Abstract
Intestinal cholesterol absorption involves the chylomicron and HDL pathways and is dependent on microsomal triglyceride transfer protein (MTP) and ABCA1, respectively. Chylomicrons transport free and esterified cholesterol, whereas HDLs transport free cholesterol. ACAT2 esterifies cholesterol for secretion with chylomicrons. We hypothesized that free cholesterol accumulated during ACAT2 deficiency may be secreted with HDLs when chylomicron assembly is blocked. To test this, we studied cholesterol absorption in mice deficient in intestinal MTP, global ACAT2, and both intestinal MTP and global ACAT2. Intestinal MTP ablation significantly increased intestinal triglyceride and cholesterol levels and reduced their transport with chylomicrons. In contrast, global ACAT2 deficiency had no effect on triglyceride absorption but significantly reduced cholesterol absorption with chylomicrons and increased cellular free cholesterol. Their combined deficiency reduced cholesterol secretion with both chylomicrons and HDLs. Thus, contrary to our hypothesis, free cholesterol accumulated in the absence of MTP and ACAT2 is unavailable for secretion with HDLs. Global ACAT2 deficiency causes mild hypertriglyceridemia and reduces hepatosteatosis in mice fed high cholesterol diets by increasing hepatic lipoprotein production by unknown mechanisms. We show that this phenotype is preserved in the absence of intestinal MTP in global ACAT2-deficient mice fed a Western diet. Further, we observed increases in hepatic MTP activity in these mice. Thus, ACAT2 deficiency might increase MTP expression to avoid hepatosteatosis in cholesterol-fed animals. Therefore, ACAT2 inhibition might avert hepatosteatosis associated with high cholesterol diets by increasing hepatic MTP expression and lipoprotein production.
Keywords: microsomal triglyceride transfer protein, acyl-CoA:cholesterol acyltransferase 2, high density lipoproteins, chylomicrons
High plasma cholesterol levels increase risk for atherosclerosis. Absorption of dietary and biliary cholesterol by the intestine is a major determinant of plasma cholesterol levels. Therefore, cholesterol absorption has been studied extensively (1–3) and reduction in cholesterol absorption is a valid target to lower plasma cholesterol concentrations (4). Cholesterol absorption involves uptake of free cholesterol by enterocytes from the apical side by a mechanism involving Niemann-Pick C1-like 1 (NPC1L1), conversion to cholesteryl esters by ACAT2 in the endoplasmic reticulum (5–8), and assembly and secretion with chylomicrons by microsomal triglyceride transfer protein (MTP) (9–12).
ACAT2 expression is restricted to the small intestine and liver (13, 14), and its deficiency reduces cholesterol absorption rendering mice resistant to diet-induced hypercholesterolemia, gallstone formation, and atherosclerosis (15, 16). Cholesterol esterified by ACAT2 is packaged into chylomicrons and secreted toward the basolateral side. Chylomicron assembly and secretion is critically dependent on MTP and apoB (9–12). Ablation of both apoB and MTP results in embryonic lethality in mice, as assembly of apoB-containing lipoproteins by the yolk sac appears to be essential for the survival of the embryo (17–19). Intestine-specific MTP (gene, Mttp) ablation significantly reduces cholesterol absorption in mice (20, 21). Thus, both ACAT2 and MTP play an important role in cholesterol absorption via the chylomicron pathway. Here, we examined their individual and combined contributions to intestinal cholesterol absorption.
Besides being converted to cholesteryl esters and secreted with the chylomicron pathway, free cholesterol taken up by the enterocytes can be secreted with HDLs (22–25). This pathway appears to be dependent on ABCA1. Ablation of intestinal ABCA1 reduces acute cholesterol absorption by ∼28%, mainly via the HDL pathway (21, 24). Using intestine-specific MTP and ABCA1-deficient mice, we showed that these two pathways are responsible for ∼95% of cholesterol absorption (21).
The role of ACAT2 in the metabolism of free cholesterol in the intestine has been studied using ACAT2 [gene, sterol O-acyltransferase (Soat)2] knockout mice. Early studies indicated that ACAT2 deficiency increases free cholesterol levels in the intestine (15). Subsequently, it was shown that ACAT2 deficiency is associated with increases in intestinal ABCA1 expression, most likely a secondary consequence of increases in cellular free cholesterol. Augmentation of intestinal ABCA1 expression in the absence of ACAT2 was hypothesized to facilitate absorption of cholesterol via the ABCA1/HDL pathway (26). Indeed, combined deficiency of ACAT2 and ABCA1 additively reduced cholesterol absorption compared with that observed individually in Abca1−/− and Soat2−/− mice (27). Thus, it appears that ACAT2 deficiency might increase free cholesterol absorption by the HDL pathway.
In this study, we examined whether increasing cellular free cholesterol by ACAT2 ablation and curtailing chylomicron assembly by intestinal MTP ablation would further enhance absorption of cholesterol via the HDL pathway. Chylomicrons transport both free and esterified cholesterol. In the presence of ACAT2, cholesterol is esterified and is packaged into chylomicrons. In the absence of ACAT2, free cholesterol may be absorbed via the chylomicron and HDL pathways. However, if the chylomicron pathway is inhibited by ablation of the Mttp gene, then free cholesterol might be secreted with the HDL pathway. Therefore, we hypothesized that ACAT2 knockout mice would increase free cholesterol transport via the HDL pathway when chylomicron assembly was curtailed. To test this hypothesis, we studied acute cholesterol absorption in mice deficient in intestinal MTP and global ACAT2. Further, we hypothesized that the phenotype of increased cholesterol secretion with HDLs related to the deficiencies of ACAT2 and MTP might be enhanced when a Western diet rich in cholesterol was fed to these mice.
Another aim of this study was to identify compensatory changes that might occur in mice fed chow and Western diets in the absence of intestinal MTP and global ACAT2. The hypothesis tested was that in the absence of intestinal MTP, the liver might upregulate transport of lipids via VLDLs and these changes might be further affected in the absence of ACAT2. It is known that ACAT2 deficiency increases free cholesterol in the intestine but not in the liver. One possibility is that VLDL assembly might be increased to alleviate free cholesterol accretion in ACAT2-deficient hepatocytes. Therefore, we examined whether ACAT2 deficiency affects hepatic lipid metabolism in mice fed chow and Western diets to compensate for lower dietary fat and cholesterol absorption in intestinal MTP-deficient mice.
MATERIALS AND METHODS
Generation of intestine-specific MTP, global ACAT2, and MTP/ACAT2 double knockout mice
Three different sets of mice were used in this study. Generation of global Soat2−/− (15) and intestine-specific MTP knockout (21) mice have been described previously. To create mice deficient in intestinal MTP and ACAT2 (I-DKO), ERT2-villin-Cre;Mttpf/f mice on a C57BL/6J background were crossed with Soat2−/− mice on a C57BL/6J background to generate ERT2-villin-Cre;Mttpf/fSoat2−/−. To induce intestinal Mttp gene ablation, tamoxifen (0.5 mg/mouse) was injected intraperitoneally three times on alternate days in 200 μl of corn oil as described before (21). For a matching control, Soat2+/+ and ERT2-villin-Cre;Mttpf/f mice were injected with corn oil (200 μl) used to dissolve tamoxifen. No significant differences were found among these two controls; therefore, they have been combined and used as WT controls. Mice were fed either chow diet (LabDiet 5001) or Western diet (TD88137, Harlan Teklad) containing 17, 48.5, 21.2, and 0.2% by weight of protein, carbohydrate, fat, and cholesterol, respectively, after the first tamoxifen injection until they were euthanized 7 days after the last tamoxifen injection. All studies were approved by the institutional animal care and use committee of SUNY Downstate Medical Center.
Plasma lipid measurements
Plasma and tissue total cholesterol and triglyceride (Thermo-Fisher Scientific), free cholesterol (Wako Chemicals), and glycerol (Sigma) levels were measured using commercial kits. Esterified cholesterol was calculated by subtracting free cholesterol from total cholesterol. Plasma free glycerol levels were subtracted from triglyceride levels. HDL lipid levels were measured after precipitating apoB lipoproteins using phosphotungstate/MgCl2 reagent (22). Plasma lipoproteins were separated by fast protein liquid chromatography (FPLC) using a Superose 6 column (flow rate of 0.2 ml/min) and 200 μl fractions were collected to measure cholesterol and triglycerides.
Short-term lipid absorption studies
Age-matched male mice (n = 3 per group) on a chow or Western diet were fasted overnight and injected intraperitoneally with poloxamer 407 (P407) (30 mg/mouse). After 1 h, mice were gavaged with 0.5 μCi of either [14C]triolein or [3H]cholesterol with 0.2 mg of unlabeled cholesterol in 15 μl of olive oil (23). After 2 h, plasma was collected to measure radioactivity.
Uptake and secretion of [3H]oleic acid and [3H]cholesterol by primary enterocytes
To study uptake, primary enterocytes isolated from overnight fasted mice (n = 3) as previously described (22, 23), were suspended in 4 ml of DMEM containing 0.5 μCi/ml of [3H]oleic acid or [3H]cholesterol and incubated at 37°C (22, 23). After 1 h, enterocytes were washed and cellular lipids were extracted to determine uptake of radiolabeled lipids. For characterization of secreted lipoproteins, enterocytes were isolated from overnight fasted mice and radiolabeled for 1 h with 0.5 μCi/ml of [3H]oleic acid or [3H]cholesterol, washed, and incubated with fresh media containing lipid/bile salt micelles consisting of 1.4 mM oleic acid, 0.14 mM sodium cholate, 0.15 mM sodium deoxycholate, 0.17 mM phosphatidylcholine, and 0.19 mM mono-oleoylglycerol (22, 28). After 2 h, enterocytes were centrifuged and supernatants were collected. In the [3H]oleic acid radiolabeling experiments, lipids were extracted from cells and media and separated by thin layer chromatography to quantify incorporation of [3H]oleic acid into triglycerides, phospholipids, and cholesteryl esters. In the [3H]cholesterol radiolabeling experiment, media were subjected to density gradient ultracentrifugation to determine radiolabeled cholesterol distribution among different lipoprotein classes (22).
Determination of MTP activity
Small pieces (0.1 g) of liver and proximal small intestine (∼1 cm) were homogenized in a low-salt buffer [1 mM Tris-HCl (pH 7.6), 1 mM EGTA, and 1 mM MgCl2], centrifuged, and supernatants were used for protein determination and the MTP assay (29, 30) using a kit (Chylos, Inc).
Histology
Aliquots of intestines were fixed overnight in 10% formalin, dehydrated in 30% sucrose, embedded in M1 cryo-preservation media at −20°C, and stored at −70°C. Sections (7 μm) were placed on Tissue-Tack (Polysciences) slides, dehydrated in 60% isopropanol, immersed in 1% Oil Red O for 30 min at 22°C, washed in 60% isopropanol, rinsed with tap water for 10 s, counterstained with Gills hematoxylin for at least 20 min, rinsed with tap water until clear, acidified in alcohol (0.4% HCl in 95% ethanol), rinsed with tap water again, and dipped in basic solution (0.03 N NaOH) until sections visibly darkened. Images were taken with a SPOT RT3 digital camera. Image analysis was performed using SPOT software from Imaging Diagnostics.
mRNA quantifications
Total RNA from tissues was isolated using TriZolTM (Invitrogen). The purity of RNA was assessed by the A260/A280 ratio and preparations with ratios more than 1.7 were used for cDNA synthesis. The first strand cDNA was synthesized using Omniscript RT (Qiagen) kit. Each reaction of quantitative (q)PCR was carried out in a volume of 20 μl, consisting of 5 μl cDNA sample (1:100 dilution of the first strand cDNA sample) and 15 μl of PCR master mix solution containing 1× PCR reaction buffer (qPCRTM Core Kit for SYBR Green I, Eurogentec) and specific primers (21). The PCR was carried out by incubating the reaction mixture first for 10 min at 95°C followed by 40 cycles of 15 s incubations at 95°C and 1 min at 60°C in an ABI 7000 SDS PCR machine. Data were analyzed using the ΔΔCT method according to the manufacturer’s instructions and presented as arbitrary units and were normalized to ARPp0 mRNA.
Statistics
Data are presented as mean ± SD. Statistical significance (P < 0.05) was determined using either Student’s t-test, one-way ANOVA and comparisons between groups were analyzed using the Newman-Keuls posttest, or two-way ANOVA with Bonferroni’s posttest (GraphPad Prism 5). For all knockout mice, WT mice served as controls. However, for I-DKO mice there were two more controls; Soat2−/− and I-Mttp−/−.
RESULTS
ACAT2 ablation reduces total plasma cholesterol
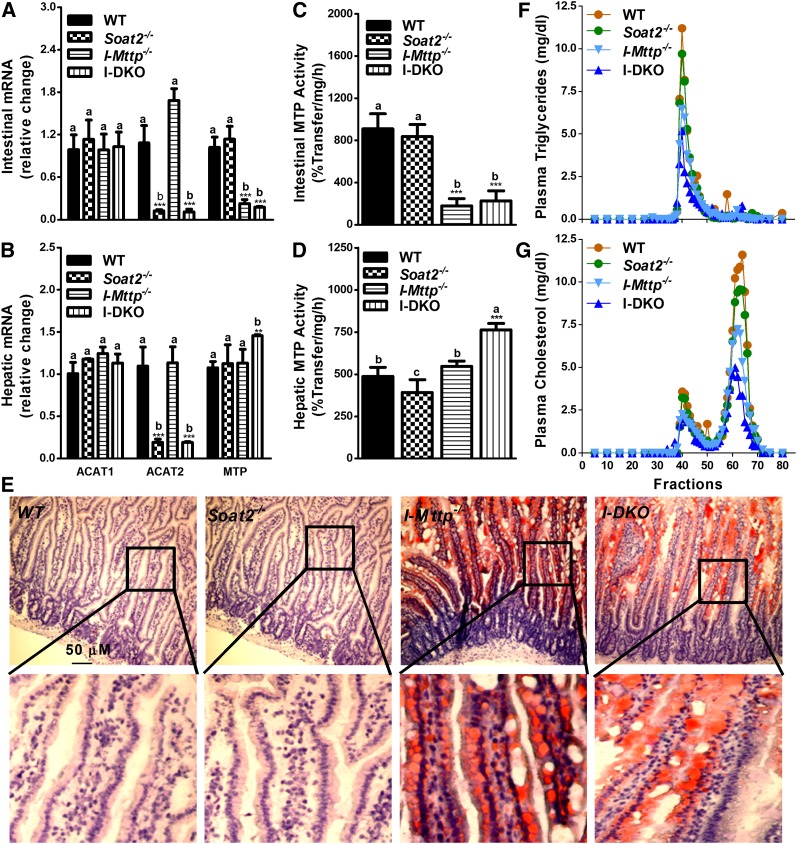
As anticipated, ACAT2 mRNA levels were very low in the intestine (Fig. 1A) and liver (Fig. 1B) of Soat2−/− mice. Deletion of ACAT2 had no effect on the relative expression of ACAT1 in both the intestine and liver (Fig. 1A, B), in agreement with other reports (15, 26), confirming that the loss of ACAT2 does not upregulate ACAT1 in either the intestine or the liver. Further, ablation of ACAT2 had no effect on intestinal and hepatic MTP mRNA (Fig. 1A, B) and activity (Fig. 1C, D) indicating that ACAT2 deficiency also does not affect MTP expression. ACAT2 deficiency had no significant effect on intestinal triglyceride (Table 1) and on lipid accumulation in enterocytes as determined by Oil Red O staining (Fig. 1E). Furthermore, ACAT2 ablation had no significant effect on total cholesterol in the intestine, but it increased free cholesterol by 46% and decreased cholesteryl esters by 43% (Table 1). ACAT2 deficiency had no effect on hepatic triglyceride, reduced hepatic total cholesterol, had no effect on free cholesterol, and decreased esterified cholesterol (Table 1) consistent with other studies (15, 31). ACAT2 deficiency had no effect on plasma triglyceride, but reduced total cholesterol concentrations by 18%, mainly due to reductions in esterified cholesterol (Table 1). FPLC analysis showed that ACAT2 deficiency had no effect on triglyceride and cholesterol in VLDL/LDL fractions (Fig. 1F, G), but reduced cholesterol in the HDL fraction (Fig. 1G). Thus, ACAT2 deficiency reduces esterified cholesterol in tissues and plasma. However, it increases free cholesterol in the intestine, but not in the liver, of chow-fed mice.
Fig. 1.
Effect of global ACAT2 and intestine-specific MTP deficiency on intestinal gene expression, lipid accumulation, and plasma lipoproteins. A–D: Total RNA isolated from the intestine (A) and the liver (B) of 12-week-old WT, Soat2−/−, I-Mttp−/−, and I-DKO (n = 5) male mice fed a chow diet was used to quantify mRNA levels of ACAT1, ACAT2, and MTP. Intestinal (C) and hepatic (D) tissues were also used to measure MTP activity. Data are presented as mean ± SD. **P < 0.01 and ***P < 0.001 compared with WT as determined by Student’s t-test. Statistically significant differences in different parameters in the four groups were evaluated by one-way ANOVA with Newman-Keuls multiple comparison test. Different letters above bars for each component indicate statistically significant differences in the mean values in different groups (P < 0.05) as determined by one-way ANOVA. E: Proximal intestinal sections were used for lipid staining by Oil Red O. A higher magnification image of the boxed area is shown under each picture to show the presence of lipids in the absorptive epithelial cells. F, G: Plasma was separated by gel filtration to determine mass of triglycerides (F) and cholesterol (G) in different lipoproteins.
TABLE 1.
Effect of global ACAT2 and intestine-specific MTP gene deletion on tissue and plasma lipids in chow- and Western diet-fed mice
| Chow Fed | Western Fed | ||||||||
| WT | Soat2−/− | I-Mttp−/− | I-DKO | WT | Soat2−/− | I-Mttp−/− | I-DKO | Interaction (P) (Genotype vs. Diet) | |
| Intestine (μg/mg protein) | |||||||||
| Triglycerides | 16.8 ± 3.0a,r | 20.7 ± 3.4a,r (+23.2) | 730.2 ± 257.6b,r (+4246.4)*** | 831.1 ± 221.0b,r (+4847.0)*** | 44.4 ± 4.2a,r | 46.3 ± 6.2a,r (+4.3) | 319.2 ± 40.0b,s (+618.9)*** | 375.9 ± 40.9b,s (+746.6)*** | <0.001 |
| Total cholesterol | 16.6 ± 2.5a,r | 18.3 ± 2.5a,r (+10.2) | 19.3 ± 2.2a,r (+16.3)* | 18.3 ± 4.8a,r (+10.2) | 23.9 ± 1.8a,s | 24.2 ± 1.8a,s (+1.3) | 28.7 ± 0.5b,s (+20.1)* | 21.2 ± 0.9a,r (−11.3) | NS |
| Free cholesterol | 9.8 ± 1.2a,r | 14.3 ± 1.7b,r (+45.9)*** | 12.6 ± 1.8b,r (+28.6)** | 14.9 ± 3.8b,r (+52.0)** | 10.8 ± 1.9a,r | 17.8 ± 0.7b,r (+64.8)** | 13.6 ± 1.4c,r (+25.9) | 18.5 ± 1.0b,r (+71.3)** | NS |
| Esterified cholesterol | 6.8 ± 2.1a,r | 3.9 ± 1.5b,r (−42.6)** | 6.7 ± 1.3a,r (−1.5) | 3.4 ± 1.5b,r (−50.0)** | 13.1 ± 0.7a,s | 6.5 ± 1.2b,r (−50.4)** | 15.1 ± 1.9a,s (+15.3) | 2.7 ± 1.2c,r (−79.4)** | <0.001 |
| Liver (μg/mg protein) | |||||||||
| Triglycerides | 25.6 ± 6.6a,r | 26.4 ± 3.5a,r (+3.1) | 13.7 ± 2.5b,r (−46.5)*** | 16.3 ± 3.9b,r (−36.3)** | 460.9 ± 60.5a,s | 183.9 ± 34.0b,s (−60.1)** | 444.5 ± 44.5a,s (−3.4) | 135.3 ± 26.0b,s (−70.6)** | <0.001 |
| Total cholesterol | 8.0 ± 0.8a,r | 5.6 ± 0.9b,r (−30.0)*** | 9.7 ± 1.0c,r (+21.3)** | 9.3 ± 1.9c,r (+16.3) | 17.1 ± 0.6a,s | 7.3 ± 0.3b,r (−57.3)*** | 18.1 ± 0.9a,s (+5.8) | 8.0 ± 1.1b,r (−53.2)*** | <0.001 |
| Free cholesterol | 5.2 ± 0.6a,r | 5.1 ± 0.8a,r (−1.9) | 4.7 ± 0.7a,r (−9.6) | 6.1 ± 0.9b,r (+17.3)* | 4.1 ± 0.3a,r | 3.5 ± 0.4a,s (−14.6)* | 6.4 ± 0.7b,s (+56.1)** | 3.5 ± 0.3a,s (−14.6)* | <0.001 |
| Esterified cholesterol | 2.8 ± 0.9a,r | 0.5 ± 0.5b,r (−82.1)*** | 4.9 ± 1.0c,r (+60.7)*** | 3.2 ± 1.2a,r (+14.3) | 13.0 ± 0.6a,s | 3.9 ± 0.1b,s (−70.0)*** | 11.7 ± 0.5a,s (−10.0)* | 4.5 ± 1.2b,r (−65.4)*** | <0.001 |
| Plasma (mg/dl) | |||||||||
| Triglycerides | 41.7 ± 6.1a,r | 46.5 ± 5.2a,r (+11.5) | 22.5 ± 5.6b,r (−46.0)c | 27.8 ± 6.3b,r (−33.3)c | 60.4 ± 6.1a,s | 82.2 ± 6.6b,s (+36.1)a | 42.3 ± 5.1c,s (−30.0)a | 102.2 ± 6.0d,s (+69.2)b | <0.001 |
| Total cholesterol | 83.7 ± 6.4a,r | 68.4 ± 2.5b,r (−18.3)*** | 37.4 ± 10.9c,r (−55.3)*** | 38.1 ± 5.5c,r (−54.5)*** | 132.3 ± 10.6a,s | 84.7 ± 5.5b,s (−36.0)** | 63.3 ± 6.5c,s (−52.2)*** | 46.7 ± 3.8d,r (−64.7)*** | <0.001 |
| Free cholesterol | 20.0 ± 1.4a,r | 19.6 ± 1.9a,r (−2.0) | 10.5 ± 1.2b,r (−47.5)*** | 10.0 ± 3.3b,r (−50.0)*** | 44.7 ± 4.4a,s | 37.7 ± 2.3b,s (−15.7) | 34.7 ± 2.5b,s (−22.4)* | 32.3 ± 1.8b,s (−27.7)* | <0.05 |
| Esterified cholesterol | 63.7 ± 6.1a,r | 48.8 ± 3.9b,r (−23.4)*** | 26.9 ± 10.5c,r (−57.8)*** | 28.4 ± 6.0c,r (−55.4)*** | 87.6 ± 8.3a,s | 47.0 ± 5.4b,r (−46.3)** | 28.5 ± 6.0c,r (−67.5)*** | 14.3 ± 2.5d,s (−83.7)*** | <0.001 |
Intestinal and hepatic tissues and plasma from WT, Soat2−/−, I-Mttp−/−, and I-DKO mice fed either chow (n = 8) or Western (n = 3) diet were used to measure triglycerides, total, free, and esterified cholesterol mass. Data are presented as mean ± SD. Values in parenthesis show percent change compared with WT. Significant differences (a, b, c, d) within chow and Western diet groups were determined by one-way ANOVA using Neuman-Keuls multiple comparison test. Differences (r, s) between chow and Western diet groups were determined by two-way ANOVA with Bonferroni posttest.
*P < 0.05 compared with WT mice as determined by Student’s t-test.
**P < 0.01 compared with WT mice as determined by Student’s t-test.
***P < 0.001 compared with WT mice as determined by Student’s t-test.
Intestinal MTP ablation increases intestinal lipids and reduces plasma lipoproteins
Intestine-specific Mttp gene ablation was obtained by injecting tamoxifen on three alternate days in chow-fed male ERT2-Villin-Cre;Mttpf/f mice as previously described (21). All studies were performed 7 days after the last injection. Tamoxifen injection reduced MTP mRNA (Fig. 1A) and activity (Fig. 1C) by ∼80% in the intestine, but had no significant effect on intestinal and hepatic ACAT1 and ACAT2 mRNA (Fig. 1A, B) and hepatic MTP mRNA (Fig. 1B) and activity (Fig. 1D). To determine the consequences of MTP deletion on tissue homeostasis, lipids in the intestine of these mice were quantified and Oil Red O staining was performed on the frozen intestinal sections. Consistent with previous reports (20, 21), conditional intestinal ablation of MTP was associated with significant increases in triglycerides, cholesterol, and free cholesterol by 42-fold, 16 and 29%, respectively; with no significant change in esterified cholesterol levels (Table 1). As expected, MTP ablation was associated with enhanced Oil Red O staining of the intestinal sections (Fig. 1E). Lipids were mostly present in the absorptive epithelial cells of the villi. Intestine-specific MTP ablation reduced triglycerides and increased cholesterol, mainly esterified cholesterol, in the livers of these mice (Table 1). Next, the effects of intestinal MTP ablation on plasma lipids were assessed. I-Mttp−/− mice had 46 and 55% less plasma triglyceride and cholesterol, respectively (Table 1). FPLC analysis of plasma showed lower triglycerides in VLDL/LDL fractions (Fig. 1F) and reduced cholesterol concentrations in both VLDL/LDL and HDL fractions (Fig. 1G). These studies showed that intestine-specific MTP ablation is associated with significant lipid accumulation in the intestine and reduced plasma lipids and lipoproteins.
Global ACAT2 deficiency and intestine-specific MTP ablation increase tissue cholesterol and reduce plasma cholesterol
I-Mttp−/−;Soat2−/− (I-DKO) mice had significantly lower levels of ACAT2 mRNA in the intestine similar to Soat2−/−, but these mice did not register any change in ACAT1 mRNA levels compared with WT mice (Fig. 1A). I-DKO mice, similar to I-Mttp−/− mice, had significantly reduced MTP mRNA (Fig. 1A) and activity (Fig. 1C) in the intestine, but increased hepatic MTP mRNA (Fig. 1B) and activity compared with WT mice (Fig. 1D). Increases in hepatic MTP mRNA and activity in I-DKO were significantly different from the individual knockout and WT mice. I-DKO mice had significantly higher amounts of intestinal triglycerides compared with WT and Soat2−/− mice, and lower esterified cholesterol levels compared with WT mice (Table 1). However, they were similar to those in I-Mttp−/− mice. Similar to I-Mttp−/− mice, intestinal sections from I-DKO mice had significant lipid stain (Fig. 1E). The increase in intestinal triglycerides in I-DKO mice was not statistically significant from I-Mttp−/− mice, suggesting that ACAT2 does not play any significant role in the accumulation of the intestinal triglycerides in chow-fed mice. The increases in intestinal free cholesterol in I-DKO mice were not significantly different from the individual knockout mice, suggesting lack of additive effects of Mttp and Soat2 genes. I-DKO mice had lower hepatic triglyceride and higher free cholesterol levels with no significant change in total and esterified cholesterol compared with WT and Soat2−/− mice (Table 1). One-way ANOVA analysis revealed that total cholesterol in the livers of I-DKO mice was significantly higher than WT and Soat2−/− mice, but not higher than I-Mttp−/− mice. These data also showed that accumulation of cholesterol in the liver of I-DKO mice was due to increased free cholesterol. I-DKO mice had significantly lower plasma levels of total triglyceride, as well as total, free, and esterified cholesterol (Table 1). Further, they had reduced lipids in both VLDL and HDL fractions (Fig. 1F, G). The plasma lipid levels were not significantly different from I-Mttp−/− mice. These studies suggest that the major effect in I-DKO mice on plasma and tissue lipids is due to MTP deficiency.
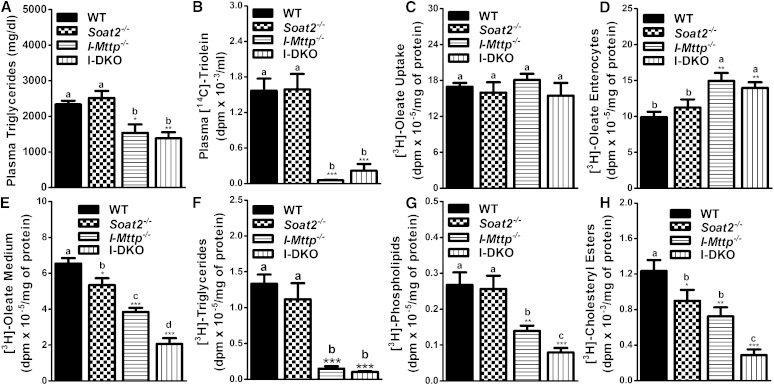
Lower plasma lipids in intestine-specific MTP-deficient and global ACAT2 knockout mice are due to reduced lipid absorption
To understand the physiological and biochemical mechanisms for lower plasma lipids in I-DKO mice, we hypothesized that reductions in plasma triglyceride and cholesterol concentrations in I-DKO mice might be secondary to lower lipid absorption during the postprandial state. To study lipid absorption, I-Mttp−/−, Soat2−/−, and I-DKO mice were injected with P407 to inhibit lipoprotein lipase and were gavaged with [14C]triolein in olive oil and cholesterol. First, we measured total plasma triglyceride concentrations. Plasma triglyceride mass was significantly lower in I-Mttp−/− and I-DKO mice but was similar in Soat2−/− mice compared with WT mice (Fig. 2A) indicating that MTP, but not ACAT2, contributes to plasma triglycerides. To gain a better understanding of the contribution of these genes in the transport of newly absorbed lipids, we followed radiolabeled triolein. Appearance of [14C]triolein-derived lipids in the plasma of ACAT2-deficient mice was not different compared with WT mice after 2 h of gavage (Fig. 2B). In contrast, deficiency of intestinal MTP reduced the appearance of [14C]triolein-derived lipids by >90% compared with WT mice (Fig. 2B). Similarly, plasma of I-DKO mice had significantly fewer triolein-derived counts and these counts were not significantly different than those in I-Mttp−/− mice. These studies indicate that ACAT2 does not, but MTP does, play a significant role in triglyceride absorption.
Fig. 2.
Intestinal MTP and global ACAT2 gene deletions decrease lipid absorption. Twelve-week-old WT, Soat2−/−, I-Mttp−/−, and I-DKO male mice (n = 3) were fasted overnight and injected intraperitoneally with P407 (30 mg/mouse). After 1 h, mice were gavaged with 0.5 μCi of [14C]triolein, as well as 0.2 mg of cholesterol, in 15 μl of olive oil. Plasma was collected after 2 h to measure triglyceride mass (A). Total plasma was also used to measure radioactivity to determine the absorption of [14C]triolein (B). To study lipid uptake, enterocytes were isolated from 12-week-old chow diet-fed overnight-fasted mice, and incubated with 0.5 μCi/ml of [3H]oleate. After 1 h, enterocytes were washed and lipids were isolated to determine uptake of radiolabeled fatty acid (C). For characterization of secreted lipoproteins, after 1 h of [3H]oleate uptake, enterocytes were washed and incubated with fresh media containing 1.4 mM oleic acid containing micelles for 2 h. Isolated lipids from the cells (D) and media (E) were counted to determine total fatty acid-derived radioactivity. Lipids were extracted from the media and separated by thin layer chromatography to determine radioactivity in triglycerides (F), phospholipids (G), and cholesteryl esters (H). Each measurement was done in triplicate with three mice per group. These data are representative of two separate experiments. Data are presented as mean ± SD. Data in (C–H) are normalized to cellular protein. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with WT as determined by Student’s t-test. Statistically significant differences in different parameters in the four groups were evaluated by one-way ANOVA with Newman-Keuls multiple comparison test. Different letters above bars indicate statistically significant differences (P < 0.05) as determined by one-way ANOVA.
Intestinal MTP and global ACAT2 deficiency does not affect fatty acid uptake, but reduces cholesteryl ester secretion by enterocytes
Next, we investigated the role of ACAT2 and MTP on the uptake and secretion of fatty acids by the enterocytes. Soat2−/− and I-Mttp−/− enterocytes took up similar amounts of [3H]oleic acid compared with WT enterocytes (Fig. 2C). Moreover, combined deficiencies of ACAT2 and MTP also had no effect on oleic acid uptake. These studies suggest that both ACAT2 and MTP do not affect fatty acid uptake by enterocytes.
We then studied the secretion of [3H]oleic acid-labeled lipids by enterocytes using a pulse-chase protocol. At the end of the chase, radiolabeled lipids in Soat2−/− enterocytes were not statistically different from WT enterocytes (Fig. 2D). In contrast, I-Mttp−/− and I-DKO enterocytes contained significantly higher labeled lipids, indicating increased retention of lipids in these enterocytes compared with controls. Next, we measured amounts of radiolabeled lipids secreted during the 2 h chase period. Soat2−/− enterocytes secreted lesser amounts of oleic acid-derived labeled lipids (Fig. 2E). Similarly, I-Mttp−/− enterocytes secreted significantly lower amounts of radiolabeled lipids compared with WT enterocytes. These studies suggest that individual absence of ACAT2 and MTP reduces secretion of 3H-oleic acid-labeled lipids. I-DKO enterocytes secreted significantly fewer lipids than those secreted by I-Mttp−/− and Soat2−/− enterocytes, indicating that ACAT2 and MTP additively contribute to lipid secretion.
Furthermore, we analyzed the effects of these gene ablations on the secretion of different types of secreted lipids labeled with oleic acid after separation by thin layer chromatography. Soat2−/− enterocytes secreted similar amounts of newly synthesized triglycerides, while I-Mttp−/− and I-DKO enterocytes secreted 89–92% less radiolabeled triglycerides (Fig. 2F). Phospholipid secretion was not reduced in Soat2−/− enterocytes, but I-Mttp−/− and I-DKO enterocytes secreted 48 and 70% less nascent phospholipids (Fig. 2G). Soat2−/− and I-Mttp−/− enterocytes secreted 27 and 41% less cholesteryl esters, respectively, and enterocytes deficient in both ACAT2 and MTP secreted 77% less cholesteryl esters (Fig. 2H). These studies indicate that ACAT2 deficiency does not affect secretion of glycerolipids but reduces secretion of cholesteryl esters, whereas MTP deficiency significantly reduces both glycerolipids and sterol secretion.
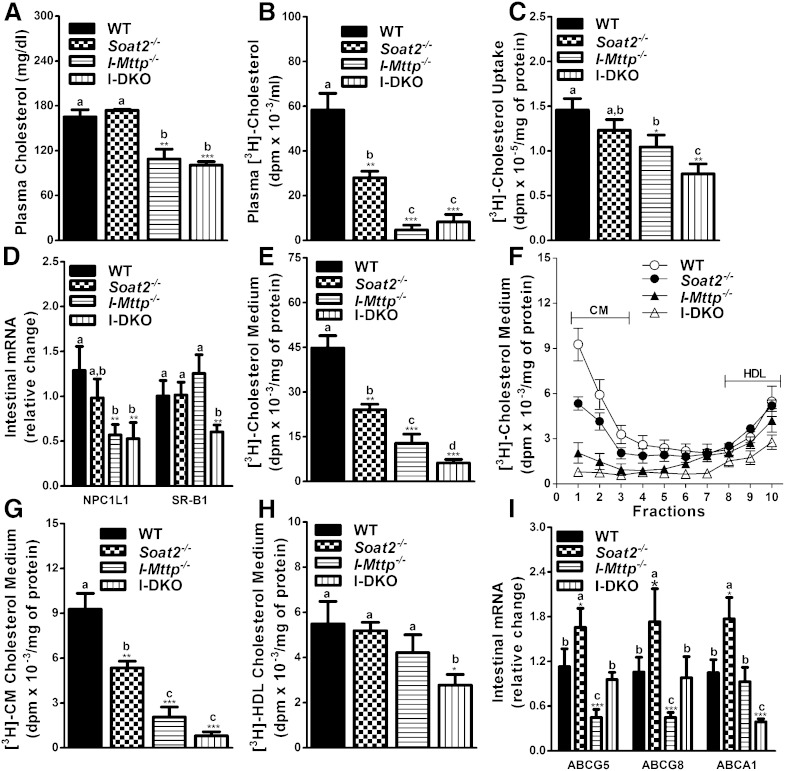
ACAT2 and MTP gene ablations reduce cholesterol secretion by enterocytes via the chylomicron pathway
After evaluating the role of ACAT2 and MTP on fatty acid and triglyceride absorption, we studied the effect of these gene deletions on the acute absorption of cholesterol in mice injected with P407 to inhibit plasma lipases. Plasma cholesterol mass remained unchanged in Soat2−/− mice after the gavage of cholesterol and olive oil, but was significantly reduced in I-Mttp−/− and I-DKO mice compared with WT controls (Fig. 3A). However, the appearance of [3H]cholesterol-derived lipids in the plasma was significantly reduced in both ACAT2- and MTP- deficient mice (Fig. 3B). Decrease in I-DKO acute cholesterol absorption was not significantly different from I-Mttp−/− mice, but both these groups showed reduced cholesterol absorption compared with Soat2−/− and WT mice. Thus, ACAT2 deficiency reduces the appearance of radiolabeled cholesterol in the plasma, whereas MTP deficiency reduces both radiolabeled and unlabeled cholesterol. We interpret these data to suggest that ACAT2 plays a significant role in the transport of newly absorbed cholesterol, whereas MTP is critical for the transport of both newly absorbed and previously stored cholesterol.
Fig. 3.
Global ACAT2 and intestine-specific MTP gene deletion decreases absorption and secretion of cholesterol. Twelve-week-old WT, Soat2−/−, I-Mttp−/−, and I-DKO male mice (n = 3) were fasted overnight and injected intraperitoneally with P407 (30 mg/mouse). After 1 h, mice were gavaged with 0.5 μCi of [3H]cholesterol as well as 0.2 mg of cholesterol in 15 μl of olive oil. Plasma was collected after 2 h to measure cholesterol mass (A). Total plasma was also used to measure total radioactivity to determine the absorption of [3H]cholesterol (B). To study cholesterol uptake, enterocytes were isolated from twelve-week-old chow diet-fed overnight-fasted mice and incubated with 0.5 μCi/ml of [3H]cholesterol. After 1 h, enterocytes were washed and lipids were isolated to determine uptake of radiolabeled cholesterol (C). Total RNA isolated from the intestine, as described in Fig. 1, was used to quantify mRNA levels of NPC1L1 and SR-B1 (D). For characterization of secreted lipoproteins, after 1 h of uptake, enterocytes were washed and incubated with fresh media containing 1.4 mM oleic acid containing micelles for 2 h. Isolated lipids from the media (E) were counted to determine total cholesterol radioactivity. [3H]cholesterol radiolabeled media were used for separating lipoproteins by density gradient ultracentrifugation and radioactivity was determined in each fraction (F). Fractions 1–3 and 8–10 represent chylomicrons (CM) and HDLs, respectively. For better representation of CMs (G) and HDLs (H), fractions 1 and 10 from (F), respectively, were plotted separately. Total RNA isolated from the intestine of 12-week-old WT, Soat2−/−, I-Mttp−/−, and I-DKO (n = 5) male mice fed a chow diet was used to quantify mRNA levels of different cholesterol absorption (I). Each measurement was done in triplicate with three mice per group. Data in (C) and (E–H) were normalized to cellular protein and are representative of two separate experiments. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with WT as determined by Student’s t-test. Statistically significant differences in different parameters in the four groups were evaluated by one-way ANOVA with Newman-Keuls multiple comparison test. Different letters above bars indicate statistically significant differences (P < 0.05) as determined by one-way ANOVA.
Next, we evaluated the role of MTP and ACAT2 in cholesterol absorption by enterocytes. Absorption involves uptake and subsequent secretion. ACAT2 deficiency insignificantly decreased cholesterol uptake by 15% compared with WT mouse enterocytes (Fig. 3C). This was surprising, as ACAT2 deficiency has been shown to reduce NPC1L1 expression in cholesterol-fed mice (26, 27). Therefore, we measured mRNA levels of NPC1L1. Levels of NPC1L1 were reduced by 23%, but the difference did not reach statistical significance (Fig. 3D). No significant reductions in NPC1L1 and cholesterol uptake might be related to the chow-fed mice used in this study. Indeed, feeding of a Western diet reduced cholesterol uptake in these mice (described later). Hence, ACAT2 deficiency and a high cholesterol diet are needed to see reductions in NPC1L1 expression and cholesterol uptake.
As reported earlier (21), uptake of cholesterol (Fig. 3C) and expression of NPC1L1 (Fig. 3D) were reduced in the absence of intestinal MTP. Combined deficiency of ACAT2 and MTP reduced cholesterol uptake by 49% compared with WT mice (Fig. 3C). This reduction was also significantly higher compared with I-Mttp−/− and Soat2−/− enterocytes. Gene expression analysis (Fig. 3D) revealed that I-DKO enterocytes had reduced expression of NPC1L1 and SR-B1, and these changes might have contributed to significant decreases in the uptake of cholesterol.
As opposed to variable results of gene ablations on cholesterol uptake, cholesterol secretion was consistently reduced by 46 and 71% in ACAT2- and MTP-deficient enterocytes, respectively, and their combined deficiency reduced cholesterol secretion by 86% compared with controls (Fig. 3E). The decrease in cholesterol secretion by the enterocytes from I-DKO was also significantly different from Soat2−/− and I-Mttp−/− enterocytes. These studies indicate that both ACAT2 and MTP additively contribute to cholesterol secretion by enterocytes.
It is known that enterocytes secrete cholesterol via the chylomicron (apoB-dependent) or HDL (apoB-independent) pathways (22). The chylomicron pathway transports both free and esterified cholesterol, whereas the HDL pathway is mainly involved in free cholesterol transport. Therefore, we hypothesized that MTP and ACAT2 deficiencies that accrete cellular free cholesterol might increase free cholesterol export via the HDL pathway. To test this hypothesis, we subjected media to ultracentrifugation. Individual deficiencies of ACAT2 and MTP significantly reduced secretion of cholesterol with chylomicrons but had no significant effect on HDL compared with controls (Fig. 3F–H). However, combined deficiency of MTP and ACAT2 reduced cholesterol secretion with chylomicrons by 91% and with HDLs by ∼49% (Fig. 3G, H). Thus, individual deficiencies of ACAT2 and MTP reduce secretion of cholesterol with chylomicrons only, but their combined deficiency additionally reduces secretion of cholesterol with HDLs.
To understand the reasons for decreased cholesterol secretion with HDL by enterocytes deficient in both ACAT2 and MTP, we measured mRNA levels of ABC transporters involved in cholesterol efflux (Fig. 3I). ACAT2 deficiency increased ABCG5, ABCG8, and ABCA1 expression. MTP deficiency reduced ABCG5 and ABCG8, but had no effect on ABCA1 expression. Surprisingly, combined deficiency of ACAT2 and MTP reduced expression of ABCA1. Thus, reduced secretion of cholesterol with HDL might be secondary to lower intestinal expression of ABCA1 in I-DKO mice.
Effect of intestine-specific MTP and global ACAT2 deficiency on the expression of hepatic lipid metabolism genes
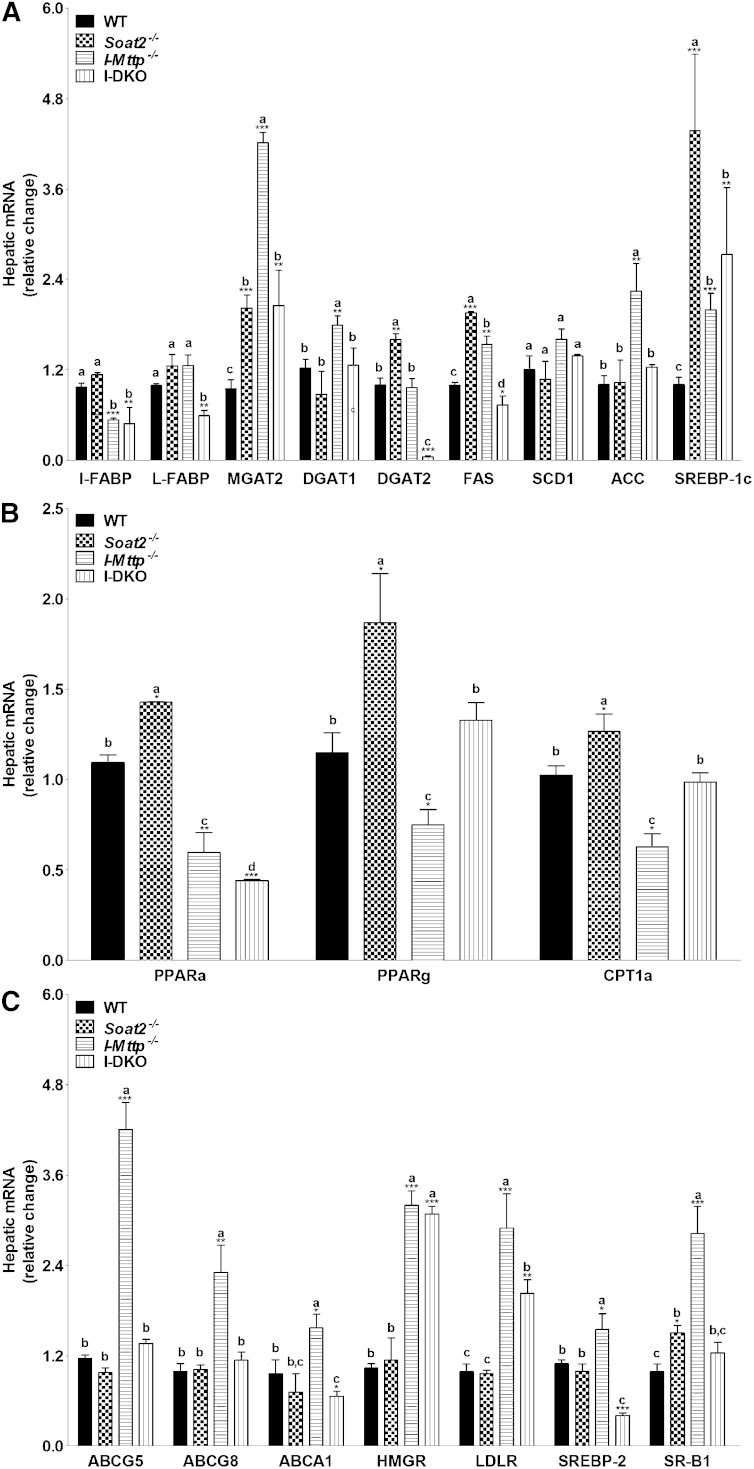
Significant changes in hepatic cholesterol metabolism have been reported in cholesterol-fed Soat2−/− mice (15, 26, 27, 32), but these changes have not been quantified in chow-fed animals. Intestinal MTP deficiency has been shown to affect hepatic gene expression (20, 21). Thus, deficiencies of ACAT2 and MTP have been shown to affect hepatic lipid metabolism. There is no data about the effects of I-Mttp and global ACAT2 deficiencies on hepatic lipid metabolism. It would be interesting to know changes in hepatic lipid metabolism associated with reduced intestinal lipid absorption. We hypothesized that reduced delivery of lipids in the absence of intestinal MTP and ACAT2 might alter hepatic lipid metabolism. Therefore, we studied the effect of intestinal MTP and global ACAT2 deficiency on the expression of hepatic genes involved in lipid metabolism. First, we studied the effects of ACAT2 deficiency on hepatic expression of genes involved in fatty acid and triglyceride synthesis. ACAT2 deficiency did not affect hepatic I-FABP, L-FABP, DGAT1, SCD1, and ACC1, but increased MGAT2, DGAT2, FAS, and SREBP-1c (Fig. 4A). Intestinal MTP deficiency had no effect on hepatic L-FABP, DGAT2, and SCD1, but increased MGAT2, DGAT1, FAS, ACC, and SREBP-1c mRNA levels. Combined deficiencies of ACAT2 and MTP reduced the expression of I-FABP, L-FABP, and DGAT2; increased the expression of MGAT2 and SREBP-1c; and had no effect on DGAT1, SCD1, and ACC. These studies indicate variable effects of ACAT2 and MTP gene ablations on the expression of genes involved in fatty acid and glycerolipid synthesis.
Fig. 4.
Relative hepatic mRNA levels of genes involved in lipid metabolism. Total RNA isolated from the liver of 12-week-old WT, Soat2−/−, I-Mttp−/−, and I-DKO (n = 5) male mice fed a chow diet was used to quantify mRNA levels of different genes involved in lipid synthesis (A), fatty acid oxidation (B), and cholesterol absorption (C). Data are presented as mean ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with WT as determined by Student’s t-test. Different letters above bars for each component indicate statistically different mean values (P < 0.05), as determined by one-way ANOVA with Newman-Keuls multiple comparison test.
Analysis of mRNA levels of genes involved in fatty acid oxidation showed that the livers of Soat2−/− mice had significantly increased expression of PPARα, PPARγ, and CPT1α (Fig. 4B). I-Mttp−/− mice had reduced expression of hepatic PPARα, PPARγ, and CPT1α (Fig. 4B). In I-DKO mice, only PPARα mRNA levels were significantly lower. These studies indicate modest, if any, effects on fatty acid oxidation in I-DKO mice.
Next, we studied the effect of ACAT2 deficiency on genes involved in cholesterol metabolism. We did not see significant changes in the expression of genes involved in cholesterol metabolism, except for increases in SR-B1, consistent with no significant changes in hepatic cholesterol (Table 1). We observed significant increases in hepatic cholesterol levels in I-Mttp−/− mice and in the expression of ABCG5, ABCG8, ABCA1, HMGR, LDL receptor, SREBP-2, and SR-B1 (Fig. 4C), but not in ACAT1 and ACAT2 (Fig. 1B). These studies suggest significant effects of intestinal MTP deficiency on cholesterol transport. In I-DKO mice, hepatic expression of ABCG5, ABCG8, HMGR, and LDL receptor was increased, but that of SR-B1 was unchanged (Fig. 4C). These findings suggest modest compensatory alterations in hepatic lipid metabolism in I-DKO mice fed a chow diet.
Effect of ACAT2 and MTP deficiency on intestinal, hepatic, and plasma lipids in Western diet-fed mice
It is known that ACAT2 deficiency increases free cholesterol in the intestine, but not in the liver, in cholesterol fed mice. Further, it has been shown that VLDL assembly is increased in these mice. It is possible that increases in VLDL assembly occur to avoid toxicity associated with hepatic free cholesterol assimilation. If this is true, then there might not be any need to increase hepatic VLDL assembly when intestinal cholesterol absorption is curtailed. Therefore, we examined whether intestinal MTP deficiency in combination with global ACAT2 deficiency affects hepatic lipid metabolism in Western diet-fed mice. We hypothesized that reduced delivery of lipids from the intestine might preclude increases in hepatic VLDL secretion.
To determine the effect of diet enriched in fat and cholesterol on lipid absorption, mice were fed a Western diet for 12 days starting with the first tamoxifen injection. First, we looked at the changes within the Western diet-fed mice. Western diet had no significant effect on intestinal triglyceride and total cholesterol, increased free cholesterol by ∼65%, and decreased esterified cholesterol by 50% in ACAT2-deficient mice compared with WT mice (Table 1). Thus, ACAT2 deficiency affects percent distribution of free and esterified cholesterol in the intestine. Intestinal MTP deficiency alone and in combination with ACAT2 deficiency increased intestinal triglycerides, but had variable effects on intestinal cholesterol. Thus, ACAT2 deficiency decreases intestinal cholesterol esters, whereas MTP deficiency increases triglycerides.
ACAT2 deficiency reduced hepatic triglycerides and cholesterol consistent with an earlier study (32). Intestinal MTP deficiency had no effect on hepatic triglyceride and total cholesterol in Western diet-fed mice. However, I-DKO mice had significantly reduced hepatic triglyceride and cholesterol. Thus, intestinal MTP and total ACAT2 deficiencies reduce hepatic lipids.
Soat2−/− mice had higher plasma triglyceride but lower cholesterol levels. I-Mttp−/− mice had lower plasma triglyceride and cholesterol levels. I-DKO mice had significantly higher plasma triglyceride but reduced plasma cholesterol (Table 1). Thus, total deficiency of ACAT2 appears to have a dominant effect on plasma and hepatic triglyceride levels than intestinal MTP deficiency has on plasma and hepatic triglycerides. These results suggest that reduced delivery of lipids from the intestine in I-DKO mice might not affect increases in hepatic VLDL secretion that occur as a consequence of ACAT2 deficiency in cholesterol-fed mice.
Second, we compared the interactions of diets and genes by two-way ANOVA. Except for intestinal total and free cholesterol, all lipid parameters showed significant interactions (Table 1). Third, we compared the effects of the Western diet on these parameters in different types of mice by applying Bonferroni posttest. Although intestinal triglyceride content tended to increase in Western diet-fed WT and Soat2−/− mice, they did not reach statistical significance compared with chow-fed animals. Surprisingly, intestines from the Western diet-fed I-Mttp−/− and I-DKO mice had significantly lower amounts of triglycerides compared with the chow-fed animals. Intestinal cholesteryl esters increased after Western diet feeding in WT and I-Mttp−/− mice. Hepatic triglycerides were significantly increased in all four different types of mice fed a Western diet compared chow-fed animals. Total hepatic cholesterol increased in WT and I-Mttp−/− mice, but not in Soat2−/− and I-DKO mice. Hepatic free cholesterol decreased in Soat2−/− and I-DKO mice, increased in I-Mttp−/− mice, and did not change in WT mice. Hepatic esterified cholesterol content increased in all mice after Western diet feeding. Plasma triglycerides, total cholesterol, and free cholesterol were increased in all four types of mice fed a Western diet. Total plasma cholesterol increased in all mice except for I-DKO mice. Plasma esterified cholesterol increased in WT mice and decreased in I-DKO mice, but remained unaffected by Western diet in I-Mttp−/− and Soat2−/− mice (Table 1). These studies indicate significant gene/diet interactions in these mice.
Effect of ACAT2 and MTP deficiency on lipid absorption in Western diet-fed mice
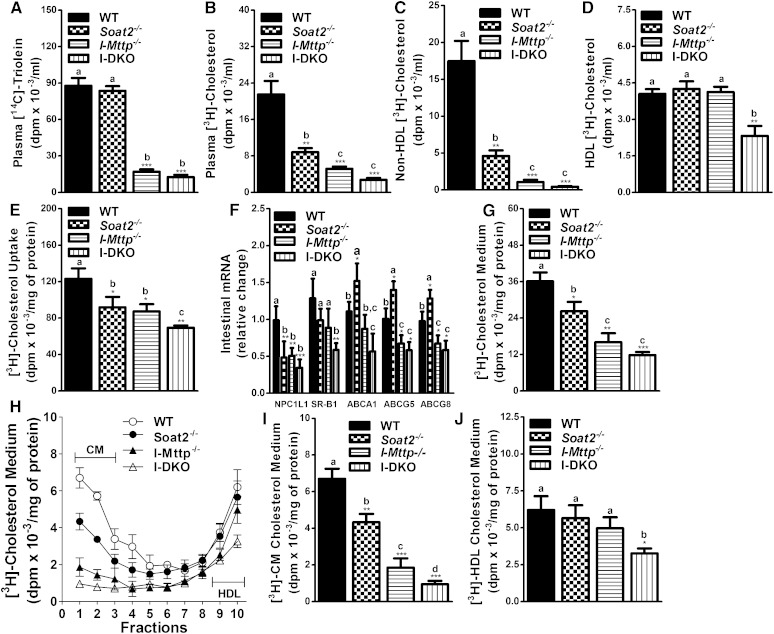
Next, we studied the effects of intestinal MTP and ACAT2 deficiency on the acute absorption of triglycerides and cholesterol in mice fed a Western diet and injected with P407 to inhibit plasma lipases. Similar to chow-fed mice, the appearance of [14C]triolein-derived lipids was unaffected by ACAT2 deficiency (Fig. 5A). However, I-Mttp−/− and I-DKO mice showed a significant decrease of 80 and 86%, respectively, in the absorption of [14C]triolein (Fig. 5A). The appearance of [3H]cholesterol-derived lipids in the plasma of Soat2−/−, I-Mttp−/−, and I-DKO mice was significantly reduced by 59, 76, and 87%, respectively, compared with WT mice (Fig. 5B). The reduction in cholesterol absorption in I-DKO mice was not statistically different from that in I-Mttp−/− mice, suggesting that ACAT2 deficiency does not affect cholesterol absorption in the absence of MTP. Cholesterol-derived counts were lower in apoB-containing nonHDL lipoproteins (Fig. 5C). Individual ablation of these genes had no effect on cholesterol transport via HDLs, but I-DKO mice showed 42% decreased cholesterol absorption with HDLs (Fig. 5D). These studies indicate that both ACAT2 and MTP play a significant role in cholesterol absorption via nonHDL pathways. However, combined deficiency of these genes also reduces cholesterol transport with HDLs.
Fig. 5.
Intestinal MTP and global ACAT2 gene deletion decreases absorption and secretion of cholesterol in Western diet-fed mice. Twelve-week-old WT, Soat2−/−, I-Mttp−/−, and I-DKO male mice (n = 3) were fed a Western diet for 12 days starting after the first tamoxifen injection. Mice were fasted overnight and injected intraperitoneally with P407 (30 mg/mouse). After 1 h, mice were gavaged with 0.5 μCi of [14C]triolein and 0.5 μCi of [3H]cholesterol, as well as 0.2 mg of cholesterol in 15 μl of olive oil. Plasma was collected after 2 h to measure the appearance of [14C]triolein (A) and [3H]cholesterol (B). ApoB-lipoproteins were precipitated as described in Materials and Methods to determine radioactive cholesterol counts in nonHDLs (C) and HDLs (D). To study cholesterol uptake, enterocytes were isolated from 12-week-old Western diet-fed overnight-fasted mice and radiolabeled for 1 h with 0.5 μCi/ml of [3H]cholesterol. After 1 h, enterocytes were washed and lipids were isolated to determine uptake of radiolabeled cholesterol (E). Total RNA isolated from the intestine was used to quantify mRNA levels of NPC1L1, SR-B1, ABCA1, ABCG5, and ABCG8 (F). For characterization of secreted lipoproteins, enterocytes were supplemented with [3H]cholesterol for 1 h, washed, and incubated with fresh media containing 1.4 mM oleic acid containing micelles for 2 h. Isolated lipids from the media (G) were counted to determine total cholesterol radioactivity. Media were also used to separate lipoproteins by density gradient ultracentrifugation and radioactivity was determined in each fraction (H). For better representation of chylomicrons (CM) (I) and HDLs (J), fractions 1 and 10 from (H), respectively, were plotted separately. Each measurement was done in triplicate with three mice per group. Data are presented as mean ± SD. Data in (E) and (G–J) were normalized to cellular protein. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with WT as determined by Student’s t-test. Statistically significant differences in different parameters in the four groups were evaluated by one-way ANOVA with Newman-Keuls multiple comparison test. Different letters above bars indicate statistically significant differences (P < 0.05) as determined by one-way ANOVA.
We then evaluated the role of MTP and ACAT2 in the uptake of cholesterol by enterocytes isolated from Western diet-fed gene-ablated mice. Cholesterol uptake was reduced by 25, 29, and 44% in Soat2−/−, I-Mttp−/−, and I-DKO mice, respectively (Fig. 5E). To understand the reasons for reduced uptake, we measured mRNA levels of genes involved in cholesterol uptake and transport. Individual and combined deficiencies of ACAT2 and MTP reduced NPC1L1 mRNA levels (Fig. 5F). These studies indicate that Western diet feeding may reduce cholesterol uptake in ACAT2- and MTP-deficient enterocytes by reducing NPC1L1 expression.
Furthermore, we studied the secretion of cholesterol by enterocytes. ACAT2 deficiency decreased secretion of cholesterol by 27%, whereas MTP deficiency decreased it by 55% and their combined deficiency decreased cholesterol secretion by 67% (Fig. 5G). The decrease in cholesterol secretion by I-DKO enterocytes was not statistically different from I-Mttp−/− mouse enterocytes. This decrease in cholesterol secretion was mainly with chylomicrons in Soat2−/− and I-Mttp−/− enterocytes (Fig. 5H, I). However, enterocytes from I-DKO mice showed reduced cholesterol secretion with both chylomicrons and HDLs (Fig. 5H–J). To find out the reasons for reduced cholesterol secretion with HDLs, we measured mRNA levels of ABCA1, ABCG5, and ABCG8 (Fig. 5F). In I-DKO mice, expression of these transporters was significantly reduced. These data suggest that feeding a Western diet to ACAT2- and MTP-deficient mice reduces uptake and secretion of cholesterol by the enterocytes.
Effect of ACAT2 and MTP deficiency on hepatic lipid accumulation in Western diet-fed mice
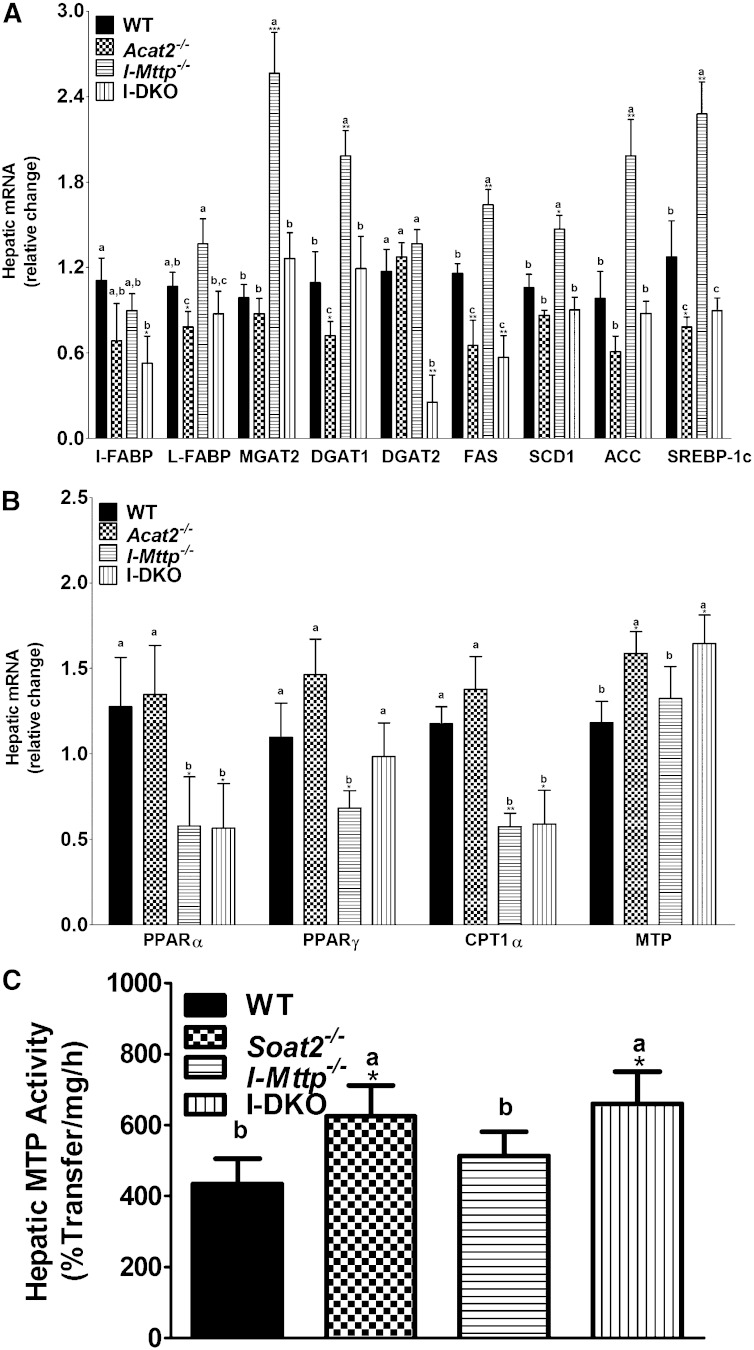
It has been reported previously that ACAT2 deficiency prevents hepatic steatosis in cholesterol-fed mice by increasing the mobilization of hepatic triglycerides (32). In this study, we observed that ACAT2 deficiency increases plasma triglyceride and decreases hepatic triglyceride even when triglyceride absorption from the intestine is significantly curtailed due to MTP deficiency (Table 1). To explain the mechanisms, we measured expression of genes involved in lipogenesis, β-oxidation, and lipoprotein production. ACAT2 deficiency had no effect on I-FABP, MGAT2, and DGAT2, but reduced L-FABP, FAS, and SREBP-1c compared with WT mice (Fig. 6A). I-DKO mice had reduced expression of I-FABP and FAS. These studies suggest that there might not be consistent reductions in lipogenesis in the absence of ACAT2.
Fig. 6.
Changes in hepatic mRNA levels of genes involved in lipid metabolism in mice fed a Western diet. Total RNA isolated from the liver of 12-week-old WT, Soat2−/−, I-Mttp−/−, and I-DKO (n = 3) male mice fed a Western diet for 12 days was used to quantify mRNA levels of different genes involved in lipid synthesis (A) and fatty acid oxidation (B). Further MTP mRNA (B) and activity (C) was measured. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with WT as determined by Student’s t-test. Statistically significant differences in different parameters in the four groups were evaluated by one-way ANOVA with Newman-Keuls multiple comparison test. Different letters above bars indicate statistically significant differences (P < 0.05) as determined by one-way ANOVA.
Next, we measured expression of genes involved in β-oxidation. ACAT2 deficiency had no significant effect on PPARα, PPARγ, and CPT1α mRNA levels (Fig. 6B). Livers of I-Mttp−/− and I-DKO mice had lower levels of PPARα and CPT1α compared with WT and Soat2−/− mice. Therefore, changes in the expression of genes involved in β-oxidation do not explain consistent reductions in hepatic triglyceride in Western diet-fed Soat2−/− and I-DKO mice.
However, we did observe a significant increase in hepatic Mttp mRNA levels in ACAT2-deficient and I-DKO mice (Fig. 6B). Further, MTP activity was increased by 20–44% in Western diet-fed Soat2−/− and I-DKO mice (Fig. 6C). Hence, it is likely that increased MTP expression might be associated with enhanced lipoprotein production in the absence of ACAT2, explaining reduced hepatosteatosis and increased plasma triglyceride in Soat2−/− mice. These results suggest that increases in hepatic MTP expression and lipoprotein production in I-DKO mice is independent of any changes in the intestinal lipid absorption.
DISCUSSION
Cholesterol secretion by enterocytes occurs by apoB-dependent (chylomicron) and apoB-independent (HDL) pathways. A hypothesis tested in this paper was that free cholesterol absorption might be increased via the HDL pathway after the inhibition of chylomicron assembly by ablating Mttp gene in the intestines of ACAT2-deficient mice. Our studies show that accumulation of free cholesterol after ACAT2 ablation and failure to assemble chylomicrons due to MTP deficiency does not increase the transport of free cholesterol with HDLs; instead, we found significant reduction in cholesterol secretion with HDLs in Western diet-fed mice. Our studies indicate that a reason for reduced cholesterol secretion with HDLs in I-DKO mice might be related to diminished expression of ABCA1. The reasons for these unexpected findings are not clear. It is possible that free cholesterol that accumulates in the absence of ACAT2 is not available for transport via the ABCA1 pathway, suggesting the existence of different pools specific for these two pathways. However, this hypothesis does not explain reduced cholesterol absorption in mice that are deficient in both ACAT2 and ABCA1 (27), and those deficient in MTP and ABCA1 (21). It is possible that free cholesterol that accretes in the absence of ACAT2 is unable to move to plasma membranes for efflux by the HDL pathway. Another possibility is that decreased cholesterol uptake by the MTP-deficient enterocytes as a result of reduced NPC1L1 expression might have precluded cholesterol secretion by the HDL pathway. Further studies are needed to explain why free cholesterol that accretes in the absence of ACAT2 is not available for secretion with HDLs when chylomicron assembly is inhibited by MTP deficiency.
These studies provide new understanding about the effects of intestinal MTP deficiency in combination with global ACAT2 deficiency on intestinal lipid metabolism. ACAT2 deficiency has no effect on intestinal fatty acid uptake and triglyceride secretion. Further, ACAT2 deficiency has no effect on cholesterol uptake in chow-fed mice, but feeding a Western diet reduces intestinal cholesterol uptake. Our studies are in agreement with earlier observations that ACAT2 deficiency increases intestinal cellular free cholesterol and decreases esterified cholesterol. We show that ACAT2 deficiency reduces secretion of cholesterol with chylomicrons, indicating that ACAT2 is a major enzyme contributing to the secretion of cholesterol with intestinal lipoproteins. These studies confirm earlier observations that MTP deficiency has no effect on fatty acid uptake but reduces glycerolipid and cholesterol secretion with chylomicrons resulting in increased accumulation of triglyceride and cholesterol in the intestine and significant reductions in plasma lipids. Here we show that combined deficiencies of ACAT2 and MTP reduce glycerolipid and cholesterol secretion by enterocytes without affecting uptake of free fatty acids and cholesterol in chow-fed animals.
ACAT2 deficiency reduces acute cholesterol absorption, but this effect is not seen when absorption studies are performed over a longer time period. Previous studies have shown that cholesterol absorption, studied over a period of 72 h using dual label gavage of 3H-cholesterol and 14C-sitosterol, is not different in Soat2−/− and WT mice (15, 26). However, Soat2−/− mice show significantly lower fractional cholesterol absorption when fed diets that contain higher cholesterol content (15, 26). These data have been interpreted to suggest that cholesterol absorption is not impaired when mice eat a low-cholesterol-containing chow diet, but is impaired when fed high cholesterol diets. In our studies, we used low-cholesterol-containing chow-fed mice and studied acute cholesterol absorption by providing a bolus of cholesterol along with tracer. Under these acute conditions, Soat2−/− mice fed a chow diet did absorb significantly less cholesterol than WT mice. Similarly, significant reductions in the acute absorption of cholesterol are also observed in enterocytes obtained from mice fed high cholesterol diets similar to those in long-term cholesterol absorption studies in mice fed diets high in cholesterol (15, 26). Hence, we suggest that Soat2−/− enterocytes do show significant defects in the acute cholesterol absorption and these effects are significantly enhanced when mice are fed high cholesterol diets. However, these effects are not apparent when studied over a period of 72 h, owing to adequate absorption of cholesterol over a longer time period.
Apart from the role ACAT2 plays in cholesterol absorption, Alger et al. (32) have shown that feeding high cholesterol diets to ACAT2-deficient mice causes mild hypertriglyceridemia, prevents hepatosteatosis, and increases hepatic lipoprotein production. Here, we observed a similar phenotype in Western diet-fed Soat2−/− mice which led to our hypothesis that increased intestinal lipid absorption in Western diet-fed mice resulting in increased delivery of fat to the liver might enhance hepatic lipoprotein production in the absence of ACAT2. However, we observed that Western diet-fed I-Mttp−/−Soat2−/− mice develop hypertriglyceridemia and have less hepatosteatosis in the absence of intestinal MTP similar to Soat2−/− mice. Therefore, increased intestinal lipid absorption is not a cause for increased hepatic lipoprotein production in these mice. Instead, we observed that Western diet increases hepatic MTP expression in ACAT2-deficient Soat2−/− and I-Mttp−/−Soat2−/− mice (Fig. 6). Thus, it is possible that increased hepatic MTP expression, independent of any changes in intestinal lipid absorption, could facilitate lipoprotein production, reduce hepatosteatosis, and cause hypertriglyceridemia. We speculate that increased hepatic MTP expression and lipoprotein production might be a mechanism to avoid accumulation of hepatic free cholesterol. It is interesting to note that this accommodation happens in the liver, but not in the intestine, probably reflecting the possibility that the liver regulates intracellular free cholesterol levels more stringently than the intestine. It is known that the liver can also convert cholesterol to bile acids. It is not known why bile acid synthesis is not increased to avoid reduced intracellular free cholesterol in ACAT2-deficient mice. We speculate that secretion of free cholesterol via VLDL biogenesis might be more efficient to reduce hepatic free cholesterol levels. Thus, ACAT2 deficiency increases hepatic MTP expression after feeding high cholesterol diets, but mechanisms involved in this regulation remain to be explained.
In summary, these studies show that combined deficiency of ACAT2 and MTP reduces cholesterol secretion compared with WT mice and mice deficient in individual genes. Biochemical studies suggest that this reduction is related to significantly reduced secretion of cholesterol by chylomicrons in chow-fed animals. In Western diet-fed mice, cholesterol secretion via both the chylomicron and HDL pathways is reduced. Further, these studies show that ACAT2 deficiency in animals fed high cholesterol diets increases hepatic MTP expression to avoid hepatosteatosis and cause hypertriglyceridemia. This might be a mechanism to avoid hepatic free cholesterol accumulation. Hence, it is likely that ACAT2 inhibitors might be able to avoid hepatosteatosis associated with high cholesterol diets. Further, they may act in combination with MTP inhibitors to lower hepatosteatosis.
Footnotes
Abbreviations:
- FPLC
- fast protein liquid chromatography
- I-DKO
- mice deficient in intestinal MTP and global ACAT2
- MTP
- microsomal triglyceride transfer protein
- Soat
- sterol O-acyltransferase
- NPC1L1
- Niemann-Pick C1-like 1
- P407
- poloxamer 407
This work was supported in part by National Institutes of Health Grant DK46900 (M.M.H.) and an American Heart Association Grant-in-Aid (J.I.).
REFERENCES
- 1.Wilson M. D., Rudel L. L. 1994. Review of cholesterol absorption with emphasis on dietary and biliary cholesterol. J. Lipid Res. 35: 943–955. [PubMed] [Google Scholar]
- 2.Dawson P. A., Rudel L. L. 1999. Intestinal cholesterol absorption. Curr. Opin. Lipidol. 10: 315–320. [DOI] [PubMed] [Google Scholar]
- 3.McGill H. C., Jr 1979. The relationship of dietary cholesterol to serum cholesterol concentration and to atherosclerosis in man. Am. J. Clin. Nutr. 32: 2664–2702. [DOI] [PubMed] [Google Scholar]
- 4.Davis H. R., Jr, Tershakovec A. M., Tomassini J. E., Musliner T. 2011. Intestinal sterol transporters and cholesterol absorption inhibition. Curr. Opin. Lipidol. 22: 467–478. [DOI] [PubMed] [Google Scholar]
- 5.Chang T. Y., Chang C. C., Cheng D. 1997. Acyl-coenzyme A:cholesterol acyltransferase. Annu. Rev. Biochem. 66: 613–638. [DOI] [PubMed] [Google Scholar]
- 6.Buhman K. K., Chen H. C., Farese R. V., Jr 2001. The enzymes of neutral lipid synthesis. J. Biol. Chem. 276: 40369–40372. [DOI] [PubMed] [Google Scholar]
- 7.Chang T. Y., Chang C. C., Lin S., Yu C., Li B. L., Miyazaki A. 2001. Roles of acyl-coenzyme A:cholesterol acyltransferase-1 and -2. Curr. Opin. Lipidol. 12: 289–296. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen T. M., Sawyer J. K., Kelley K. L., Davis M. A., Rudel L. L. 2012. Cholesterol esterification by ACAT2 is essential for efficient intestinal cholesterol absorption: evidence from thoracic lymph duct cannulation. J. Lipid Res. 53: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussain M. M., Rava P., Walsh M., Rana M., Iqbal J. 2012. Multiple functions of microsomal triglyceride transfer protein. Nutr. Metab. (Lond). 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussain M. M., Kancha R. K., Zhou Z., Luchoomun J., Zu H., Bakillah A. 1996. Chylomicron assembly and catabolism: role of apolipoproteins and receptors. Biochim. Biophys. Acta. 1300: 151–170. [DOI] [PubMed] [Google Scholar]
- 11.Berriot-Varoqueaux N., Aggerbeck L. P., Samson-Bouma M., Wetterau J. R. 2000. The role of the microsomal triglyceride transfer protein in abetalipoproteinemia. Annu. Rev. Nutr. 20: 663–697. [DOI] [PubMed] [Google Scholar]
- 12.Yao Z., McLeod R. S. 1994. Synthesis and secretion of hepatic apolipoprotein B-containing lipoproteins. Biochim. Biophys. Acta. 1212: 152–166. [DOI] [PubMed] [Google Scholar]
- 13.Lee R. G., Willingham M. C., Davis M. A., Skinner K. A., Rudel L. L. 2000. Differential expression of ACAT1 and ACAT2 among cells within liver, intestine, kidney, and adrenal of nonhuman primates. J. Lipid Res. 41: 1991–2001. [PubMed] [Google Scholar]
- 14.Parini P., Davis M., Lada A. T., Erickson S. K., Wright T. L., Gustafsson U., Sahlin S., Einarsson C., Eriksson M., Angelin B., et al. 2004. ACAT2 is localized to hepatocytes and is the major cholesterol-esterifying enzyme in human liver. Circulation. 110: 2017–2023. [DOI] [PubMed] [Google Scholar]
- 15.Buhman K. K., Accad M., Novak S., Choi R. S., Wong J. S., Hamilton R. L., Turley S., Farese R. V., Jr 2000. Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat. Med. 6: 1341–1347. [DOI] [PubMed] [Google Scholar]
- 16.Willner E. L., Tow B., Buhman K. K., Wilson M., Sanan D. A., Rudel L. L., Farese R. V., Jr 2003. Deficiency of acyl CoA:cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proc. Natl. Acad. Sci. USA. 100: 1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farese R. V., Jr, Ruland S. L., Flynn L. M., Stokowski R. P., Young S. G. 1995. Knockout of the mouse apolipoprotein B gene results in embryonic lethality in homozygotes and protection against diet-induced hypercholesterolemia in heterozygotes. Proc. Natl. Acad. Sci. USA. 92: 1774–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raabe M., Flynn L. M., Zlot C. H., Wong J. S., Véniant M. M., Hamilton R. L., Young S. G. 1998. Knockout of the abetalipoproteinemia gene in mice: Reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proc. Natl. Acad. Sci. USA. 95: 8686–8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farese R. V., Jr, Cases S., Ruland S. L., Kayden H. J., Wong J. S., Young S. G., Hamilton R. L. 1996. A novel function for apolipoprotein B: Lipoprotein synthesis in the yolk sac is critical for maternal-fetal lipid transport in mice. J. Lipid Res. 37: 347–360. [PubMed] [Google Scholar]
- 20.Xie Y., Newberry E. P., Young S. G., Robine S., Hamilton R. L., Wong J. S., Luo J., Kennedy S., Davidson N. O. 2006. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J. Biol. Chem. 281: 4075–4086. [DOI] [PubMed] [Google Scholar]
- 21.Iqbal J., Parks J. S., Hussain M. M. 2013. Lipid absorption defects in intestine-specific microsomal triglyceride transfer protein and ATP-binding cassette transporter A1-deficient mice. J. Biol. Chem. 288: 30432–30444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iqbal J., Anwar K., Hussain M. M. 2003. Multiple, independently regulated pathways of cholesterol transport across the intestinal epithelial cells. J. Biol. Chem. 278: 31610–31620. [DOI] [PubMed] [Google Scholar]
- 23.Iqbal J., Hussain M. M. 2005. Evidence for multiple complementary pathways for efficient cholesterol absorption in mice. J. Lipid Res. 46: 1491–1501. [DOI] [PubMed] [Google Scholar]
- 24.Brunham L. R., Kruit J. K., Iqbal J., Fievet C., Timmins J. A., Pape T. D., Coburn B. A., Bissada N., Staels B., Groen A. K., et al. 2006. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 116: 1052–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams K. J. 2008. Molecular processes that handle–and mishandle–dietary lipids. J. Clin. Invest. 118: 3247–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Repa J. J., Buhman K. K., Farese R. V., Jr, Dietschy J. M., Turley S. D. 2004. ACAT2 deficiency limits cholesterol absorption in the cholesterol-fed mouse: impact on hepatic cholesterol homeostasis. Hepatology. 40: 1088–1097. [DOI] [PubMed] [Google Scholar]
- 27.Temel R. E., Lee R. G., Kelley K. L., Davis M. A., Shah R., Sawyer J. K., Wilson M. D., Rudel L. L. 2005. Intestinal cholesterol absorption is substantially reduced in mice deficient in both ABCA1 and ACAT2. J. Lipid Res. 46: 2423–2431. [DOI] [PubMed] [Google Scholar]
- 28.Anwar K., Iqbal J., Hussain M. M. 2007. Mechanisms involved in vitamin E transport by primary enterocytes and in vivo absorption. J. Lipid Res. 48: 2028–2038. [DOI] [PubMed] [Google Scholar]
- 29.Athar H., Iqbal J., Jiang X. C., Hussain M. M. 2004. A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J. Lipid Res. 45: 764–772. [DOI] [PubMed] [Google Scholar]
- 30.Rava P., Athar H., Johnson C., Hussain M. M. 2005. Transfer of cholesteryl esters and phospholipids as well as net deposition by microsomal triglyceride transfer protein. J. Lipid Res. 46: 1779–1785. [DOI] [PubMed] [Google Scholar]
- 31.Turley S. D., Valasek M. A., Repa J. J., Dietschy J. M. 2010. Multiple mechanisms limit the accumulation of unesterified cholesterol in the small intestine of mice deficient in both ACAT2 and ABCA1. Am. J. Physiol. Gastrointest. Liver Physiol. 299: G1012–G1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alger H. M., Brown J. M., Sawyer J. K., Kelley K. L., Shah R., Wilson M. D., Willingham M. C., Rudel L. L. 2010. Inhibition of acyl-coenzyme A:cholesterol acyltransferase 2 (ACAT2) prevents dietary cholesterol-associated steatosis by enhancing hepatic triglyceride mobilization. J. Biol. Chem. 285: 14267–14274. [DOI] [PMC free article] [PubMed] [Google Scholar]