Abstract
Recent genome-wide association studies have identified multiple loci robustly associated with plasma lipids, which also contribute to extreme lipid phenotypes. However, these common genetic variants explain <12% of variation in lipid traits. Adiposity is also an important determinant of plasma lipoproteins, particularly plasma TGs and HDL cholesterol (HDLc) concentrations. Thus, interactions between genes and clinical phenotypes may contribute to this unexplained heritability. We have applied a weighted genetic risk score (GRS) for both plasma TGs and HDLc in two large cohorts at the extremes of BMI. Both BMI and GRS were strongly associated with these lipid traits. A significant interaction between obese/lean status and GRS was noted for each of TG (PInteraction = 2.87 × 10−4) and HDLc (PInteraction = 1.05 × 10−3). These interactions were largely driven by SNPs tagging APOA5, glucokinase receptor (GCKR), and LPL for TG, and cholesteryl ester transfer protein (CETP), GalNAc-transferase (GALNT2), endothelial lipase (LIPG), and phospholipid transfer protein (PLTP) for HDLc. In contrast, the GRSLDL cholesterol × adiposity interaction was not significant. Sexual dimorphism was evident for the GRSHDL on HDLc in obese (PInteraction = 0.016) but not lean subjects. SNP by BMI interactions may provide biological insight into specific genetic associations and missing heritability.
Keywords: obesity, genetic risk score, lipoproteins, single nucleotide polymorphism, statistical interaction
Recent genome-wide association studies (GWASs) have identified multiple genetic variants robustly associated with plasma lipid traits. The Global Lipids Consortium reported 157 significant loci (P < 5 × 10−8) (1, 2). Many are novel, and several encompass genes not previously implicated in plasma lipid metabolism. Furthermore, these loci were shown to contribute not only to general variation in plasma lipids, but also to extreme lipid phenotypes (3). Notably, for TGs, individuals in the top quartile of the TG risk score were 44 times more likely to have hypertriglyceridemia as compared with individuals in the bottom quartile (P = 4 × 10−28). For HDL cholesterol (HDLc), individuals in the top quartile of the risk score were four times more likely to have high HDLc as compared with those in the bottom quartile (1).
Although family-based association studies indicate that 40% to 60% of variation in plasma TG and HDLc is genetically based (4, 5), the identified loci explain <12% of variation in each of these lipid traits (1). Environmental and clinical factors including BMI, physical activity, and alcohol intake are also important determinants of plasma TG and HDLc (6).
Thus, interactions between genetic risk factors and clinical phenotypes may account for some of the unexplained heritability of plasma lipid traits. Here we have examined whether the effect of a weighted genetic risk score (GRS) on each of TG and HDLc is modified by adiposity, as assessed by BMI. This study provides biological insight into specific genetic associations and may aid in the identification of dyslipidemic subjects for whom weight loss is likely to be an important intervention.
METHODS
Study subjects
Subjects with a BMI ≥30 kg/m2 were defined as obese, those with a BMI ≤23 kg/m2 as lean, and intermediate subjects (30 kg/m2 ≥ BMI ≥ 23 kg/m2) as normal range. The BMI cutoff of ≤23 for the lean subgroup is below the 25th percentile for the majority of individuals studied. Two cohorts were studied.
Obese versus lean.
Obese, unrelated subjects of strictly European ancestry were recruited from the University of Ottawa Weight Management Clinic. Obese individuals displayed a BMI of >35 kg/m2 and a history of at least 10 years of adult obesity with no medical or psychiatric predisposing factors. Unrelated lean subjects were recruited from the Ottawa community. These healthy individuals had a lifelong BMI of less than the 25th percentile for sex and age, and no medical or psychiatric conditions affecting body weight (7, 8). Body weight was measured using a Tanita electronic scale to the nearest 0.3 kg. BMI was defined as weight in kilograms divided by height in meters squared (kg/m2). Height was measured to the nearest 0.5 cm. Plasma lipid fractions were measured using standard procedures. For coronary artery disease controls (CAD-C) subjects on lipid modifying medication, written documentation of pretreatment plasma lipids was obtained from the primary care physician and used for these analyses. These data were not available for 6.4% of the CAD-C subjects, none of whom were treated with a fibrate or niacin. In the obese versus lean (OBLE) cohort, 2.6% of lean and 14.8% of obese subjects were on low- to moderate-dose statin therapy, not expected to have major effects on TG or HDLc. The study was approved by the Human Ethics Experimentation Committees of the University of Ottawa Heart Institute and the Ottawa Hospital and written informed consent was obtained from all subjects.
CAD-C.
Details of the CAD-C cohorts have been previously described (9). Briefly, CAD-C included healthy controls recruited as part of the Ottawa Heart Genomics Study in collaboration with the Cleveland Clinic Gene Bank (OHGS_A and OHGS_CCGB_B). These subsets were combined together to form a single CAD-C sample. Subjects were collected under human research protocols approved by their respective committees.
Genotyping and imputation
SNP genotyping of the OBLE and CAD-C cohorts was performed on Affymetrix 6.0 or 500K Arrays at the University of Ottawa Heart Institute using the standard protocol recommended by the manufacturer and processed as described (10, 11). Imputation was performed using IMPUTE2 and the August 2009 1000 Genomes European reference panel (12). After imputation, ∼5.5 M SNPs passed post-quality control (QC) measures (info >0.5, Hardy Weinberg Equilibrium >1e–6, missing <10%).
Selection of GWAS SNPs
To create weighted GRSs for TG (GRSTG) and HDLc (GRSHDLc), we applied the findings of the Global Lipids Consortium 2010 study, which performed a fixed-effects meta-analysis on 46 separate GWASs comprising >100,000 individuals of European descent at a total of ∼2.6 million imputed or directly genotyped (1). Because the Global Lipids SNPs were identified in populations separate from those being considered here, we have avoided the bias inherent in performing discovery and effect size estimation in the same data set.
GRS
SNPs were individually coded as 0, 1, or 2, according to the number of trait-increasing alleles at that particular SNP. To generate the GRSTG, 20 SNPs were analyzed in the population; to generate the GRSHDL, 34 SNPs were analyzed. To generate GRS for LDL cholesterol (GRSLDLc), 11 SNPs were analyzed. Several SNPs for each trait failed to pass QC in our populations and were thus excluded from analysis. If a particular SNP failed QC in a particular subgroup, it was coded as missing in the total population. A weighted GRS (Ŝ) was constructed for each individual by taking a sum across SNPs of the number of reference alleles (0, 1, or 2) at that SNP and multiplying by the β effect score of that allele. Thus, we define G as an m-vector of coded markers (0, 1, or 2) and β as the effect size at that allele defined by the Global Lipids Consortium (1, 13, 14).
After experimentation with various methodologies, we concluded that a weighted GRS outperforms allele counting or a merely additive model (9, 13, 15). GRSs were constructed in PLINK: whole-genome association analysis toolset (14). SNPs and corresponding effect sizes for each of TG and HDLc are provided in supplementary Table I. Effect sizes provided are for the primary trait only.
Statistical analysis
Individual post-QC genotyped SNPs were coded as 0, 1, or 2 according to the number of effect alleles present, and a weighted GRS was constructed for each individual according to the previously described procedure for each of TG and HDLc. Multiple general linear regression models (GLMs) were used to test for the association between genotypes and HDLc and TGs. Data were adjusted for age, sex, and age2. Response data were broken down into lean, obese and normal range categories in order to investigate the effect of genetic risk across the BMI spectrum. Each SNP was tested for associations to phenotype separately from the GRS using GLMs, and interaction scores were constructed for SNP × obese/lean status and SNP × sex by including an interaction term in the respective models. The same covariates, which were used to analyze the data, were also controlled for when determining SNP × obese/lean status and SNP × sex interaction terms. Data were further stratified by gender. All analyses were conducted in PLINK (14) and R version 3.0.0 (http://www.r-project.org/).
RESULTS
The general characteristics of obese and lean subjects in each of the two main cohorts are shown in Table 1. Within the OBLE and CAD-C cohorts, subjects were well matched for age and sex. The OBLE cohort was younger and exhibited greater extremes of BMI [mean 43.1 ± 0.3 (obese); 20.3 ± 0.1 kg/m2 (lean)] as compared with the CAD-C group [mean BMI 34.6 ± 0.2 (obese); 21.3 ± 0.1 kg/m2 (lean)].
TABLE 1.
Characteristics of the study sample separated by cohort and by trait under study
| n | Male (%) | Age (years) | BMI (kg/m22) | Risk Score | |
| OBLE | |||||
| TG | |||||
| n | 1,784 | 34.1 | 45.4 ± 0.3 | 31.8 ± 0.3 | −0.198 ± 0.01 |
| Lean | 868 | 39.4 | 44.5 ± 0.5 | 20.3 ± 0.1 | −0.192 ± 0.014 |
| Obese | 916 | 28.9 | 46.4 ± 0.4 | 43.1 ± 0.3 | −0.204 ± 0.013 |
| HDL | |||||
| n | 1,779 | 34.3 | 45.5 ± 0.3 | 31.7 ± 0.3 | 0.007 ± 0.001 |
| Lean | 868 | 39.4 | 44.5 ± 0.5 | 20.3 ± 0.1 | 0.009 ± 0.002 |
| Obese | 911 | 25.1 | 46.4 ± 0.4 | 43.0 ± 0.3 | 0.005 ± 0.002 |
| CAD-C | |||||
| TG | |||||
| n | 2,966 | 49.4 | 75 ± 0.1 | 26.3 ± 0.1 | −0.149 ± 0.006 |
| Lean | 788 | 46.4 | 75.8 ± 0.2 | 21.6 ± 0.1 | −0.142 ± 0.011 |
| Obese | 338 | 37.1 | 73.4 ± 0.2 | 34.6 ± 0.2 | −0.145 ± 0.016 |
| Normal | 1,840 | 55.2 | 74.9 ± 0.1 | 26.8 ± 0.1 | −0.153 ± 0.007 |
| HDL | |||||
| n | 2,937 | 49.3 | 74.9 ± 0.1 | 26.3 ± 0.1 | −0.006 ± 0.001 |
| Lean | 596 | 48.2 | 76.1 ± 0.2 | 21.1 ± 0.1 | −0.006 ± 0.002 |
| Obese | 498 | 32.6 | 73.9 ± 0.2 | 33.4 ± 0.1 | −0.005 ± 0.002 |
| Normal | 1,843 | 55.0 | 74.8 ± 0.1 | 26.1 ± 0.1 | −0.005 ± 0.001 |
| Total | |||||
| TG | |||||
| n | 4,718 | 43.7 | 63.9 ± 0.2 | 28.4 ± 0.1 | −0.167 ± 0.005 |
| Lean | 1,656 | 38.3 | 59.4 ± 0.5 | 20.9 ± 0.1 | −0.168 ± 0.009 |
| Obese | 1,222 | 33.7 | 53.8 ± 0.4 | 40.7 ± 0.2 | −0.188 ± 0.011 |
| Normal | 1,840 | 55.3 | 74.9 ± 0.1 | 26.8 ± 0.1 | −0.153 ± 0.007 |
| HDL | |||||
| n | 4,683 | 43.6 | 63.8 ± 0.2 | 28.3 ± 0.1 | −0.001 ± 0.001 |
| Lean | 1,464 | 36.6 | 57.3 ± 0.5 | 20.6 ± 0.1 | 0.003 ± 0.001 |
| Obese | 1,376 | 35.6 | 56.3 ± 0.4 | 39.5 ± 0.2 | 0.001 ± 0.001 |
| Normal | 1,843 | 55.0 | 74.8 ± 0.1 | 26.1 ± 0.1 | −0.005 ± 0.001 |
Values represent mean ± standard deviation, unless otherwise indicated. Lean: BMI <23 kg/m2 and less than 25th percentile. Obese: BMI >30 kg/m2 for >10 years. Normal: 23 kg/m2 ≤ BMI ≤ 30 kg/m2. Risk score corresponds to the sum of the effect size per risk gene multiplied by the effect size of that risk gene, divided by the total number of risk genes. Data are provided as mean ± standard deviation. See supplementary Table I for further details.
For the entire group, the mean difference in TG for subjects above or below the 50th percentile of the weighted GRSTG was 0.191 mM [95% confidence interval (CI) = 0.140–0.241, P = 1.92 × 10−13]. For obese subjects, this difference was 0.325 mM (95% CI = 0.250–0.399, P < 2.20 × 10−16) and for lean subjects 0.114 mM (95% CI = 0.250–0.399, P < 2.20 × 10−16). The mean difference in HDLc for all subjects above or below the 50th percentile of the GRSHDL (based on HDLc-raising alleles) was 0.129 mM (95% CI = 0.106–0.153, P < 2.2 × 10−16). This value was lower for the obese (0.108 mM; 95% CI = 0.075–0.141, P = 2.25 × 10−10) and higher for the lean (0.166 mM; 95% CI = 0.124–0.208, P = 1.97 × 10−14) subjects.
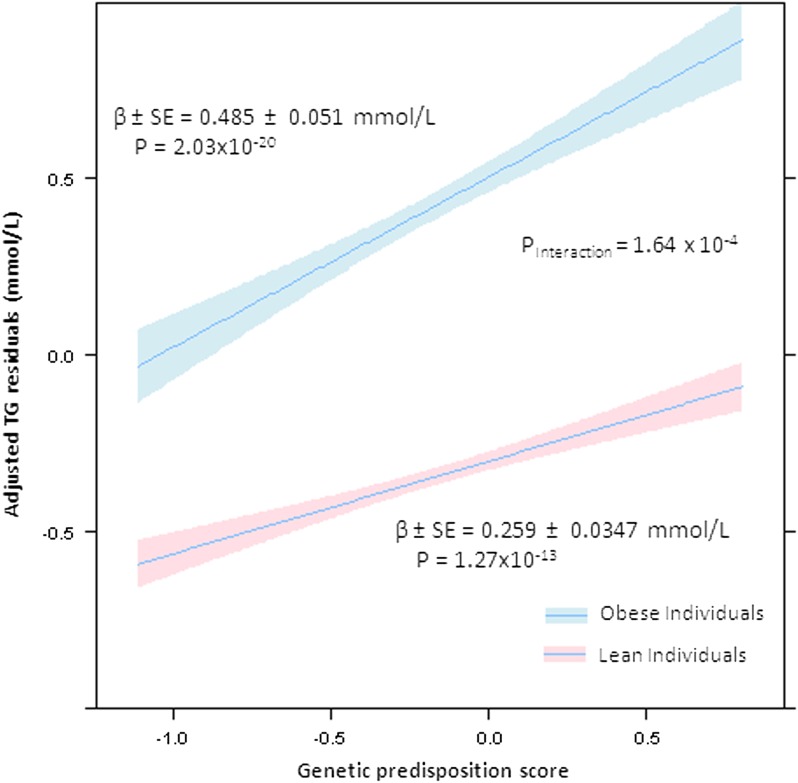
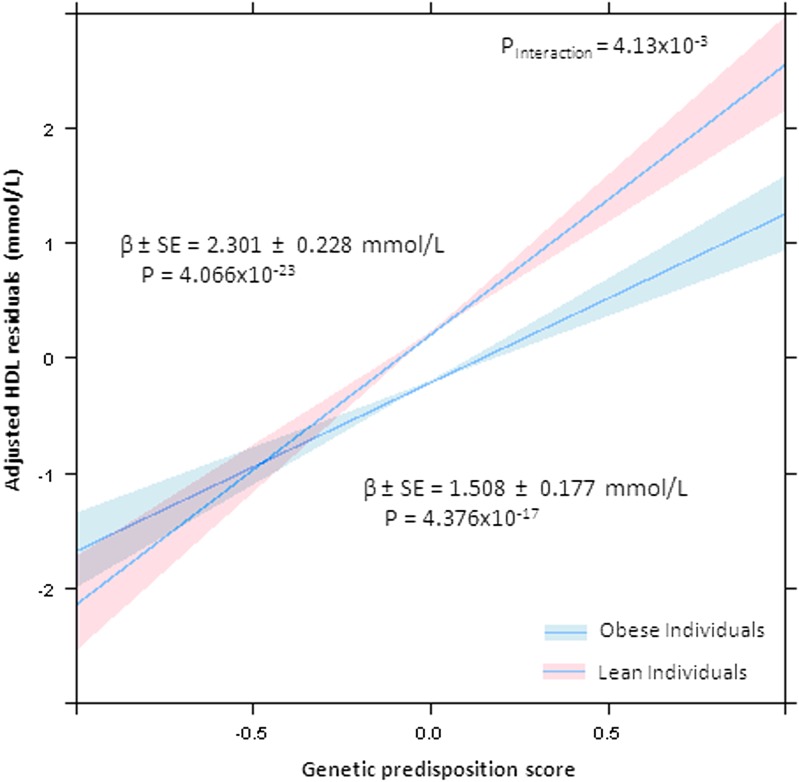
As shown in Table 2, subsequent analysis by covariate adjusted multiple linear models revealed a significant difference in the effect size (β) of the GRS on each of TG and HDLc in the obese versus lean subgroups. For GRSTG on TG in the obese population, β = 0.480 mM (SE = 0.0533, P = 8.97 × 10−19), versus for the lean subgroup, β = 0.261 mM (SE = 0.0336, P = 1.52 × 10−14), with a significant interaction term (PInteraction = 2.87 × 10−4) (Fig. 1). For GRSHDL and HDLc in the obese sample, β = 1.466 mM (SE = 0.166, P = 2.49 × 10−18) versus β = 2.347 mM (SE = 0.209, P = 3.41 × 10−28) in the lean, demonstrating significant interactions for obese/lean status × GRSHDL (PInteraction = 1.05 × 10−3) (Fig. 2). For GRSLDLc in the obese population, β = 0.434 mM (SE = 0.0831, P = 2.14 × 10−7), similar to the lean population where β = 0.390 mM (SE = 0.0715, P = 5.63 × 10−8). As expected, no significant interaction between GRSLDLc and obese/lean status was found (PInteraction = 0.689). Subjects with a BMI in the normal range (23 kg/m2 < BMI < 30 kg/m2) exhibited a value between the lean and obese for TG, β = 0.354 mM (SE = 0.0289, P = 4.68 × 10−34); for HDLc, β = 1.91 mM (SE = 0.126, P = 2.16 × 10−50); but not for LDL, β = 0.464 mM (SE = 0.0473, P = 1.54 × 10−22). Subjects with a BMI in the normal range (23 kg/m2 < BMI < 30 kg/m2) exhibited a value between the lean and obese for TG, β = 0.354 mM (SE = 0.0289, P = 4.68 × 10−34); for HDLc, β = 1.91 mM (SE = 0.126, P = 2.16 × 10−50); but not for LDL, β = 0.464 (SE = 0.0473, P = 1.54 × 10−22).
TABLE 2.
Associations of GRS with adjusted lipid trait stratified by adiposity
| Obese | Lean | ||||||||
| Trait | na | βb (SE) | P | R2 | n | β (SE) | P | R2 | PInteraction |
| TG | 1,222 | 0.480 (0.053) | 8.98E–19 | 0.0614 | 1,656 | 0.261 (0.034) | 1.52E–14 | 0.0345 | 0.000287 |
| HDL | 1,376 | 1.466 (0.165) | 2.49E–18 | 0.0533 | 1,464 | 2.347 (0.209) | 3.41E–28 | 0.0790 | 0.00105 |
Number of nonmissing individuals with complete information included in analysis.
β coefficient for regression, measured in mM.
Fig. 1.
TG residuals compared with GRS stratified by lean versus obese status. Significantly differently slope coefficients with 95% CIs are displayed, demonstrating a significant interaction between obesity status and a GRS. The rate of increased TG residuals for an increased predisposition is displayed for obese (broken line) and lean (solid line) individuals. Increased risk in obese individuals corresponds to an increased expression of lipid levels above what would normally be expected. This dimorphic effect was dependent on three SNPs tagging APOA5, glucokinase receptor (GCKR), and LPL, not before observed to have adiposity-dependent dimorphic effects.
Fig. 2.
HDLc residuals compared with GRS stratified by lean versus obese status. Significantly differing slope coefficients with 95% CIs are displayed for obese and lean populations. Lean individuals exhibit a greater response to a larger number of HDLc-raising alleles. The dimorphic effect in HDL is due to SNPs tagging cholesteryl ester transfer protein (CETP), endothelial lipase (LIPG), GalNAc-transferase (GALNT2), and phospholipid transfer protein (PLTP), loci not previously noted to exhibit adiposity-dependent dimorphism.
Because obesity status significantly influenced the clinical expression of these lipid trait loci, we determined the explained variance (R2) of the GRSTG and GRSHDLc in obese versus lean subjects. For GRSTG on TG, R2 = 0.0614 for obese versus R2 = 0.0345 for lean subjects, a 2-fold difference. An opposite trend was observed for GRSHDLc (based on HDLc-raising alleles) on HDLc, R2 = 0.0790 for lean versus R2 = 0.0533 for obese. In contrast, for the GRSLDLc on LDLc, explained variance was only slightly higher in the obese (R2 = 0.0215) versus lean (R2 = 0.0172) populations.
We next examined the individual SNPs included in the GRSTG and GRSHDLc. Three TG SNPs (APOA5, GCKR, and LPL) and four HDLc SNPs (CETP, LIPG, GALNT2, and PLTP) were found to have a significant obese/lean status × SNP effect interaction term at a false discovery rate (FDR) of 20% (Table 3). LPL and APOA5 achieved a 10% FDR for TG, and CETP, GALNT2, and LIPG reached a 10% FDR for HDLc. However, at a 5% FDR, only LPL and CETP were significant. Statistical correction for multiple testing was achieved by ordering each tested SNP from least to greatest PInteraction value. The largest interaction term that was less than the PFDR [i.e., the ratio of the position of the SNP (i) divided by the number of SNPs analyzed (number of tests performed) multiplied by the FDR] was determined to be the cutoff at which results were classified as significant (16). Further details regarding SNP × obese/lean status analyses are provided in supplementary Table II. To test whether these SNPs were the major contributors to the overall obese/lean status × GRS interaction, a new score was constructed for each group omitting these SNPs. As expected, the interaction term was no longer significant (TG: PInteraction = 0.196; HDLc: PInteraction = 0.321).
TABLE 3.
Individual loci that exert differing effects in obese versus lean subjects
| Obese | Lean | Interaction | |||||||||||
| Locus | Lead SNP | Allelea | Trait | nb | β (SE)c | P | n | β (SE) | P | n | β (SE) | PInteraction | d |
| LPL | rs12678919 | G | TG | 945 | –0.148 (0.03) | 4.03E–06 | 932 | –0.050 (0.03) | 1.13E–01 | 1,877 | –0.21 (0.06) | 6.99E–04 | 0.01 |
| APOA5 | rs964184 | G | TG | 1,078 | 0.159 (0.03) | 1.31E–07 | 1,282 | 0.14 (0.03) | 1.91E–07 | 2,360 | 0.15 (0.06) | 8.87E–03 | 0.02 |
| GCKR | rs1260326 | T | TG | 1,189 | 0.0932 (0.03) | 1.21E–03 | 1,569 | 0.067 (0.02) | 6.84E–03 | 2,758 | 0.12 (0.05) | 2.82E–02 | 0.03 |
| CETP | rs3764261 | A | HDL | 1,083 | 0.132 (0.03) | 1.67E–06 | 1,238 | 0.189 (0.03) | 3.15E–13 | 2,415 | –0.21 (0.05) | 1.14E–05 | 0.005882 |
| GALNT2 | rs4846914 | G | HDL | 1,212 | –0.065 (0.03) | 1.24E–02 | 1,463 | –0.002 (0.02) | 9.39E–01 | 2,829 | –0.13 (0.05) | 3.03E–03 | 0.011765 |
| LIPG | rs7241918 | G | HDL | 1,061 | –0.004 (0.03) | 8.96E–01 | 1,178 | –0.102 (0.03) | 1.35E–04 | 2,329 | 0.13 (0.05) | 7.00E–03 | 0.017647 |
| PLTP | rs6065906 | C | HDL | 1,184 | –0.034 (0.03) | 2.01E–01 | 1,435 | –0.107 (0.02) | 1.19E–05 | 2,769 | 0.10 (0.04) | 2.08E–02 | 0.023529 |
Active allele analyzed.
Number of nonmissing individuals with complete information used in analysis.
β coefficient for regression; measured in mM (standard error).
FDR of 20% displayed. All achieved FDR <20%; APOA5, GALNT2, and LIPG achieved FDR <10%; LPL and CETP achieved FDR <5%.
Sex × lipid trait interactions
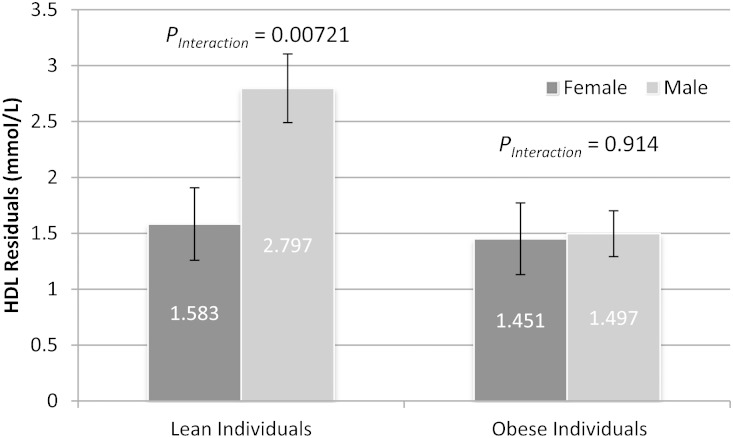
Next, we investigated whether GRS effects differed by sex. Of note, sex did not significantly influence the effect of the GRS on any trait in the whole population (TG: PInteraction = 0.0925; HDLc: PInteraction = 0.0868; LDL: PInteraction = 0.189). However, for GRSHDLc on HDLc, there was a significant interaction with sex in the obese (PInteraction = 0.016) but not the lean (PInteraction = 0.369) population. A sex dimorphic effect by obese/lean stratification was not found for the other lipid traits. Further analysis of individual SNPs failed to identify significant interaction terms in the whole population for either TG or HDLc. However, one sexually dimorphic locus for HDLc was found in each of the lean (rs4846914 tagging GALNT2) and obese [rs605066 tagging Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain, 2 (CITED2)] populations. However, after correction for multiple testing, these loci were only nominally significant (FDR = 15%) (Table 4). More complete SNP × sex interaction data may be found in supplementary Table III.
TABLE 4.
Individual SNPs with suggestive evidence of sexual dimorphism
| Male | Female | Interaction | |||||||||||
| Locus | Lead SNP | Allelea | Popb | nc | β (SE)d | P | n | β (SE) | P | n | β (SE) | PInteraction | e |
| GALNT2 | rs4846914 | G | OB | 405 | 0.060 (0.05) | 2.15E–01 | 807 | –0.12 (0.03) | 3.97E–04 | 1,212 | 0.14(0.04) | 2.09E–03 | 4.84E–03 |
| CITED2 | rs605066 | C | LE | 515 | 0.694 (0.29) | 1.75E–02 | 894 | –0.058 (0.03) | 7.77E–02 | 1,409 | 0.12(0.04) | 4.57E–03 | 4.84E–03 |
Active allele analyzed.
Population where loci are active.
Number of nonmissing individuals with complete information used in analysis.
β coefficient for regression; measured in mM (standard error).
FDR of 15% displayed. GALNT2 is significant at <10% FDR.
DISCUSSION
Lifestyle and clinical factors may modify genetic risk. For example, the effect of a GRS on BMI was found to be significantly attenuated in physically active versus sedentary individuals (17). To explore the effects of adiposity on genetic risk for dyslipidemia, we have utilized a GRS constructed from loci previously reported by the Global Lipids Consortium. We demonstrate that obesity status significantly alters the effect of genetic variants associated with increased TGs as well as those associated with higher levels of HDLc, but not LDLc.
For TG, the effect size (β) of a weighted GRSTG in the obese population was nearly double that of the lean population (β = 0.480 vs. 0.261). As shown in Fig. 1, for any GRSTG, plasma TG levels are greater for obese versus lean subjects. This is not surprising given known effects of substrate availability on hepatic TG synthesis; in obese individuals, the effect of nutrient excess outweighs the effect of known genetic variants at any GRSTG. In both obese and lean individuals, GRSTG associates with higher TGs, but the slope of the line for GRSTG versus TG differs for obese as compared with lean yielding a significant interaction coefficient. Overall, the variance in plasma TG concentrations explained by the GRSTG was 6.14% for the obese subjects, nearly double that found for the lean population (3.45%).
In contrast to TGs, the effect of a GRSHDL, consisting of HDLc-raising alleles, on HDLc was greater for the lean (β = 2.347) than the obese (β = 1.466) population. For HDLc, it is important to note that we created a GRSHDL composed of HDLc-raising alleles (a genetic protective score for HDL). As shown, obese individuals have higher circulating levels of TG-rich lipoproteins, leading to TG enrichment of HDL and more rapid HDL clearance. Thus, as shown in Fig. 2, it is likely that the metabolic effect of hypertriglyceridemia acts to attenuate the effect of HDL-raising alleles, for example near genes encoding CETP, LIPG, and PLTP. The GRSHDL for HDLc explained 7.89% of HDLc variation in the lean versus 5.33% in the obese subjects.
Thus, the genetic risk for hypertriglyceridemia is significantly worsened by the obese state, whereas the beneficial effect of HDLc-raising genetic variants is attenuated. These data demonstrate that the gene × adiposity interaction contributes to part of the hitherto unexplained genetic variance in plasma lipids levels.
Here, we utilized an aggregated, weighted risk score rather than the more common allele counting method. In the past, allele counting, also known as an additive model, has been used due to a lack of well-established effect sizes (14). However, a weighted, aggregated risk score has been shown to improve power (7, 10). We did not perform receiver operating characteristic area-under-curve analysis because hypertriglyceridemia (high TG) and hypoalphalipoproteinemia (low HDL) are defined by age- and sex-dependent quantiles.
Although we lack the statistical power necessary to detect the individual effects of all loci, we identified seven novel loci not previously reported to have obesity-related dimorphic effects. SNPs tagging APOA5 (PInteraction = 8.87 × 10−3), GCKR (PInteraction = 2.82 × 10−2), and LPL (PInteraction = 6.69 × 10−4) showed interaction with obese/lean status for TG. These encompass genes encoding proteins altering both hepatic TG synthesis and peripheral lipolysis. The GCKR gene product, the glucokinase regulatory protein, regulates glucokinase (GCK) activity competitively with respect to the substrate glucose, inhibiting GCK activity. Hepatic GCK activity enhances glycolytic flux, promoting hepatic glucose metabolism and increasing malonyl CoA availability, a major substrate for de novo hepatic lipogenesis (18). LPL and APOA5 encode major determinants of peripheral lipolysis of TG-rich lipoproteins, LPL and ApoA5, the latter a regulator of LPL activity (19). The effect sizes of the previously discussed TG loci were among the highest in this study (APOA5β = 16.95, GCKRβ = 8.76, and LPLβ = –13.64) and not surprisingly were responsible for the significant obese/lean status × GRS interaction. Consistently, in a Filipino population the APOA5 effect on plasma TG levels was found to be modified by waist circumference (20), another measure of adiposity.
For HDLc, interactions were noted for SNPs tagging CETP (PInteraction = 1.14 × 10−5), LIPG (PInteraction = 7.00 × 10−3), GALNT2 (PInteraction = 3.03 × 10−3), and PLTP (PInteraction = 2.08 × 10−2). The roles of CETP, LIPG, and PLTP in HDL remodeling in the intravascular space are well known. GALNT2 encodes GalNAc-transferase believed to play a critical role in O-glycosylation of proteins involved in lipid metabolism, including angiopoietin-like 3 (21). In the mouse, altered hepatic GALNT2 expression significantly modifies circulating HDLc levels (1). Although these HDLc loci exhibited lower effect sizes (CETPβ = 3.39, LIPGβ = –1.31, PLTPβ = –0.93, and GALNT2β = –0.61) as compared with the top TG SNPs, they were similarly responsible for the significant GRSHDLc × obese/lean status interaction term. In contrast, no significant interaction was found for GRSLDLc × obese/lean status.
In a second stage, we performed a sex-stratified analysis. The effect of neither weighted GRSTG nor GRSHDLc was found to be significantly different for males versus females for the population as a whole. Importantly, sexual dimorphism for genetic effects on HDLc was entirely driven by the obese subjects (PInteraction = 0.016) and was not evident in the lean (PInteraction = 0.914) or all (PInteraction = 0.0868) groups. Obese men showed an attenuated increase in HDLc in response to GRSHDLc as compared with women (Fig. 3). Loci in each subpopulation (CITED2 for obese and GALNT2 for lean) were found to be dimorphic (Table 4). However, after correction for multiple testing, these remained only nominally significant (FDR <15%), thus requiring confirmation in additional populations.
Fig. 3.
Regression coefficients for HDLc (mM) for male versus female subjects stratified by lean versus obese status. Data are shown for males and females stratified by adiposity, and bars represent SE. In the lean population, women and men display a similar response to GRSHDLc. In contrast, obese men demonstrate an attenuated effect of GRSHDLc as compared with obese women. One locus was found to be exhibit sexually dimorphic effects in each of obese (rs4846914 tagging GALNT2) and lean (rs605066 tagging CITED2) populations as shown in Table 4.
In summary, we have created weighted GRSs for each of TG and HDLc based on loci identified by the Global Lipids Consortium and tested effects in separate large, well-defined obese and lean populations; thus, our results are without discovery bias. Neither GRSTG nor GRSHDLc showed an association with adiposity (BMI) per se. Here we demonstrate convincing gene-adiposity trait interactions. Notably, lean subjects have an ∼50% reduction in the genetic predisposition for increased TGs and an ∼35% greater response to HDLc-raising alleles, as compared with obese subjects. These effects are mainly driven by SNPs tagging APOA5, GCKR, and LPL for TG, and CETP, LIPG, GALNT2, and PLTP for HDLc. We also report sexual dimorphism for genetic effects on HDLc that is confined to the obese group of subjects. These findings demonstrate that obese individuals are more susceptible to genetic risk for dyslipidemia. SNP by BMI interactions may provide biological insight into specific genetic associations and missing heritability.
Supplementary Material
Footnotes
Abbreviations:
- CAD-C
- coronary artery disease controls
- CETP
- cholesteryl ester transfer protein
- CI
- confidence interval
- CITED2
- Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain, 2
- FDR
- false discovery rate
- GALNT2
- GalNAc-transferase
- GCK
- glucokinase
- GCKR
- glucokinase receptor
- GRS
- genetic risk score
- GWAS
- genome-wide association study
- HDLc
- HDL cholesterol
- LDLc
- LDL cholesterol
- LIPG
- endothelial lipase
- OBLE
- obese versus lean study
- PLTP
- phospholipid transfer protein
- QC
- quality control
This work was supported by Canadian Institutes for Health Research (CIHR) Grants MOP2390941 and OPB134211 (R.M. and R.D.), and a CIHR Genetics Computational Biology studentship (C.B.C.).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three tables.
REFERENCES
- 1.Teslovich T. M., Musunuru K., Smith A. V., Edmondson A. C., Stylianou I. M., Koseki M., Pirruccello J. P., Ripatti S., Chasman D. I., Willer C. J., et al. 2010. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 466: 707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willer C. J., Schmidt E. M., Sengupta S., Peloso G. M., Gustafsson S., Kanoni S., Ganna A., Chen J., Buchkovich M. L., Mora S., et al. 2013. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 45: 1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansen C. T., Wang J., Lanktree M. B., McIntyre A. D., Ban M. R., Martins R. A., Kennedy B. A., Hassell R. G., Visser M. E., Schwartz S. M., et al. 2011. An increased burden of common and rare lipid-associated risk alleles contributes to the phenotypic spectrum of hypertriglyceridemia. Arterioscler. Thromb. Vasc. Biol. 31: 1916–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Namboodiri K. K., Kaplan E. B., Heuch I., Elston R. C., Green P. P., Rao D. C., Laskarzewski P., Glueck C. J., Rifkind B. M. 1985. The Collaborative Lipid Research Clinics Family Study: biological and cultural determinants of familial resemblance for plasma lipids and lipoproteins. Genet. Epidemiol. 2: 227–254. [DOI] [PubMed] [Google Scholar]
- 5.Yu Y., Wyszynski D. F., Waterworth D. M., Wilton S. D., Barter P. J., Kesaniemi Y. A., Mahley R. W., McPherson R., Waeber G., Bersot T. P., et al. 2005. Multiple QTLs influencing triglyceride and HDL and total cholesterol levels identified in families with atherogenic dyslipidemia. J. Lipid Res. 46: 2202–2213. [DOI] [PubMed] [Google Scholar]
- 6.Howard B. V., Ruotolo G., Robbins D. C. 2003. Obesity and dyslipidemia. Endocrinol. Metab. Clin. North Am. 32: 855–867. [DOI] [PubMed] [Google Scholar]
- 7.Ahituv N., Kavaslar N., Schackwitz W., Ustaszewska A., Martin J., Hebert S., Doelle H., Ersoy B., Kryukov G., Schmidt S., et al. 2007. Medical sequencing at the extremes of human body mass. Am. J. Hum. Genet. 80: 779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies R. W., Lau P., Naing T., Nikpay M., Doelle H., Harper M. E., Dent R., McPherson R. 2013. A 680 kb duplication at the FTO locus in a kindred with obesity and a distinct body fat distribution. Eur. J. Hum. Genet. 21: 1417–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies R. W., Wells G. A., Stewart A. F., Erdmann J., Shah S. H., Ferguson J. F., Hall A. S., Anand S. S., Burnett M. S., Epstein S. E., et al. 2012. A genome-wide association study for coronary artery disease identifies a novel susceptibility locus in the major histocompatibility complex. Circ. Cardiovasc. Genet. 5: 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dandona S., Stewart A. F., Chen L., Williams K., So D., O’Brien E., Glover C., Lemay M., Assogba O., Vo L., et al. 2010. Gene dosage of the common variant 9p21 predicts severity of coronary artery disease. J. Am. Coll. Cardiol. 56: 479–486. [DOI] [PubMed] [Google Scholar]
- 11.Schunkert H., Konig I. R., Kathiresan S., Reilly M. P., Assimes T. L., Holm H., Preuss M., Stewart A. F., Barbalic M., Gieger C., et al. 2011. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 43: 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howie B. N., Donnelly P., Marchini J. 2009. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 5: e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudbridge F. 2013. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 9: e1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M. A., Bender D., Maller J., Sklar P., de Bakker P. I., Daly M. J., et al. 2007. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81: 559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies R. W., Dandona S., Stewart A. F., Chen L., Ellis S. G., Tang W. H., Hazen S. L., Roberts R., McPherson R., Wells G. A. 2010. Improved prediction of cardiovascular disease based on a panel of SNPs identified through genome wide association studies. Circ. Cardiovasc. Genet. 3: 468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benjamini Y., Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B. 57: 289–300. [Google Scholar]
- 17.Li S., Zhao J. H., Luan J., Ekelund U., Luben R. N., Khaw K. T., Wareham N. J., Loos R. J. 2010. Physical activity attenuates the genetic predisposition to obesity in 20,000 men and women from EPIC-Norfolk prospective population study. PLoS Med. 7: e1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beer N. L., Tribble N. D., McCulloch L. J., Roos C., Johnson P. R., Orho-Melander M., Gloyn A. L. 2009. The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum. Mol. Genet. 18: 4081–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grosskopf I., Baroukh N., Lee S. J., Kamari Y., Harats D., Rubin E. M., Pennacchio L. A., Cooper A. D. 2005. Apolipoprotein A-V deficiency results in marked hypertriglyceridemia attributable to decreased lipolysis of triglyceride-rich lipoproteins and removal of their remnants. Arterioscler. Thromb. Vasc. Biol. 25: 2573–2579. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y., Marvelle A. F., Li J., Croteau-Chonka D. C., Feranil A. B., Kuzawa C. W., Li Y., Adair L. S., Mohlke K. L. 2013. Genetic association with lipids in Filipinos: waist circumference modifies an APOA5 effect on triglyceride levels. J. Lipid Res. 54: 3198–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schjoldager K. T., Vester-Christensen M. B., Bennett E. P., Levery S. B., Schwientek T., Yin W., Blixt O., Clausen H. 2010. O-glycosylation modulates proprotein convertase activation of angiopoietin-like protein 3: possible role of polypeptide GalNAc-transferase-2 in regulation of concentrations of plasma lipids. J. Biol. Chem. 285: 36293–36303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.