 28: 435–492. [DOI] [PubMed] [Google Scholar]
- 4.Vitner E. B., Platt F. M., Futerman A. H. 2010. Common and uncommon pathogenic cascades in lysosomal storage diseases. J. Biol. Chem. 285: 20423–20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platt F. M., Boland B., van der Spoel C. 2012. The cell biology of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction. J. Cell Biol. 199: 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkegaard T. 2013. Emerging therapies and therapeutic concepts for lysosomal storage diseases. Expert Opin. Orphan Drugs. 1: 385–404. [Google Scholar]
- 7.Mu T. W., Ong D. S., Wang Y. J., Balch W. E., Yates J. R., Segatori L., Kelly J. W. 2008. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell. 134: 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartl F. U., Bracher A., Hayer-Hartl M. 2011. Molecular chaperones in protein folding and proteostasis. Nature. 475: 324–332. [DOI] [PubMed] [Google Scholar]
- 9.Nollen E. A.A., Morimoto R. I. 2002. Chaperoning signaling pathways: molecular chaperones as stress-sensing “heat shock” proteins. J. Cell Sci. 115: 2809–2816. [DOI] [PubMed] [Google Scholar]
- 10.Morimoto R. I. 2008. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 22: 1427–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bivik C., Rosdahl I., Ollinger K. 2007. Hsp70 protects against UVB induced apoptosis by preventing release of cathepsins and cytochrome c in human melanocytes. Carcinogenesis. 28: 537–544. [DOI] [PubMed] [Google Scholar]
- 12.Nylandsted J., Gyrd-Hansen M., Danielewicz A., Fehrenbacher N., Lademann U., Høyer-Hansen M., Weber E., Multhoff G., Rohde M., Jäättelä M. 2004. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J. Exp. Med. 200: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirkegaard T., Jäättelä M. 2009. Lysosomal involvement in cell death and cancer. Biochim. Biophys. Acta. 1793: 746–754. [DOI] [PubMed] [Google Scholar]
- 14.Jäättelä M. 1999. Heat shock proteins as cellular lifeguards. Ann. Med. 31: 261–271. [DOI] [PubMed] [Google Scholar]
- 15.Mu T-W., Fowler D. M., Kelly J. W. 2008. Partial restoration of mutant enzyme homeostasis in three distinct lysosomal storage disease cell lines by altering calcium homeostasis. PLoS Biol. 6: e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong D. S. T., Kelly J. W. 2011. Chemical and/or biological therapeutic strategies to ameliorate protein misfolding diseases. Curr. Opin. Cell Biol. 23: 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muchowski P. J., Wacker J. L. 2005. Modulation of neurodegeneration by molecular chaperones. Nat. Rev. Neurosci. 6: 11–22. [DOI] [PubMed] [Google Scholar]
- 18.Kalmar B., Lu C-H., Greensmith L. 2014. The role of heat shock proteins in amyotrophic lateral sclerosis: the therapeutic potential of Arimoclomol. Pharmacol. Ther. 141: 40–54. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg S. A. 2012. Pathogenesis and therapy of inclusion body myositis. Curr. Opin. Neurol. 25: 630–639. [DOI] [PubMed] [Google Scholar]
- 20.Vabulas R. M., Raychaudhuri S., Hayer-Hartl M., Hartl F. U. 2010. Protein folding in the cytoplasm and the heat shock response. Cold Spring Harb. Perspect. Biol. 2: a004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartl F. U. 1996. Molecular chaperones in cellular protein folding. Nature. 381: 571–579. [DOI] [PubMed] [Google Scholar]
- 22.Hartl F. U., Hayer-Hartl M. 2009. Converging concepts of protein folding in vitro and in vivo. Nat. Struct. Mol. Biol. 16: 574–581. [DOI] [PubMed] [Google Scholar]
- 23.Scheufler C., Brinker A., Bourenkov G., Pegoraro S., Moroder L., Bartunik H., Hartl F. U., Moarefi I. 2000. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell. 101: 199–210. [DOI] [PubMed] [Google Scholar]
- 24.Buchberger A., Bukau B., Sommer T. 2010. Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol. Cell. 40: 238–252. [DOI] [PubMed] [Google Scholar]
- 25.Matlack K. E., Misselwitz B., Plath K., Rapoport T. A. 1999. BiP acts as a molecular ratchet during posttranslational transport of prepro-alpha factor across the ER membrane. Cell. 97: 553–564. [DOI] [PubMed] [Google Scholar]
- 26.Kabani M., Kelley S. S., Morrow M. W., Montgomery D. L., Sivendran R., Rose M. D., Gierasch L. M., Brodsky J. L. 2003. Dependence of endoplasmic reticulum-associated degradation on the peptide binding domain and concentration of BiP. Mol. Biol. Cell. 14: 3437–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ron D., Walter P. 2007. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8: 519–529. [DOI] [PubMed] [Google Scholar]
- 28.Kaushik S., Cuervo A. M. 2012. Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol. 22: 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anckar J., Sistonen L. 2011. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu. Rev. Biochem. 80: 1089–1115. [DOI] [PubMed] [Google Scholar]
- 30.Zou J., Guo Y., Guettouche T., Smith D. F., Voellmy R. 1998. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 94: 471–480. [DOI] [PubMed] [Google Scholar]
- 31.Prodromou C., Roe S. M., O’Brien R., Ladbury J. E., Piper P. W., Pearl L. H. 1997. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 90: 65–75. [DOI] [PubMed] [Google Scholar]
- 32.Murakami Y., Uehara Y., Yamamoto C., Fukazawa H., Mizuno S. 1991. Induction of hsp 72/73 by herbimycin A, an inhibitor of transformation by tyrosine kinase oncogenes. Exp. Cell Res. 195: 338–344. [DOI] [PubMed] [Google Scholar]
- 33.Kieran D., Kalmar B., Dick J. R. T., Riddoch-Contreras J., Burnstock G., Greensmith L. 2004. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat. Med. 10: 402–405. [DOI] [PubMed] [Google Scholar]
- 34.Crul T., Toth N., Piotto S., Literati-Nagy P., Tory K., Haldimann P., Kalmar B., Greensmith L., Torok Z., Balogh G., et al. 2013. Hydroximic acid derivatives: pleiotropic HSP co-inducers restoring homeostasis and robustness. 19: 309–346. [DOI] [PubMed] [Google Scholar]
- 35.Malik B., Nirmalananthan N., Gray A. L., La Spada A. R., Hanna M. G., Greensmith L. 2013. Co-induction of the heat shock response ameliorates disease progression in a mouse model of human spinal and bulbar muscular atrophy: implications for therapy. Brain. 136: 926–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh L. R., Gupta S., Honig N. H., Kraus J. P., Kruger W. D. 2010. Activation of mutant enzyme function in vivo by proteasome inhibitors and treatments that induce Hsp70. PLoS Genet. 6: e1000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gehrig S. M., van der Poel C., Sayer T. A., Schertzer J. D., Henstridge D. C., Church J. E., Lamon S., Russell A. P., Davies K. E., Febbraio M. A., et al. 2012. Hsp72 preserves muscle function and slows progression of severe muscular dystrophy. Nature. 484: 394–398. [DOI] [PubMed] [Google Scholar]
- 38.Vígh L., Literáti P. N., Horváth I., Török Z., Balogh G., Glatz A., Kovács E., Boros I., Ferdinándy P., Farkas B., et al. 1997. Bimoclomol: a nontoxic, hydroxylamine derivative with stress protein-inducing activity and cytoprotective effects. Nat. Med. 3: 1150–1154. [DOI] [PubMed] [Google Scholar]
- 39.Auluck P. K., Chan H. Y. E., Trojanowski J. Q., Lee V. M. Y., Bonini N. M. 2002. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science. 295: 865–868. [DOI] [PubMed] [Google Scholar]
- 40.Ron I., Horowitz M. 2005. ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum. Mol. Genet. 14: 2387–2398. [DOI] [PubMed] [Google Scholar]
- 41.Desnick R. J., Schuchman E. H. 2012. Enzyme replacement therapy for lysosomal diseases: lessons from 20 years of experience and remaining challenges. [DOI] [PubMed] [Google Scholar]
- 42.Fan J. Q., Ishii S., Asano N., Suzuki Y. 1999. Accelerated transport and maturation of lysosomal alpha-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat. Med. 5: 112–115. [DOI] [PubMed] [Google Scholar]
- 43.Zampieri S., Bembi B., Rosso N., Filocamo M., Dardis A. 2012. Treatment of human fibroblasts carrying NPC1 missense mutations with MG132 leads to an improvement of intracellular cholesterol trafficking. JIMD Rep. 2: 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pipalia N. H., Cosner C. C., Huang A., Chatterjee A., Bourbon P., Farley N., Helquist P., Wiest O., Maxfield F. R. 2011. Histone deacetylase inhibitor treatment dramatically reduces cholesterol accumulation in Niemann-Pick type C1 mutant human fibroblasts. Proc. Natl. Acad. Sci. USA. 108: 5620–5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang C., Rahimpour S., Lu J., Pacak K., Ikejiri B., Brady R. O., Zhuang Z. 2013. Histone deacetylase inhibitors increase glucocerebrosidase activity in Gaucher disease by modulation of molecular chaperones. Proc. Natl. Acad. Sci. USA. 110: 966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baumeister P., Dong D., Fu Y., Lee A. S. 2009. Transcriptional induction of GRP78/BiP by histone deacetylase inhibitors and resistance to histone deacetylase inhibitor-induced apoptosis. Mol. Cancer Ther. 8: 1086–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meriin A. B., Gabai V. L., Yaglom J., Shifrin V. I., Sherman M. Y. 1998. Proteasome inhibitors activate stress kinases and induce Hsp72. Diverse effects on apoptosis. J. Biol. Chem. 273: 6373–6379. [DOI] [PubMed] [Google Scholar]
- 48.Yang C., Swallows C. L., Zhang C., Lu J., Xiao H., Brady R. O., Zhuang Z. 2014. Celastrol increases glucocerebrosidase activity in Gaucher disease by modulating molecular chaperones. Proc. Natl. Acad. Sci. USA. 111: 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Leary E. M., Igdoura S. A. 2012. The therapeutic potential of pharmacological chaperones and proteosomal inhibitors, Celastrol and MG132 in the treatment of sialidosis. Mol. Genet. Metab. 107: 173–185. [DOI] [PubMed] [Google Scholar]
- 50.Lu J., Yang C., Chen M., Ye D. Y., Lonser R. R., Brady R. O., Zhuang Z. 2011. Histone deacetylase inhibitors prevent the degradation and restore the activity of glucocerebrosidase in Gaucher disease. Proc. Natl. Acad. Sci. USA. 108: 21200–21205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fritah S., Col E., Boyault C., Govin J., Sadoul K., Chiocca S., Christians E., Khochbin S., Jolly C., Vourc’h C. 2009. Heat-shock factor 1 controls genome-wide acetylation in heat-shocked cells. Mol. Biol. Cell. 20: 4976–4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kolter T., Sandhoff K. 2010. Lysosomal degradation of membrane lipids. FEBS Lett. 584: 1700–1712. [DOI] [PubMed] [Google Scholar]
- 53.Xu Y.-H., Barnes S., Sun Y., Grabowski G. A. 2010. Multi-system disorders of glycosphingolipid and ganglioside metabolism. J. Lipid Res. 51: 1643–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walkley S. U., Vanier M. T. 2009. Secondary lipid accumulation in lysosomal disease. Biochim. Biophys. Acta. 1793: 726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cox T. M., Cachón-González M. B. 2012. The cellular pathology of lysosomal diseases. J. Pathol. 226: 241–254. [DOI] [PubMed] [Google Scholar]
- 56.Lloyd-Evans E., Platt F. M. 2010. Lipids on trial: the search for the offending metabolite in Niemann-Pick type C disease. Traffic. 11: 419–428. [DOI] [PubMed] [Google Scholar]
- 57.Kirkegaard T., Roth A. G., Petersen N. H. T., Mahalka A. K., Olsen O. D., Moilanen I., Zylicz A., Knudsen J., Sandhoff K., Arenz C., et al. 2010. Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature. 463: 549–553. [DOI] [PubMed] [Google Scholar]
- 58.Petersen N. H. T., Olsen O. D., Groth-Pedersen L., Ellegaard A-M., Bilgin M., Redmer S., Ostenfeld M. S., Ulanet D., Dovmark T. H., Lønborg A., et al. 2013. Transformation-associated changes in sphingolipid metabolism sensitize cells to lysosomal cell death induced by inhibitors of acid sphingomyelinase. Cancer Cell. 24: 379–393. [DOI] [PubMed] [Google Scholar]
- 59.Gabandé-Rodríguez E., Boya P., Labrador V., Dotti C. G., Ledesma M. D. 2014. High sphingomyelin levels induce lysosomal damage and autophagy dysfunction in Niemann Pick disease type A. Cell Death Differ. 21: 864–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petersen N. H. T., Kirkegaard T., Olsen O. D., Jäättelä M. 2010. Connecting Hsp70, sphingolipid metabolism and lysosomal stability. Cell Cycle. 9: 2305–2309. [DOI] [PubMed] [Google Scholar]
- 61.Devlin C., Pipalia N. H., Liao X., Schuchman E. H., Maxfield F. R., Tabas I. 2010. Improvement in lipid and protein trafficking in Niemann-Pick C1 cells by correction of a secondary enzyme defect. Traffic. 11: 601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petersen N. H. T., Kirkegaard T. 2010. HSP70 and lysosomal storage disorders: novel therapeutic opportunities. Biochem. Soc. Trans. 38: 1479–1483. [DOI] [PubMed] [Google Scholar]
- 63.Ballabio A., Gieselmann V. 2009. Lysosomal disorders: from storage to cellular damage. Biochim. Biophys. Acta. 1793: 684–696. [DOI] [PubMed] [Google Scholar]
- 64.Nixon R. A. 2013. The role of autophagy in neurodegenerative disease. Nat. Med. 19: 983–997. [DOI] [PubMed] [Google Scholar]
- 65.Neefjes J., van der Kant R. 2014. Stuck in traffic: an emerging theme in diseases of the nervous system. Trends Neurosci. 37: 66–76. [DOI] [PubMed] [Google Scholar]
- 66.Pereira V. G., Gazarini M. L., Rodrigues L. C., da Silva F. H., Han S. W., Martins A. M., Tersariol I. L. S., D’Almeida V. 2010. Evidence of lysosomal membrane permeabilization in mucopolysaccharidosis type I: rupture of calcium and proton homeostasis. J. Cell. Physiol. 223: 335–342. [DOI] [PubMed] [Google Scholar]
- 67.Micsenyi M. C., Sikora J., Stephney G., Dobrenis K., Walkley S. U. 2013. Lysosomal membrane permeability stimulates protein aggregate formation in neurons of a lysosomal disease. J. Neurosci. 33: 10815–10827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kollmann K., Damme M., Markmann S., Morelle W., Schweizer M., Hermans-Borgmeyer I., Röchert A. K., Pohl S., Lübke T., Michalski J-C., et al. 2012. Lysosomal dysfunction causes neurodegeneration in mucolipidosis II “knock-in” mice. Brain. 135: 2661–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amritraj A., Peake K., Kodam A., Salio C., Merighi A., Vance J. E., Kar S. 2009. Increased activity and altered subcellular distribution of lysosomal enzymes determine neuronal vulnerability in Niemann-Pick type C1-deficient mice. Am. J. Pathol. 175: 2540–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Acker G. J., Perides G., Steer M. L. 2006. Co-localization hypothesis: a mechanism for the intrapancreatic activation of digestive enzymes during the early phases of acute pancreatitis. World J. Gastroenterol. 12: 1985–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aits S., Jäättelä M. 2013. Lysosomal cell death at a glance. J. Cell Sci. 126: 1905–1912. [DOI] [PubMed] [Google Scholar]
- 72.Kurz T., Eaton J. W., Brunk U. T. 2010. Redox activity within the lysosomal compartment: implications for aging and apoptosis. Antioxid. Redox Signal. 13: 511–523. [DOI] [PubMed] [Google Scholar]
- 73.Kiselyov K., Colletti G. A., Terwilliger A., Ketchum K., Lyons W. P., Quinn J., Muallem S. 2011. TRPML: Transporters of metals in lysosomes essential for cell survival? Cell Calcium. 50: 288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kurz T., Gustafsson B., Brunk U. T. 2006. Intralysosomal iron chelation protects against oxidative stress-induced cellular damage. FEBS J. 273: 3106–3117. [DOI] [PubMed] [Google Scholar]
- 75.Shen J-S. 2008. Globotriaosylceramide induces oxidative stress and up-regulates cell adhesion molecule expression in Fabry disease endothelial cells. Mol. Genet. Metab. 95: 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vitner E. B., Farfel-Becker T., Eilam R., Biton I., Futerman A. H. 2012. Contribution of brain inflammation to neuronal cell death in neuronopathic forms of Gaucher’s disease. Brain. 135: 1724–1735. [DOI] [PubMed] [Google Scholar]
- 77.Zampieri S., Mellon S. H., Butters T. D., Nevyjel M., Douglas F., Metaboliche M., Garofolo I. B. 2009. Oxidative stress in NPC1 deficient cells: Protective effect of allopregnanolone. J. Cell. Mol. Med. 13: 3786–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jeyakumar M., Thomas R., Elliot-Smith E., Smith D. A., van der Spoel A. C., D’Azzo A., Perry V. H., Butters T. D., Dwek R. A., Platt F. M. 2003. Central nervous system inflammation is a hallmark of pathogenesis in mouse models of GM1 and GM2 gangliosidosis. Brain. 126: 974–987. [DOI] [PubMed] [Google Scholar]
- 79.Doulias P-T., Kotoglou P., Tenopoulou M., Keramisanou D., Tzavaras T., Brunk U., Galaris D., Angelidis C. 2007. Involvement of heat shock protein-70 in the mechanism of hydrogen peroxide-induced DNA damage: the role of lysosomes and iron. Free Radic. Biol. Med. 42: 567–577. [DOI] [PubMed] [Google Scholar]
- 80.Jayakumar J., Suzuki K., Sammut I. A., Smolenski R. T., Khan M., Latif N., Abunasra H., Murtuza B., Amrani M., Yacoub M. H. 2001. Heat shock protein 70 gene transfection protects mitochondrial and ventricular function against ischemia-reperfusion injury. Circulation. 104: I303–I307. [DOI] [PubMed] [Google Scholar]
- 81.de Jong P. R., Schadenberg A. W. L., Jansen N. J. G., Prakken B. J. 2009. Hsp70 and cardiac surgery: molecular chaperone and inflammatory regulator with compartmentalized effects. Cell Stress Chaperones. 14: 117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kågedal K., Zhao M., Svensson I., Brunk U. T. 2001. Sphingosine-induced apoptosis is dependent on lysosomal proteases. Biochem. J. 359: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Appelqvist H., Sandin L., Björnström K., Saftig P., Garner B., Ollinger K., Kågedal K. 2012. Sensitivity to lysosome-dependent cell death is directly regulated by lysosomal cholesterol content. PLoS ONE. 7: e50262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ullio C., Casas J., Brunk U. T., Sala G., Fabriàs G., Ghidoni R., Bonelli G., Baccino F. M., Autelli R. 2012. Sphingosine mediates TNFα-induced lysosomal membrane permeabilization and ensuing programmed cell death in hepatoma cells. J. Lipid Res. 53: 1134–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feldstein A. E., Werneburg N. W., Li Z., Bronk S. F., Gores G. J. 2006. Bax inhibition protects against free fatty acid-induced lysosomal permeabilization. Am. J. Physiol. Gastrointest. Liver Physiol. 290: G1339–G1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang G., Yi Y-P., Zhang G-J. 2006. Effects of arachidonic acid on the lysosomal ion permeability and osmotic stability. J. Bioenerg. Biomembr. 38: 75–82. [DOI] [PubMed] [Google Scholar]
- 87.Stancevic B., Kolesnick R. 2010. Ceramide-rich platforms in transmembrane signaling. FEBS Lett. 584: 1728–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gyrd-Hansen M., Farkas T., Fehrenbacher N., Bastholm L., Høyer-Hansen M., Elling F., Wallach D., Flavell R., Kroemer G., Nylandsted J., et al. 2006. Apoptosome-independent activation of the lysosomal cell death pathway by caspase-9. Mol. Cell. Biol. 26: 7880–7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jäättelä M., Wissing D., Bauer P. A., Li G. C. 1992. Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J. 11: 3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Calderwood S. K., Mambula S. S., Gray P. J., Jr 2007. Extracellular heat shock proteins in cell signaling and immunity. Ann. N. Y. Acad. Sci. 1113: 28–39. [DOI] [PubMed] [Google Scholar]
- 91.Huynh C., Roth D., Ward D. M., Kaplan J., Andrews N. W. 2004. Defective lysosomal exocytosis and plasma membrane repair in Chediak-Higashi/beige cells. Proc. Natl. Acad. Sci. USA. 101: 16795–16800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fehrenbacher N., Bastholm L., Kirkegaard-Sørensen T., Rafn B., Bøttzauw T., Nielsen C., Weber E., Shirasawa S., Kallunki T., Jäättelä M. 2008. Sensitization to the lysosomal cell death pathway by oncogene-induced down-regulation of lysosome-associated membrane proteins 1 and 2. Cancer Res. 68: 6623–6633. [DOI] [PubMed] [Google Scholar]
- 93.Guicciardi M. E., Leist M., Gores G. J. 2004. Lysosomes in cell death. Oncogene. 23: 2881–2890. [DOI] [PubMed] [Google Scholar]
- 94.Cirman T., Oresic K., Mazovec G. D., Turk V., Reed J. C., Myers R. M., Salvesen G. S., Turk B. 2004. Selective disruption of lysosomes in HeLa cells triggers apoptosis mediated by cleavage of Bid by multiple papain-like lysosomal cathepsins. J. Biol. Chem. 279: 3578–3587. [DOI] [PubMed] [Google Scholar]
- 95.Brunk U. T., Dalen H., Roberg K., Hellquist H. B. 1997. Photo-oxidative disruption of lysosomal membranes causes apoptosis of cultured human fibroblasts. Free Radic. Biol. Med. 23: 616–626. [DOI] [PubMed] [Google Scholar]
- 96.Antunes F., Cadenas E., Brunk U. T. 2001. Apoptosis induced by exposure to a low steady-state concentration of H2O2 is a consequence of lysosomal rupture. Biochem. J. 356: 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boya P., Andreau K., Poncet D., Zamzami N., Perfettini J. L., Metivier D., Ojcius D. M., Jaattela M., Kroemer G. 2003. Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J. Exp. Med. 197: 1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jäättelä M., Wissing D., Kokholm K., Kallunki T., Egeblad M. 1998. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 17: 6124–6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hwang J-H., Ryu J. K., Yoon Y. B., Lee K. H., Park Y-S., Kim J-W., Kim N., Lee D. H., Jeong J. B., Seo J-S., et al. 2005. Spontaneous activation of pancreas trypsinogen in heat shock protein 70.1 knock-out mice. Pancreas. 31: 332–336. [DOI] [PubMed] [Google Scholar]
- 100.Garrido C., Gurbuxani S., Ravagnan L., Kroemer G. 2001. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem. Biophys. Res. Commun. 286: 433–442. [DOI] [PubMed] [Google Scholar]
- 101.Li W., Yuan X., Nordgren G., Dalen H., Dubowchik G. M., Firestone R. A., Brunk U. T. 2000. Induction of cell death by the lysosomotropic detergent MSDH. FEBS Lett. 470: 35–39. [DOI] [PubMed] [Google Scholar]
- 102.Lloyd-Evans E., Morgan A. J., He X., Smith D. A., Elliot-Smith E., Sillence D. J., Churchill G. C., Schuchman E. H., Galione A., Platt F. M. 2008. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat. Med. 14: 1247–1255. [DOI] [PubMed] [Google Scholar]
- 103.Deleted in proof. [Google Scholar]
- 104.Smith B., Galbiati F., Castelvetri L. C., Givogri M. I., Lopez-Rosas A., Bongarzone E. R. 2011. Peripheral neuropathy in the Twitcher mouse involves the activation of axonal caspase 3. ASN Neuro. 3: 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hawkins-Salsbury J. A., Parameswar A. R., Jiang X., Schlesinger P. H., Bongarzone E., Ory D. S., Demchenko A. V., Sands M. S. 2013. Psychosine, the cytotoxic sphingolipid that accumulates in globoid cell leukodystrophy, alters membrane architecture. J. Lipid Res. 54: 3303–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Won J-S., Kim J., Paintlia M. K., Singh I., Singh A. K. 2013. Role of endogenous psychosine accumulation in oligodendrocyte differentiation and survival: implication for Krabbe disease. Brain Res. 1508: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Giri S., Khan M., Rattan R., Singh I., Singh A. K. 2006. Krabbe disease: psychosine-mediated activation of phospholipase A2 in oligodendrocyte cell death. J. Lipid Res. 47: 1478–1492. [DOI] [PubMed] [Google Scholar]
- 108.Foghsgaard L., Wissing D., Mauch D., Lademann U., Bastholm L., Boes M., Elling F., Leist M., Jäättelä M. 2001. Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J. Cell Biol. 153: 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guicciardi M. E., Deussing J., Miyoshi H., Bronk S. F., Svingen P. A., Peters C., Kaufmann S. H., Gores G. J. 2000. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J. Clin. Invest. 106: 1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brunk U. T., Svensson I. 1999. Oxidative stress, growth factor starvation and Fas activation may all cause apoptosis through lysosomal leak. Redox Rep. 4: 3–11. [DOI] [PubMed] [Google Scholar]
- 111.Nakayama M., Ishidoh K., Kayagaki N., Kojima Y., Yamaguchi N., Nakano H., Kominami E., Okumura K., Yagita H. 2002. Multiple pathways of TWEAK-induced cell death. J. Immunol. 168: 734–743. [DOI] [PubMed] [Google Scholar]
- 112.Wiegmann K., Schutze S., Machleidt T., Witte D., Kronke M. 1994. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell. 78: 1005–1015. [DOI] [PubMed] [Google Scholar]
- 113.Ségui B., Cuvillier O., Adam-Klages S., Garcia V., Malagarie-Cazenave S., Lévêque S., Caspar-Bauguil S., Coudert J., Salvayre R., Krönke M., et al. 2001. Involvement of FAN in TNF-induced apoptosis. J. Clin. Invest. 108: 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Taha T. A., Kitatani K., Bielawski J., Cho W., Hannun Y. A., Obeid L. M. 2005. Tumor necrosis factor induces the loss of sphingosine kinase-1 by a cathepsin B-dependent mechanism. J. Biol. Chem. 280: 17196–17202. [DOI] [PubMed] [Google Scholar]
- 115.Werneburg N. W., Guicciardi M. E., Bronk S. F., Gores G. J. 2002. Tumor necrosis factor-alpha-associated lysosomal permeabilization is cathepsin B dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 283: G947–G956. [DOI] [PubMed] [Google Scholar]
- 116.Foghsgaard L., Lademann U., Wissing D., Poulsen B., Jäättelä M. 2002. Cathepsin B mediates tumor necrosis factor-induced arachidonic acid release in tumor cells. J. Biol. Chem. 277: 39499–39506. [DOI] [PubMed] [Google Scholar]
- 117.Ribeil J. A., Zermati Y., Vandekerckhove J., Cathelin S., Kersual J., Dussiot M., Coulon S., Moura I. C., Zeuner A., Kirkegaard-Sorensen T., et al. 2007. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature. 445: 102–105. [DOI] [PubMed] [Google Scholar]
- 118.Deleted in proof. [Google Scholar]
- 119.Pelled D., Lloyd-Evans E., Riebeling C., Jeyakumar M., Platt F. M., Futerman A. H. 2003. Inhibition of calcium uptake via the sarco/endoplasmic reticulum Ca2+-ATPase in a mouse model of Sandhoff disease and prevention by treatment with N-butyldeoxynojirimycin. J. Biol. Chem. 278: 29496–29501. [DOI] [PubMed] [Google Scholar]
- 120.Ginzburg L., Futerman A. H. 2005. Defective calcium homeostasis in the cerebellum in a mouse model of Niemann-Pick A disease. J. Neurochem. 95: 1619–1628. [DOI] [PubMed] [Google Scholar]
- 121.Yamashima T. 2012. Hsp70.1 and related lysosomal factors for necrotic neuronal death. J. Neurochem. 120: 477–494. [DOI] [PubMed] [Google Scholar]
- 122.Sahara S., Yamashima T. 2010. Calpain-mediated Hsp70.1 cleavage in hippocampal CA1 neuronal death. Biochem. Biophys. Res. Commun. 393: 806–811. [DOI] [PubMed] [Google Scholar]
- 123.Yamashima T., Kohda Y., Tsuchiya K., Ueno T., Yamashita J., Yoshioka T., Kominami E. 1998. Inhibition of ischaemic hippocampal neuronal death in primates with cathepsin B inhibitor CA-074: a novel strategy for neuroprotection based on “calpain-cathepsin hypothesis”. Eur. J. Neurosci. 10: 1723–1733. [DOI] [PubMed] [Google Scholar]
- 124.Syntichaki P., Xu K., Driscoll M., Tavernarakis N. 2002. Specific aspartyl and calpain proteases are required for neurodegeneration in C. elegans. Nature. 419: 939–944. [DOI] [PubMed] [Google Scholar]
- 125.Churchill G. C., Okada Y., Thomas J. M., Genazzani A. A., Patel S., Galione A. 2002. NAADP mobilizes Ca(2+) from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell. 111: 703–708. [DOI] [PubMed] [Google Scholar]
- 126.Calcraft P. J., Ruas M., Pan Z., Cheng X., Arredouani A., Hao X., Tang J., Rietdorf K., Teboul L., Chuang K-T., et al. 2009. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 459: 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shen D., Wang X., Li X., Zhang X., Yao Z., Dibble S., Dong X., Yu T., Lieberman A. P., Showalter H. D., et al. 2012. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat. Commun. 3: 731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tupling A. R., Gramolini A. O., Duhamel T. A., Kondo H., Asahi M., Tsuchiya S. C., Borrelli M. J., Lepock J. R., Otsu K., Hori M., et al. 2004. HSP70 binds to the fast-twitch skeletal muscle sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA1a) and prevents thermal inactivation. J. Biol. Chem. 279: 52382–52389. [DOI] [PubMed] [Google Scholar]
- 129.Chung K. M., Yu S-W. 2013. Interplay between autophagy and programmed cell death in mammalian neural stem cells. BMB Rep. 46: 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ballabio A. 2009. Disease pathogenesis explained by basic science: lysosomal storage diseases as autophagocytic disorders. Int. J. Clin. Pharmacol. Ther. 47(Suppl 1): S34–S38. [DOI] [PubMed] [Google Scholar]
- 131.Raben N., Shea L., Hill V., Plotz P. 2009. Monitoring autophagy in lysosomal storage disorders. Methods Enzymol. 453: 417–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gamerdinger M., Kaya A. M., Wolfrum U., Clement A. M., Behl C. 2011. BAG3 mediates chaperone-based aggresome-targeting and selective autophagy of misfolded proteins. EMBO Rep. 12: 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jacobs A. T., Marnett L. J. 2009. HSF1-mediated BAG3 expression attenuates apoptosis in 4-hydroxynonenal-treated colon cancer cells via stabilization of anti-apoptotic Bcl-2 proteins. J. Biol. Chem. 284: 9176–9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dokladny K., Zuhl M. N., Mandell M., Bhattacharya D., Schneider S., Deretic V., Moseley P. L. 2013. Regulatory coordination between two major intracellular homeostatic systems: heat shock response and autophagy. J. Biol. Chem. 288: 14959–14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vidal-Donet J. M., Cárcel-Trullols J., Casanova B., Aguado C., Knecht E. 2013. Alterations in ROS activity and lysosomal pH account for distinct patterns of macroautophagy in LINCL and JNCL fibroblasts. PLoS ONE. 8: e55526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chung E. S., Bok E., Sohn S., Lee Y. D., Baik H. H., Jin B. K. 2010. GT1b-induced neurotoxicity is mediated by the Akt/GSK-3/tau signaling pathway but not caspase-3 in mesencephalic dopaminergic neurons. BMC Neurosci. 11: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ryu J. K., Shin W. H., Kim J., Joe E. H., Lee Y. B., Cho K. G., Oh Y. J., Kim S. U., Jin B. K. 2002. Trisialoganglioside GT1b induces in vivo degeneration of nigral dopaminergic neurons: role of microglia. Glia. 38: 15–23. [DOI] [PubMed] [Google Scholar]
- 138.Schnaar R. L. 2010. Brain gangliosides in axon-myelin stability and axon regeneration. FEBS Lett. 584: 1741–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou J., Shao H., Cox N. R., Baker H. J., Ewald S. J. 1998. Gangliosides enhance apoptosis of thymocytes. Cell. Immunol. 183: 90–98. [DOI] [PubMed] [Google Scholar]
- 140.Chen X., Chi S., Liu M., Yang W., Wei T., Qi Z., Yang F. 2005. Inhibitory effect of ganglioside GD1b on K+ current in hippocampal neurons and its involvement in apoptosis suppression. J. Lipid Res. 46: 2580–2585. [DOI] [PubMed] [Google Scholar]
- 141.Santos A. X. S., Maia J. E., Crespo P. M., Pettenuzzo L. F., Daniotti J. L., Barbé-Tuana F. M., Martins L. M., Trindade V. M. T., Borojevic R., Guma F. C. R. 2011. GD1a modulates GM-CSF-induced cell proliferation. Cytokine. 56: 600–607. [DOI] [PubMed] [Google Scholar]
- 142.Tessitore A., del P Martin M., Sano R., Ma Y., Mann L., Ingrassia A., Laywell E. D., Steindler D. A., Hendershot L. M., d’Azzo A. 2004. GM1-ganglioside-mediated activation of the unfolded protein response causes neuronal death in a neurodegenerative gangliosidosis. Mol. Cell. 15: 753–766. [DOI] [PubMed] [Google Scholar]
- 143.Sano R., Annunziata I., Patterson A., Moshiach S., Gomero E., Opferman J., Forte M., d’Azzo A. 2009. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca2+-dependent mitochondrial apoptosis. Mol. Cell. 36: 500–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang Y., Tsui Z., Yang F. 1999. Antagonistic effect of ganglioside GM1 and GM3 on the activity and conformation of sarcoplasmic reticulum Ca(2+)-ATPase. FEBS Lett. 457: 144–148. [DOI] [PubMed] [Google Scholar]
- 145.Tanaka Y., Waki H., Kon K., Ando S. 1997. Gangliosides enhance KCl-induced Ca2+ influx and acetylcholine release in brain synaptosomes. Neuroreport. 8: 2203–2207. [DOI] [PubMed] [Google Scholar]
- 146.Mutoh T., Tokuda A., Miyadai T., Hamaguchi M., Fujiki N. 1995. Ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc. Natl. Acad. Sci. USA. 92: 5087–5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ledeen R. W., Wu G. 2006. Gangliosides of the nuclear membrane: a crucial locus of cytoprotective modulation. J. Cell. Biochem. 97: 893–903. [DOI] [PubMed] [Google Scholar]
- 148.Furuse H., Waki H., Kaneko K., Fujii S., Miura M., Sasaki H., Ito K-I., Kato H., Ando S. 1998. Effect of the mono- and tetra-sialogangliosides, GM1 and GQ1b, on long-term potentiation in the CA1 hippocampal neurons of the guinea pig. Exp. Brain Res. 123: 307–314. [DOI] [PubMed] [Google Scholar]
- 149.Wu G., Lu Z-H., Xie X., Ledeen R. W. 2004. Susceptibility of cerebellar granule neurons from GM2/GD2 synthase-null mice to apoptosis induced by glutamate excitotoxicity and elevated KCl: rescue by GM1 and LIGA20. Glycoconj. J. 21: 305–313. [DOI] [PubMed] [Google Scholar]
- 150.Usuki S., Ren J., Utsunomiya I., Cashman N. R., Inokuchi J., Miyatake T. 2001. GM2 ganglioside regulates the function of ciliary neurotrophic factor receptor in murine immortalized motor neuron-like cells (NSC-34). Neurochem. Res. 26: 375–382. [DOI] [PubMed] [Google Scholar]
- 151.Usuki S., Cashman N. R., Miyatake T. 1999. GM2 promotes ciliary neurotrophic factor-dependent rescue of immortalized motor neuron-like cell (NSC-34). Neurochem. Res. 24: 281–286. [DOI] [PubMed] [Google Scholar]
- 152.Sohn H., Kim Y-S., Kim H-T., Kim C-H., Cho E-W., Kang H-Y., Kim N-S., Kim C-H., Ryu S. E., Lee J-H., et al. 2006. Ganglioside GM3 is involved in neuronal cell death. FASEB J. 20: 1248–1250. [DOI] [PubMed] [Google Scholar]
- 153.Bieberich E., MacKinnon S., Silva J., Yu R. K. 2001. Regulation of apoptosis during neuronal differentiation by ceramide and b-series complex gangliosides. J. Biol. Chem. 276: 44396–44404. [DOI] [PubMed] [Google Scholar]
- 154.Inokuchi J., Mizutani A., Jimbo M., Usuki S., Yamagishi K., Mochizuki H., Muramoto K., Kobayashi K., Kuroda Y., Iwasaki K., et al. 1997. Up-regulation of ganglioside biosynthesis, functional synapse formation, and memory retention by a synthetic ceramide analog (L-PDMP). Biochem. Biophys. Res. Commun. 237: 595–600. [DOI] [PubMed] [Google Scholar]
- 155.Inokuchi J. 2009. Neurotrophic and neuroprotective actions of an enhancer of ganglioside biosynthesis. Int. Rev. Neurobiol. 85: 319–336. [DOI] [PubMed] [Google Scholar]
- 156.Lluis J. M., Llacuna L., von Montfort C., Bárcena C., Enrich C., Morales A., Fernandez-Checa J. C. 2009. GD3 synthase overexpression sensitizes hepatocarcinoma cells to hypoxia and reduces tumor growth by suppressing the cSrc/NF-kappaB survival pathway. PLoS ONE. 4: e8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sa G., Das T., Moon C., Hilston C. M., Rayman P.A., Rini B. I., Tannenbaum C. S., Finke J. H. 2009. GD3, an overexpressed tumor-derived ganglioside, mediates the apoptosis of activated but not resting T cells. Cancer Res. 69: 3095–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Castro-Palomino J. C., Simon B., Speer O., Leist M., Schmidt R. R. 2001. Synthesis of ganglioside GD3 and its comparison with bovine GD3 with regard to oligodendrocyte apoptosis mitochondrial damage. Chemistry. 7: 2178–2184. [DOI] [PubMed] [Google Scholar]
- 159.Hasegawa T., Sugeno N., Takeda A., Matsuzaki-Kobayashi M., Kikuchi A., Furukawa K., Miyagi T., Itoyama Y. 2007. Role of Neu4L sialidase and its substrate ganglioside GD3 in neuronal apoptosis induced by catechol metabolites. FEBS Lett. 581: 406–412. [DOI] [PubMed] [Google Scholar]
- 160.Glaros E. N., Kim W. S., Quinn C. M., Wong J., Gelissen I., Jessup W., Garner B. 2005. Glycosphingolipid accumulation inhibits cholesterol efflux via the ABCA1/apolipoprotein A-I pathway: 1-phenyl-2-decanoylamino-3-morpholino-1-propanol is a novel cholesterol efflux accelerator. J. Biol. Chem. 280: 24515–24523. [DOI] [PubMed] [Google Scholar]
- 161.Utermöhlen O., Herz J., Schramm M., Krönke M. 2008. Fusogenicity of membranes: the impact of acid sphingomyelinase on innate immune responses. Immunobiology. 213: 307–314. [DOI] [PubMed] [Google Scholar]
- 162.Lloyd-Evans E., Pelled D., Riebeling C., Bodennec J., De-Morgan A., Waller H., Schiffmann R., Futerman A. H. 2003. Glucosylceramide and glucosylsphingosine modulate calcium mobilization from brain microsomes via different mechanisms. J. Biol. Chem. 278: 23594–23599. [DOI] [PubMed] [Google Scholar]
- 163.Jana A., Hogan E. L., Pahan K. 2009. Ceramide and neurodegeneration: susceptibility of neurons and oligodendrocytes to cell damage and death. J. Neurol. Sci. 278: 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pahan K. 1998. Sphingomyelinase and ceramide stimulate the expression of inducible nitric-oxide synthase in rat primary astrocytes. J. Biol. Chem. 273: 2591–2600. [DOI] [PubMed] [Google Scholar]
- 165.Separovic D. 2008. Suppression of sphingomyelin synthase 1 by small interference RNA is associated with enhanced ceramide production and apoptosis after photodamage. Exp. Cell Res. 314: 1860–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Young M. M., Kester M., Wang H-G. 2013. Sphingolipids: regulators of crosstalk between apoptosis and autophagy. J. Lipid Res. 54: 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Smith E. L., Schuchman E. H. 2008. The unexpected role of acid sphingomyelinase in cell death and the pathophysiology of common diseases. FASEB J. 22: 3419–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Veiga M. P., Arrondo J. L., Goñi F. M., Alonso A. 1999. Ceramides in phospholipid membranes: effects on bilayer stability and transition to nonlamellar phases. Biophys. J. 76: 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Edvardson S., Hama H., Shaag A., Gomori J. M., Berger I., Soffer D., Korman S. H., Taustein I., Saada A., Elpeleg O. 2008. Mutations in the fatty acid 2-hydroxylase gene are associated with leukodystrophy with spastic paraparesis and dystonia. Am. J. Hum. Genet. 83: 643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Meixner M., Jungnickel J., Grothe C., Gieselmann V., Eckhardt M. 2011. Myelination in the absence of UDP-galactose:ceramide galactosyl-transferase and fatty acid 2 -hydroxylase. BMC Neurosci. 12: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stroobants S., Gerlach D., Matthes F., Hartmann D., Fogh J., Gieselmann V., D’Hooge R., Matzner U. 2011. Intracerebroventricular enzyme infusion corrects central nervous system pathology and dysfunction in a mouse model of metachromatic leukodystrophy. Hum. Mol. Genet. 20: 2760–2769. [DOI] [PubMed] [Google Scholar]
- 172.Nishihori Y., Kato K., Tanaka M., Okamoto T., Hagiwara S., Araki N., Kogawa K., Kuribayashi K., Nakamura K., Niitsu Y. 2009. Interleukin-2 gene transfer potentiates the alpha-galactosylceramide-stimulated antitumor effect by the induction of TRAIL in NKT and NK cells in mouse models of subcutaneous and metastatic carcinoma. Cancer Biol. Ther. 8: 1763–1770. [DOI] [PubMed] [Google Scholar]
- 173.De Francesco P. N., Mucci J. M., Ceci R., Fossati C. A., Rozenfeld P. A. 2011. Higher apoptotic state in Fabry disease peripheral blood mononuclear cells: effect of globotriaosylceramide. Mol. Genet. Metab. 104: 319–324. [DOI] [PubMed] [Google Scholar]
- 174.Lee M. H., Choi E. N., Jeon Y. J., Jung S-C. 2012. Possible role of transforming growth factor-β1 and vascular endothelial growth factor in Fabry disease nephropathy. Int. J. Mol. Med. 30: 1275–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rombach S. M., Dekker N., Bouwman M. G., Linthorst G. E., Zwinderman A. H., Wijburg F. A., Kuiper S., van den Bergh Weerman M. A., Groener J. E., Poorthuis B. J., et al. 2010. Plasma globotriaosylsphingosine: diagnostic value and relation to clinical manifestations of Fabry disease. Biochim. Biophys. Acta. 1802: 741–748. [DOI] [PubMed] [Google Scholar]
- 176.Aerts J. M., Groener J. E., Kuiper S., Donker-Koopman W. E., Strijland A., Ottenhoff R., van Roomen C., Mirzaian M., Wijburg F. A., Linthorst G. E., et al. 2008. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc. Natl. Acad. Sci. USA. 105: 2812–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kondo Y., Ikeda K., Tokuda N., Nishitani C., Ohto U., Akashi-Takamura S. 2013. TLR4-MD-2 complex is negatively regulated by an endogenous ligand, globotetraosylceramide. Proc. Natl. Acad. Sci. USA. 110: 4714–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yu W., Gong J-S., Ko M., Garver W. S., Yanagisawa K., Michikawa M. 2005. Altered cholesterol metabolism in Niemann-Pick type C1 mouse brains affects mitochondrial function. J. Biol. Chem. 280: 11731–11739. [DOI] [PubMed] [Google Scholar]
- 179.Aqul A., Liu B., Ramirez C. M., Pieper A. A., Estill S. J., Burns D. K., Liu B., Repa J. J., Turley S. D., Dietschy J. M. 2011. Unesterified cholesterol accumulation in late endosomes/lysosomes causes neurodegeneration and is prevented by driving cholesterol export from this compartment. J. Neurosci. 31: 9404–9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Huang Z., Hou Q., Cheung N. S., Li Q-T. 2006. Neuronal cell death caused by inhibition of intracellular cholesterol trafficking is caspase dependent and associated with activation of the mitochondrial apoptosis pathway. J. Neurochem. 97: 280–291. [DOI] [PubMed] [Google Scholar]