Abstract
ABCA1 mediates the efflux of cholesterol and phospholipids into apoA-I to form HDL, which is important in the prevention of atherosclerosis. To develop a novel method for the evaluation of HDL formation, we prepared an apoA-I-POLARIC by labeling the specific residue of an apoA-I variant with a hydrophobicity-sensitive fluorescence probe that detects the environmental change around apoA-I during HDL formation. apoA-I-POLARIC possesses the intact ABCA1-dependent HDL formation activity and shows 4.0-fold higher fluorescence intensity in HDL particles than in the lipid-free state. Incubation of apoA-I-POLARIC with ABCA1-expressing cells, but not ABCA1-non-expressing cells, caused a 1.7-fold increase in fluorescence intensity. Gel filtration analysis demonstrated that the increase in fluorescence intensity of apoA-I-POLARIC represents the amount of apoA-I incorporated into the discoidal HDL particles rather than the amount of secreted cholesterol. THP-1 macrophage-mediated HDL formation and inhibition of HDL formation by cyclosporine A could also be measured using apoA-I-POLARIC. Furthermore, HDL formation-independent lipid release induced by microparticle formation or cell death was not detected by apoA-I-POLARIC. These results demonstrate that HDL formation by ABCA1-expressing cells can be specifically detected by sensing hydrophobicity change in apoA-I, thus providing a novel method for assessing HDL formation and screening of the HDL formation modulator.
Keywords: cholesterol efflux, POLARIC, hydrophobicity-sensitive fluorescence probe, ATP binding cassette transporter A1, high density lipoprotein, apolipoprotein A-I
HDL formation is a primary way to eliminate the excess cholesterol from peripheral tissues and plays a critical role in the prevention of coronary artery disease (1–3). ABCA1 mediates HDL formation by secreting cellular cholesterol and phospholipids into an extracellular acceptor, apoA-I (4–7). Defects in ABCA1 cause Tangier disease, which is characterized by having a minimal to negligible level of circulating HDL, prominent cholesterol-ester accumulation in tissue macrophages, and premature atherosclerotic vascular disease (8–10). Recently, it has been reported that the capacity of serum to mediate cholesterol efflux from macrophages by ABCA1 is strongly and inversely associated with both carotid intima-media thickness and the likelihood of angiographic coronary artery disease, independent of HDL cholesterol levels (11). Therefore, enhancing HDL formation is one of the promising mechanistic approaches of drug therapies for treating and preventing atherosclerosis.
Several methods have been employed in measuring the rate of HDL formation in ABCA1-expressing cells. Because of its high sensitivity, the most widely used method is measurement of [3H]cholesterol release into medium from cells (12–14). However, this method requires the labeling of cellular cholesterol with a radioisotope and is not suitable for high-throughput screening assays. To avoid the use of radioisotopes, Sankaranarayanan et al. (15) substituted a fluorescent sterol, BODIPY-cholesterol, for the radiolabeled cholesterol, and developed a sensitive assay for ABCA1-mediated cholesterol efflux. Although the use of the fluorescent-labeled sterol provides an efficient measurement of efflux compared with the use of radiolabeled cholesterol, this method still requires the labeling of cellular sterol and is specially designed for the analysis of efflux capacity of serum. To analyze cholesterol efflux without the need for labeling of cellular sterol, gas-liquid chromatography (16) and colorimetric enzyme assays (17) have also been utilized to determine the amount of secreted cholesterol. However, these methods have lower sensitivities and require lipid extraction from culture medium prior to the measurement of cholesterol. Therefore, a convenient and highly sensitive assay is required for the high-throughput screening of ABCA1 modulators and the evaluation of HDL formation.
To develop a novel method for measurement of HDL formation by ABCA1-expressing cells, we focused on the environmental change around apoA-I protein in the HDL formation process. Human apoA-I (243 amino acid residues) contains 11- and 22-amino acid repeats that form amphipathic α-helices (18). Although lipid-free apoA-I is exposed to the hydrophilic environment, the hydrophobic surfaces of the amphipathic α-helices of apoA-I interact with the hydrophobic part of lipids in the HDL particles (19). Thus, the hydrophobicity of some residues of apoA-I is increased by the incorporation into HDL particles. Therefore, we hypothesized that HDL formation could be measured by sensing the hydrophobicity change in specific residues of apoA-I. Previously, we introduced a V53C mutation into human apoA-I and labeled the cysteine residue with N-(1-pyrene) maleimide, a hydrophobicity-sensitive fluorescence probe, to monitor the lipid binding behavior of apoA-I (20). A significant increase in the fluorescence intensity of pyrene-labeled apoA-I was observed upon binding to lipids, indicating that the pyrene moiety is embedded in the hydrophobic environment of apoA-I in the lipid-bound state. However, pyrene was not suitable for the analysis of HDL formation by ABCA1-expressing cells because it is excited by UV light, which excites numerous other compounds in culture media, and thus we could not detect the ABCA1-dependent HDL formation using pyrene-labeled apoA-I (data not shown). In this study, the cysteine residue introduced at the position 53 of apoA-I was labeled with a hydrophobicity-sensitive fluorescence probe, POLARIC-maleimide, which can be excited by visible light. We demonstrate that HDL formation by ABCA1-expressing cells can be specifically detected by sensing a hydrophobicity change in POLARIC-labeled apoA-I, thus providing a novel method for the evaluation of HDL formation and the screening of the HDL formation modulator.
MATERIALS AND METHODS
Materials
Human wild-type apoA-I and V53C variant were expressed as thioredoxin fusion proteins in Escherichia coli strain BL21-DE3 host and then cleaved and purified as previously described (21). The apoA-I preparations were at least 95% pure, as assessed by SDS-PAGE. In all experiments, apoA-I was freshly dialyzed from 4 M guanidine hydrochloride (GdnHCl) solution into the appropriate buffer before use. The mouse anti-ABCA1 monoclonal antibody KM3110 was generated against the C-terminal 20 amino acids of ABCA1 (22). POLARIC-maleimide in which POLARIC is directly connected to maleimide was obtained from Goryo Chemical (Hokkaido, Japan). The remaining chemicals were purchased from Sigma-Aldrich (St. Louis, MO), Wako Pure Chemical Industries (Osaka, Japan), and Nacalai Tesque (Kyoto, Japan).
Labeling of apoA-I with POLARIC-maleimide and preparation of discoidal HDL
The apoA-I V53C variant was incubated with 10-fold molar excess of tris(2-carboxyethyl)phosphine hydrochloride for 1.5 h to reduce the sulfhydryl group. The 10 mg/ml stock solution of POLARIC-maleimide in DMSO was added to the final molar ratio of probe to protein of 2:1 and then the reaction mixture was stirred overnight at 17°C in the dark. Unreacted POLARIC-maleimide was removed by extensive dialysis at 4°C in PBS. The degree of labeling was determined using the extinction coefficient for POLARIC of 34,000 M−1 cm−1 at a wavelength of 480 nm and ranged from 55 to 70%. Discoidal HDL (dHDL) particles consisting of POPC or 1,2-di-palmitoyl phosphatidylcholine (DPPC) were prepared by the cholate dialysis method (23). dHDL particles consisting of 1,2-di-myristoyl phosphatidylcholine (DMPC) and cholesterol were prepared by solubilization of multilamellar vesicles (MLVs) (1.2 mg/ml) containing 5 mol% cholesterol with apoA-I-POLARIC (0.6 mg/ml) at 24.5°C.
Circular dichroism spectroscopy
Far-UV circular dichroism (CD) spectra were recorded from 185 to 260 nm at 25°C using a Jasco J-600 spectropolarimeter. The apoA-I solutions in 10 mM Tris buffer (pH 7.4) were subjected to CD measurements in a 2 mm quartz cuvette, and the results were corrected by subtracting the buffer base line. The α-helix content was derived from the molar ellipticity at 222 nm ([θ]222) using the following equation: percent of α-helix = {(−[θ]222 + 3,000)/(36,000 + 3,000)} × 100 (24).
Fluorescence emission spectrum of apoA-I-POLARIC
The fluorescence emission spectrum of apoA-I-POLARIC (5 μg/ml) in the lipid-free state or dHDL particles was recorded from 500 to 700 nm using a 480 nm excitation wavelength by Jasco FP-6600 fluorescence spectrophotometer at 25°C in PBS, and the results were corrected by subtracting the buffer base-line.
Cell culture
Baby hamster kidney (BHK)/ABCA1 cells (gift from Dr. John F. Oram) (25) were grown in a humidified incubator (5% CO2) at 37°C in DMEM supplemented with 10% heat-inactivated FBS. THP-1 monocytes were cultured in RPMI-1640 medium supplemented with 10% FBS at 37°C in 5% CO2.
Detection of ABCA1-dependent HDL formation by apoA-I-POLARIC
BHK/ABCA1 cells were subcultured in 24-well plates at a density of 5 × 104 cells in DMEM containing 10% FBS. After a 24 h incubation, the cells were treated with or without 10 nM mifepristone in DMEM containing 0.02% BSA for 20 h. The cells were washed twice with PBS and incubated with apoA-I-POLARIC (0.625–5 μg/ml) in HBSS containing 0.02% BSA for 6 h. After centrifugation of the medium, the fluorescence intensity of the medium was measured using a TECAN infinite M200 microplate reader (excitation at 480 nm, emission at 575 nm). THP-1 monocytes were treated with 200 ng/ml phorbol 12-myristate 13-acetate for 2 days to facilitate differentiation into macrophages. The adherent macrophages were incubated with 10 μM TO901317 to induce ABCA1 expression for 24 h. Cells were washed twice with PBS and incubated with HBSS containing apoA-I-POLARIC (2.5 μg/ml) and 0.02% BSA for 6 h. The fluorescence intensity of the medium was determined as described above.
Cellular lipid release assay
Cells were incubated with apoA-I in DMEM or HBSS containing 0.02% BSA for 6 h. The cholesterol content in the medium was determined using a fluorescence enzyme assay (26) after lipid extraction from the medium (17).
Production of monoclonal anti-apoA-I antibody
A female BALB/c mouse (8 weeks of age; Japan SLC) was immunized biweekly (a total of four times) with the wild-type apoA-I. The antigen (50 μg) was subcutaneously injected at multiple sites on the back with an emulsion of Freund’s complete (for primary immunization) or incomplete adjuvant (for booster immunizations) (DIFCO) and sterile saline (1:1; 0.2 ml). Then, the mouse received intraperitoneal and intrasplenic injections of the antigen (each 50 μg) dissolved in sterile saline (0.5 ml and 0.2 ml, respectively). After 3 days, splenocytes (1.3 × 108 cells) were obtained from this mouse, which were fused with P3/NS1/1-Ag4-1 myeloma cells (2.6 × 107 cells) using 40% polyethylene glycol 4000 in sterile PBS containing 10% (v/v) DMSO and a 0.001% poly-L-arginine-HCl solution (1 ml). The fused cells were cultured in HAT medium supplemented with 10% Briclone (Archport) under 5% CO2/95% air at 37°C for approximately 10 days. Hybridomas secreting anti-apoA-I antibodies were assessed by ELISA using microplates on which wild-type apoA-I (conjugated with BSA) had been coated, expanded in HT medium, and then cloned by limiting dilution. A monoclonal anti-apoA-I antibody, which was secreted in culture medium from one of these hybridoma clones (clone#19-17), was used as indicator in the Western blotting assay.
Western blot analysis
Cells were washed with PBS and lysed in lysis buffer [20 mM Tris-Cl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, and 1% sodium deoxycholate] containing a protease inhibitor cocktail (Calbiochem, La Jolla, CA) (27). Samples were electrophoresed on SDS-polyacrylamide gels, blotted, and probed with the indicated antibodies. Western blots were analyzed using a Fujifilm LAS-4000 mini imaging system.
Characterization of HDL particles by gel filtration
Conditioned medium was concentrated using an Amicon ultracel-10K centrifugal filter (Millipore). Concentrated medium was fractionated by gel filtration chromatography on a Superdex 200 column calibrated by the proteins of known diameter (particle diameter range, 6.1–17.0 nm), and 1.25 ml fractions were collected (28). Fluorescence intensity and the amount of cholesterol in each fraction were analyzed as described above and normalized using BSA. The amount of apoA-I in every two fractions was determined by Western blot analysis.
Depletion of extracellular Ca2+ and Mg2+
Cells were incubated with or without 0.1 mg/ml CaCl2 and 0.1 mg/ml MgCl26H2O in PBS containing 1 mg/ml glucose, 0.02% BSA, and 2.5 μg/ml apoA-I-POLARIC for 6 h.
Statistical analysis
Values are presented as means ± SD. The statistical significance of differences between the mean values was analyzed using the nonpaired t-test. Multiple comparisons were performed using Bonferroni’s test following ANOVA. P < 0.05 was considered statistically significant.
RESULTS
Effect of POLARIC labeling on the structure and function of apoA-I
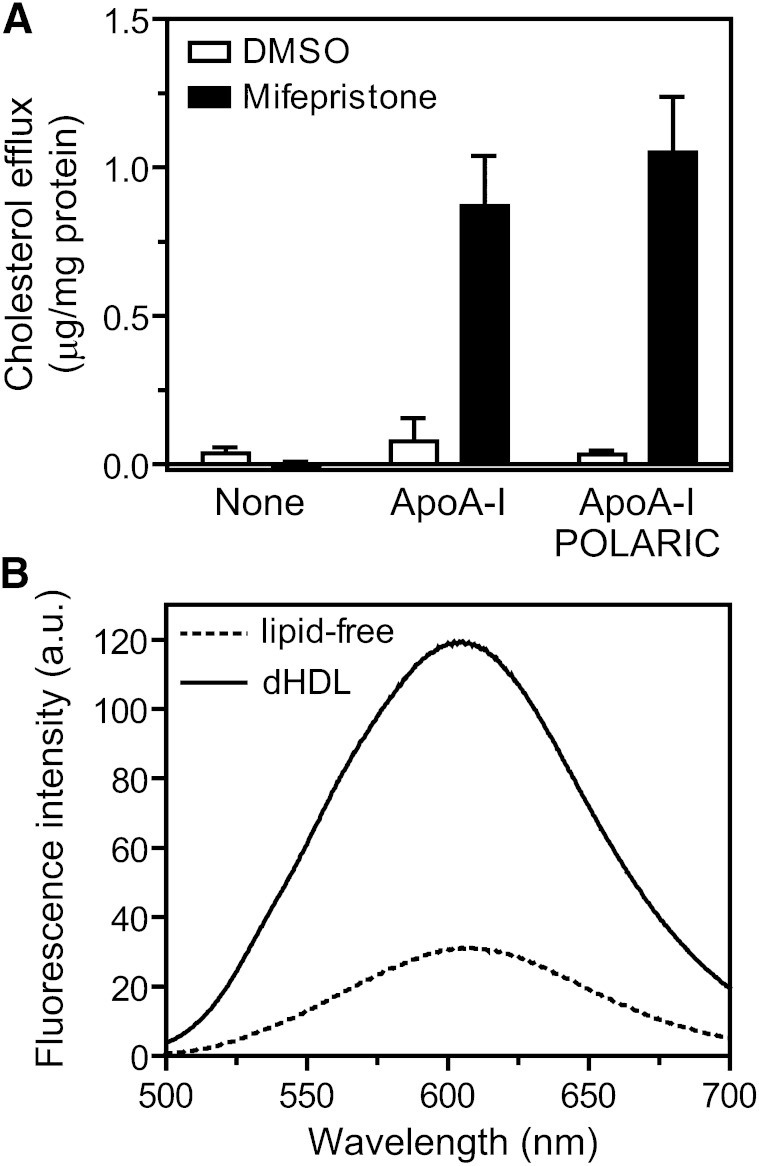
The apoA-I V53C variant was labeled with POLARIC-maleimide (supplementary Fig. I) to prepare a novel HDL formation detection probe, “apoA-I-POLARIC”. To analyze the effect of labeling with POLARIC on the secondary structure of apoA-I, the lipid-free structure was analyzed by far-UV CD spectroscopy (supplementary Fig. II). apoA-I-POLARIC exhibited a CD spectrum typical of an α-helical structure (α-helical content of 42%) and comparable to a wild-type apoA-I (21). Next, the effect of labeling with POLARIC on HDL formation by ABCA1-expressing cells was examined using a fluorescence enzyme assay (Fig. 1A). As previously reported, cholesterol efflux from BHK/ABCA1 cells in which human ABCA1 is expressed under the control of mifepristone-induced promoter was dependent on both the induction of ABCA1 and the addition of apoA-I (25, 29). The amount of cholesterol secreted from ABCA1-expressing cells (mifepristone-treated cells) was indistinguishable between wild-type apoA-I and apoA-I-POLARIC, and no cholesterol efflux into apoA-I-POLARIC was observed in ABCA1-non-expressing cells (DMSO-treated cells). These results demonstrate that labeling with POLARIC affects neither the structure nor the function of apoA-I.
Fig. 1.
Labeling of apoA-I-V53C variant by POLARIC-maleimide. A: Effect of POLARIC-labeling on ABCA1-dependent cholesterol efflux. BHK/ABCA1 cells were treated with (filled bars) or without (open bars) 10 nM mifepristone for 20 h and incubated with indicated acceptors (5 μg/ml) for 6 h. Cholesterol content in the medium was determined using a fluorescence enzyme assay. B: Fluorescence emission spectra of apoA-I-POLARIC in the lipid-free state (dotted line) or dHDL particles (solid line). Protein concentrations were 5 μg/ml.
Increase in fluorescence of apoA-I-POLARIC by incorporation into dHDL
To evaluate the ability of apoA-I-POLARIC in the detection of HDL formation, apoA-I-POLARIC was incorporated into the dHDL particles by the cholate dialysis method. Structural properties of dHDL particles containing apoA-I-POLARIC were evaluated by CD spectroscopy (supplementary Fig. II) and gel filtration chromatography (supplementary Fig. III). α-Helical content (78%) and diameter (10.2 nm) of dHDL particles containing apoA-I-POLARIC were comparable to the reported values for wild-type apoA-I (30), indicating that POLARIC labeling has no effect on the structure and function of apoA-I. Figure 1B shows typical fluorescence emission spectra of apoA-I-POLARIC in the lipid-free state and dHDL particles. The incorporation into dHDL caused a 4.0-fold increase in fluorescence intensity of apoA-I-POLARIC, suggesting that the POLARIC moiety was transferred into a more hydrophobic environment in dHDL compared with that in the lipid-free state. These results demonstrate that HDL formation in vitro can be detected through an increase in the fluorescence intensity of apoA-I-POLARIC.
The fluorescence intensity of apoA-I-POLARIC linearly increased with increasing the concentrations of apoA-I-POLARIC both in dHDL particles and in the lipid-free state within the concentration range used in the HDL formation assay (data not shown). However, at higher concentrations (>100 μg/ml), the fluorescence intensity of apoA-I-POLARIC did not increase linearly (supplementary Fig. IV). Because lipid-free apoA-I self-associates to form dimers, tetramers, and octamers at high concentration (>100 μg/ml) (31, 32), it is likely that the decreased fluorescence intensity at high concentration is due to self-quenching of POLARIC.
Detection of HDL formation by apoA-I-POLARIC in ABCA1-expressing cells
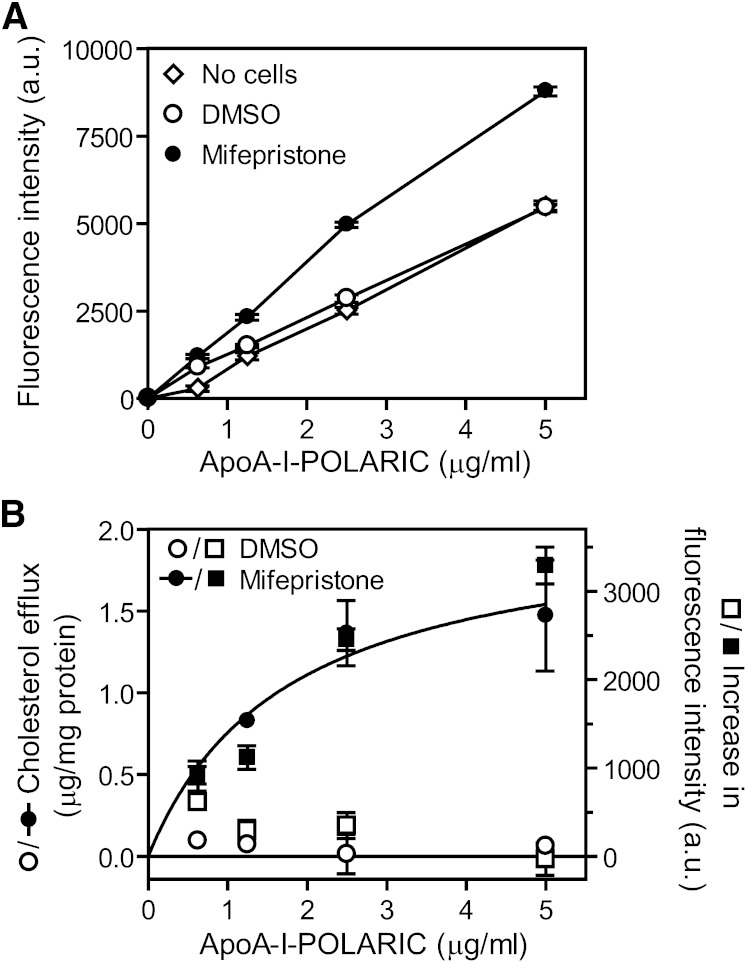
Next, we examined whether HDL formation by ABCA1-expressing cells is also measurable by apoA-I-POLARIC. BHK/ABCA1 cells were incubated with an increasing amount of apoA-I-POLARIC for 6 h, and fluorescence intensity of the medium was analyzed. The fluorescence intensity of the medium did not change in ABCA1-non-expressing cells (Fig. 2A), reflecting the ABCA1 dependence in cholesterol efflux into apoA-I (Fig. 1A). On the other hand, fluorescence intensity increased in all apoA-I-POLARIC concentrations in ABCA1-expressing cells, and a 74% increase was observed using 2.5 μg/ml of apoA-I-POLARIC compared with ABCA1-non-expressing cells. Because a change in the amount of apoA-I-POLARIC in culture medium affects the fluorescence intensity of apoA-I-POLARIC, we measured the amount of apoA-I-POLARIC before and after 6 h of incubation with BHK/ABCA1 cells. As shown in supplementary Fig. V, the amount of apoA-I-POLARIC was not decreased by 6 h of incubation with BHK/ABCA1 cells. Furthermore, the ABCA1-dependent increase in fluorescence intensity was well-fitted with the amount of cholesterol secreted into apoA-I-POLARIC (Fig. 2B), indicating that the fluorescence change in apoA-I-POLARIC reflects HDL formation by ABCA1.
Fig. 2.
Detection of HDL formation by apoA-I-POLARIC in BHK/ABCA1 cells. BHK/ABCA1 cells were treated with or without 10 nM mifepristone for 20 h and incubated with the indicated concentrations of apoA-I-POLARIC for 6 h. A: Fluorescence intensity of the medium was determined. No cells (open diamonds), DMSO-treated cells (open circles), mifepristone-treated cells (closed circles). B: Cholesterol efflux from DMSO-treated cells (open circles) and mifepristone-treated cells (closed circles) was determined. Increase in fluorescence intensity of apoA-I-POLARIC was calculated by subtracting the value of no-cells from that of DMSO-treated cells (open squares) or that of mifepristone-treated cells (closed squares).
HDL formation-dependent increase in apoA-I-POLARIC fluorescence
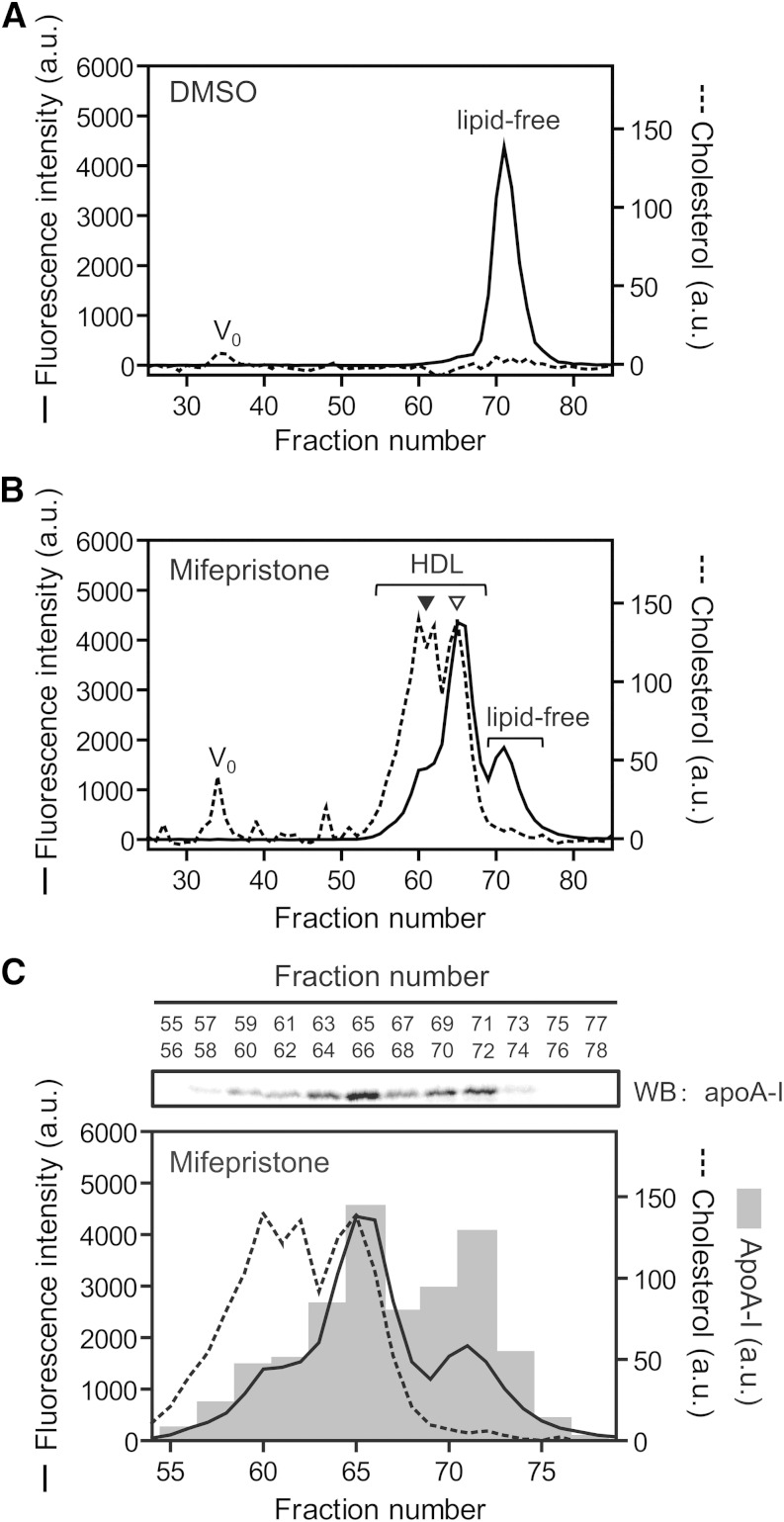
To confirm that the increase in apoA-I-POLARIC fluorescence in ABCA1-expressing cells reflects the HDL formation, HDL particles were separated from the conditioned medium by gel filtration chromatography (Fig. 3). In ABCA1-non-expressing cells, most of the fluorescence of apoA-I-POLARIC was observed in the lipid-free fraction, and cholesterol was not detected in any fraction (Fig. 3A). Incubation of apoA-I-POLARIC with ABCA1-expressing cells led to the formation of two populations of nascent HDL particles (8.3 and 10.0 nm diameter) (Fig. 3B), as previously reported for wild-type apoA-I (33). Despite the similar cholesterol content in each HDL population, a stronger fluorescence intensity was observed in smaller HDL particles. Because it has been reported that the larger HDL particles are highly cholesterol-enriched relative to the smaller HDL particles (34), it is highly likely that the increase in fluorescence intensity of the apoA-I-POLARIC represents the amount of apoA-I protein incorporated into HDL particles rather than the amount of secreted cholesterol. Supporting our interpretation, the amount of apoA-I in the dHDL fraction was well-correlated with the fluorescence intensity of apoA-I-POLARIC (Fig. 3C). Furthermore, the fluorescence intensity in the lipid-free apoA-I fraction was lower in ABCA1-expressing cells (Fig. 3B) than in ABCA1-non-expressing cells (Fig. 3A). These results establish that the increase in the fluorescence intensity in ABCA1-expressing cells represents the formation of HDL particles.
Fig. 3.
Separation of HDL particles by gel filtration. BHK/ABCA1 cells were treated without (A) or with (B, C) 10 nM mifepristone for 20 h and incubated with 5 μg/ml apoA-I-POLARIC for 6 h. Concentrated medium was separated by gel filtration chromatography [Superdex 200 column (16/60 PG)] as described in the Materials and Methods. Fluorescence intensity (solid line) and cholesterol content (dotted line) in each fraction were determined (A–C). apoA-I protein in fractions 55–78 was detected with anti-apoA-I antibody (upper panel), and the amount of apoA-I (bars) was analyzed using a Fujifilm LAS-4000 mini imaging system (C). ▽, 8.3 nm; ▼, 10.0 nm; WB, Western blot.
It has been reported that ABCA1 mediates the formation of microparticles that are larger than HDL and are not involved in HDL formation (35, 36). As previously reported, cholesterol-containing particles were observed in the conditioned medium from ABCA1-expressing cells around the void volume of the Superdex 200 column (Fig. 3B). These particles had no detectable apoA-I protein (supplementary Fig. VI) and apoA-I-POLARIC fluorescence (Fig. 3B), suggesting that the increase in the fluorescence of apoA-I-POLARIC does not represent microparticle formation, which does not contribute to HDL formation. These results show that measuring an increase in the fluorescence of apoA-I-POLARIC is a more direct method to detect ABCA1-mediated HDL formation than conventional methods.
Effect of size and lipid composition of dHDL on apoA-I-POLARIC fluorescence
The effect of the size and lipid composition of dHDL on the fluorescence intensity of apoA-I-POLARIC was analyzed using DMPC/cholesterol dHDL particles prepared by solubilization of DMPC/cholesterol MLVs with apoA-I-POLARIC. As previously reported (37), solubilization of DMPC/cholesterol MLVs produced two populations of dHDL particles (11.0 nm and 13.6 nm diameter) (supplementary Fig. VIIA). The ratio of fluorescence intensity to the amount of apoA-I-POLARIC in each dHDL population was comparable between the two populations of dHDL particles, demonstrating that the fluorescence intensity of apoA-I-POLARIC in the HDL fraction represents the amount of apoA-I protein incorporated into HDL particles. Furthermore, the ratio of fluorescence intensity to the amount of apoA-I-POLARIC in the dHDL fractions was 4.2-fold higher than that in lipid-free apoA-I (supplementary Fig. VIIA). As shown in supplementary Fig. VIIB, apoA-I-POLARIC also showed 4.0-fold higher fluorescence intensity in dHDL particles consisting of DPPC than in the lipid-free state. These increases in the ratio of fluorescence intensity to the amount of apoA-I-POLARIC (supplementary Fig. VII) were comparable to those by incorporation into POPC dHDL (4.0-fold increase) (Fig. 1B), demonstrating that the lipid composition of HDL particles had no effect on the fluorescence intensity of apoA-I-POLARIC.
Detection of HDL formation in THP-1 macrophages by apoA-I-POLARIC
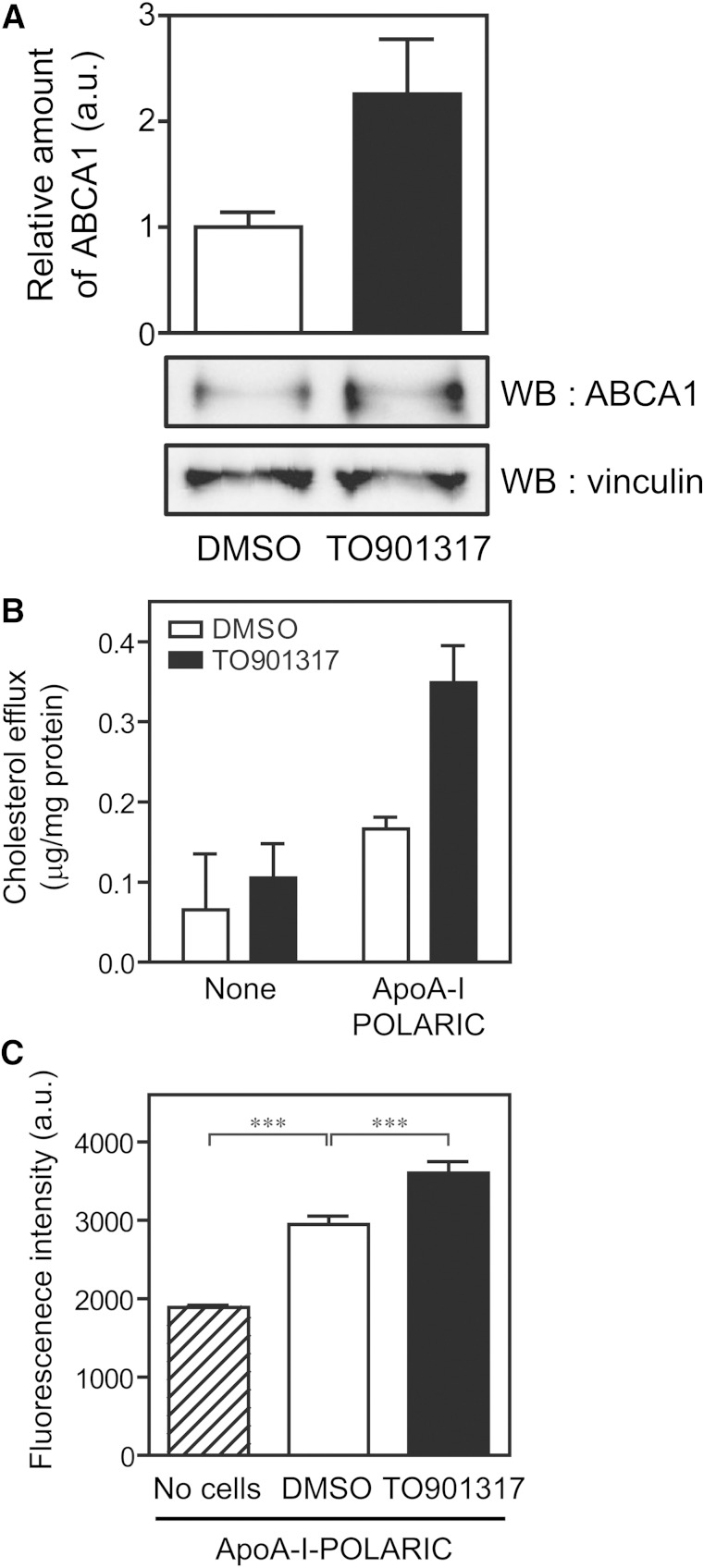
To confirm that apoA-I-POLARIC can detect the HDL formation by endogenously expressed ABCA1, THP-1 macrophages were examined. Western blot analysis showed that ABCA1 was substantially expressed in THP-1 macrophages cultured under normal conditions, and its expression increased 2-fold by the treatment with TO901317, a liver X receptor (LXR) agonist (Fig. 4A). Reflecting the expression levels of ABCA1, the increase in the fluorescence intensity of apoA-I-POLARIC (Fig. 4C), as well as cholesterol efflux (Fig. 4B), was higher in TO901317-treated cells than in control cells. Therefore, apoA-I-POLARIC is suited for the detection of HDL formation both by exogenously and endogenously expressed ABCA1. Because apoE expression is induced by the activation of LXR (38), the finding that the increase in cholesterol efflux (Fig. 4B) was larger than the increase in apoA-I-POLARIC fluorescence (Fig. 4C) in LXR-activated cells may have been due to the increased amount of lipid acceptors in the medium.
Fig. 4.
Detection of HDL formation by apoA-I-POLARIC in THP-1 macrophages. A: THP-1 macrophages were treated with 10 μM TO901317 for 24 h to induce the expression of ABCA1. Cell lysates (10 μg) were separated by 7% polyacrylamide gel electrophoresis, and ABCA1 and vinculin (loading control) were detected using the indicated antibodies. The amount of ABCA1 was normalized against vinculin. B, C: THP-1 macrophages were treated with (filled bars) or without (open bars) 10 μM TO901317 for 24 h and incubated with or without apoA-I-POLARIC (2.5 μg/ml) for 6 h. Cholesterol content in the medium (B) and fluorescence intensity of the medium (C) were determined (n = 3). ***P < 0.001; WB, Western blot.
Application of apoA-I-POLARIC to the evaluation of HDL formation modulator
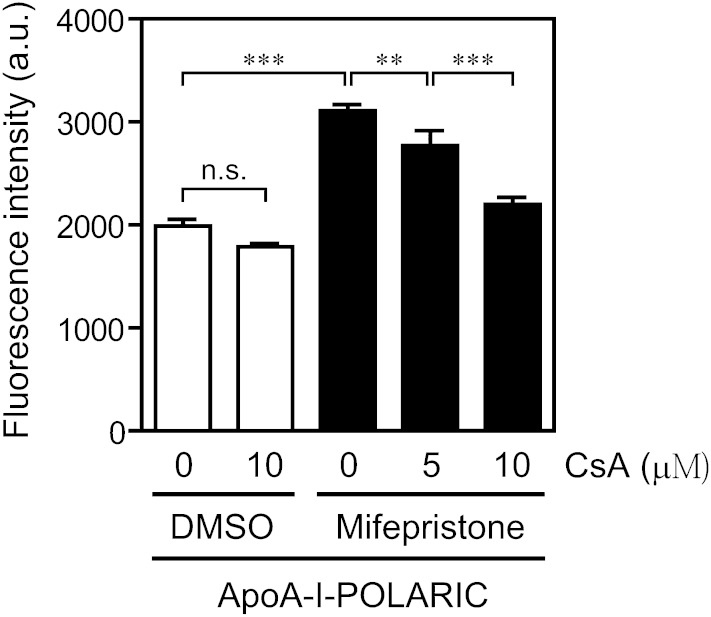
To confirm whether apoA-I-POLARIC is suitable for the evaluation of ABCA1 modulators, an ABCA1 inhibitor (cyclosporine A) was applied (39). Previously, we reported that apoA-I-dependent [3H]cholesterol efflux from BHK/ABCA1 cells is efficiently inhibited by cyclosporine A (IC50 of 7.6 μM) (40). As shown in Fig. 5, despite no effect in ABCA1-non-expressing cells, the ABCA1-dependent increase in fluorescence intensity of apoA-I-POLARIC was abolished by cyclosporine A in a concentration-dependent manner (30 and 81% inhibition by 5 μM and 10 μM cyclosporine A, respectively; estimated IC50 = 6.5 μM), demonstrating the capability of apoA-I-POLARIC in the evaluation of the ABCA1 modulator.
Fig. 5.
Inhibition of HDL formation by cyclosporine A. BHK/ABCA1 cells were treated with (filled bars) or without (open bars) 10 nM mifepristone for 20 h and incubated with 2.5 μg/ml apoA-I-POLARIC in the presence of the indicated concentrations of cyclosporine A for 6 h. Fluorescence intensity of the medium was determined. CsA, cyclosporine A. **P < 0.01; ***P < 0.001; n.s., not significant.
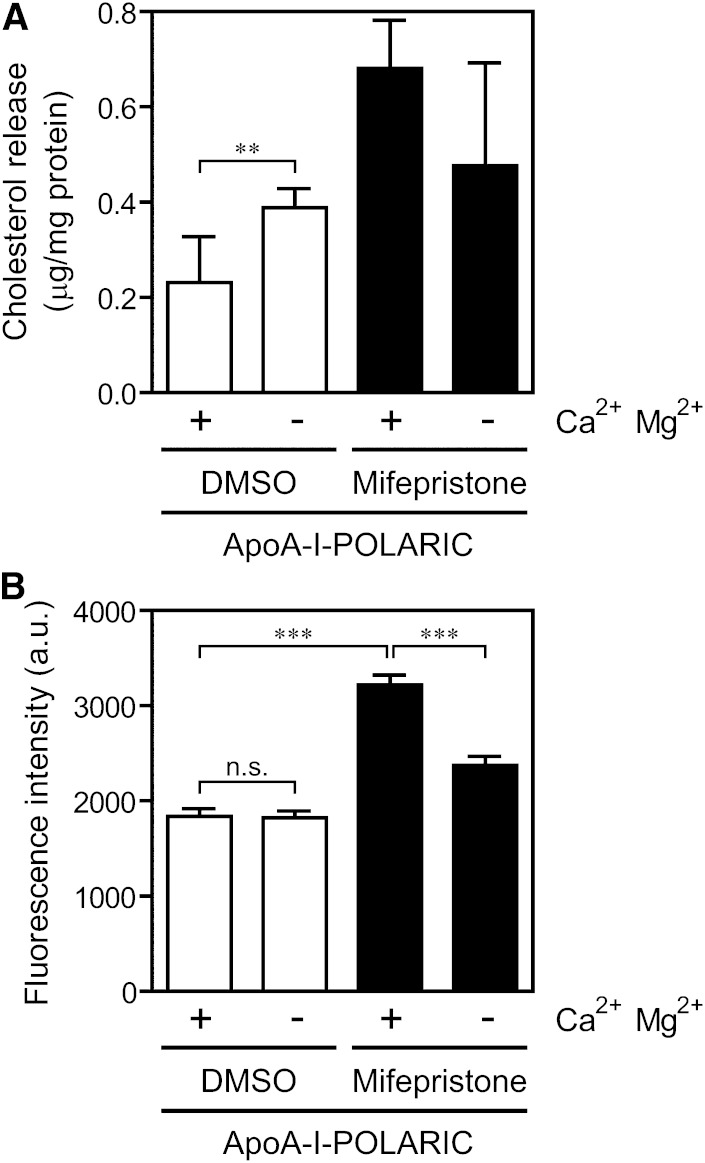
The release of lipids unrelated to HDL formation occurs in some culture conditions (e.g., cell death) and hinders the screening of HDL formation modulators. Because apoA-I-POLARIC detects the environmental change around apoA-I and not cholesterol content, we expected that the exact HDL formation would be measurable by apoA-I-POLARIC even in such a culture condition. Depletion of Ca2+ and Mg2+ from the incubation medium induced cell death, and a substantial amount of cholesterol was released from cells even in the absence of ABCA1 (Fig. 6A). In contrast to the amount of cholesterol, no increase in fluorescence of apoA-I-POLARIC was observed in ABCA1-non-expressing cells cultured in the Ca2+- and Mg2+-free condition (Fig. 6B), suggesting that apoA-I-POLARIC distinguishes the HDL formation-dependent and -independent cholesterol release. Due to decreased cellular viability, ABCA1-dependent HDL formation detected by apoA-I-POLARIC decreased by depletion of extracellular Ca2+ and Mg2+ (Fig. 6B). These results demonstrate that the increase in the fluorescence intensity of apoA-I-POLARIC is specific to HDL formation, and apoA-I-POLARIC is useful for the evaluation of HDL formation modulator.
Fig. 6.
Effect of cell death on the detection of HDL formation by apoA-I-POLARIC. BHK/ABCA1 cells were treated with (filled bars) or without (open bars) 10 nM mifepristone for 20 h and incubated with 2.5 μg/ml apoA-I-POLARIC in the presence or absence of 0.1 mg/ml CaCl2 and 0.1 mg/ml MgCl26H2O for 6 h. Cholesterol content in the medium (A) and fluorescence intensity of the medium (B) were determined. **P < 0.01; ***P < 0.001; n.s., not significant.
DISCUSSION
In this study, we developed a novel method for the analysis of HDL formation by ABCA1-expressing cells. This approach is based on the environmental change around apoA-I molecules, but not the increase of cholesterol content in the culture medium. Because the hydrophobicity of some regions of apoA-I is increased by incorporation into HDL particles (19), we hypothesized that ABCA1-dependent HDL formation could be measured by sensing the hydrophobicity change in specific residues of apoA-I. To detect this environmental change, a novel HDL formation probe, apoA-I-POLARIC, was prepared by labeling an apoA-I V53C variant with a hydrophobicity-sensitive fluorescence probe, POLARIC-maleimide. We evaluated the ability of apoA-I-POLARIC in detecting ABCA1-dependent HDL formation compared with the conventional method and demonstrated that apoA-I-POLARIC is a valuable tool for the analysis of HDL formation.
We initially evaluated the effects of POLARIC labeling on the structure and function of apoA-I. CD analysis showed that the α-helical content of apoA-I-POLARIC is comparable to that of wild-type apoA-I in both the lipid-free state and dHDL particles (supplementary Fig. II) (21, 30), demonstrating POLARIC labeling had no effects on the structure of apoA-I. In the cholesterol efflux assay, similar amounts of cholesterol were secreted into the wild-type apoA-I and apoA-I-POLARIC in an ABCA1-dependent manner (Fig. 1A). Furthermore, the apoA-I-POLARIC concentration dependence of cholesterol efflux (Km, 1.8 ± 0.6 μg/ml) was consistent with the reported property of wild-type apoA-I (Fig. 2B) (7, 28). Therefore, labeling with POLARIC-maleimide affected neither the structure nor the function of apoA-I, and apoA-I-POLARIC possessed the intact ABCA1-dependent HDL formation activity.
Taking advantage of the no-cysteine residue in human apoA-I, we introduced a V53C mutation into the apoA-I and site-specifically labeled the cysteine residue with POLARIC-maleimide to prepare apoA-I-POLARIC. In the proposed dHDL structure model (41), hydrophobic surfaces of amphipathic helices are exposed to lipids constituting the HDL particles. Based on the helical wheel projection, the 53rd residue of apoA-I is predicted to be located in the hydrophobic surface of the amphipathic helix [helix 1 (18)]. Although it is difficult to define the position of the 53rd residue of apoA-I in the lipid-free structure due to the limitation of structural information, the 53rd residue of apoA-I is shown to reside in a nonhelical region in the lipid-free state (42). Thus, it is likely that the introduced POLARIC moiety in the 53rd residue of apoA-I is embedded in the more hydrophobic environment in HDL particles, and the hydrophobicity-sensitive fluorescence of apoA-I-POLARIC is increased by its incorporation into the HDL particles. Recently, Wang et al. (43) created the apoA-I lipidation indicator by labeling the free amines of human apoA-I with the lipid-sensitive dye 7-nitrobenz-2-oxa-1,3-diazole (NBD) and observed an ABCA1-dependent lipidation by NBD fluorescence. Because human apoA-I contains 21 lysine residues, the fluorescence of NBD labeled on free amines of apoA-I represents the average hydrophobicity of several lysine residues and N-terminal amino group. Furthermore, due to the localization of lysine residues of apoA-I in the hydrophilic surfaces of the amphipathic helices, hydrophobicity changes around the lysine residues may be smaller than those in the 53rd residue of apoA-I. Therefore, site-specific labeling of the apoA-I V53C variant with POLARIC-maleimide is a rational approach to the detection of HDL formation by sensing hydrophobicity change in apoA-I. As expected, the fluorescence intensity of apoA-I-POLARIC in dHDL particles was four times higher than that in the lipid-free state (Fig. 1B). Furthermore, the fluorescence intensity of apoA-I-POLARIC increased with incubation with ABCA1-expressing cells (Figs. 2A, 4C). These results demonstrate that HDL formation in vitro and in ABCA1-expressing cells can be detected by fluorescence change in apoA-I-POLARIC.
It has been reported that dHDL particles produced by ABCA1 have different sizes and compositions, and the larger dHDL particles are highly cholesterol-enriched relative to the smaller ones (34). It was also reported that the cholesterol loading of macrophages leads to the formation of larger dHDL particles, which were highly enriched in cholesterol (36). Furthermore, the number of apoA-I proteins involved in each dHDL particle varies among different sizes of dHDL (33, 44). Therefore, the amount of secreted cholesterol into the medium is not linearly correlated with the number of formed dHDL particles and the amount of apoA-I incorporated into dHDL. Because the conversion of lipid-free apoA-I to dHDL is the first and essential step of formation of circulating HDL and completely depends on the function of ABCA1, the evaluation of the lipidation of apoA-I by ABCA1-expressing cells is important for the understanding of the HDL formation process. As shown in Fig. 3C, the fluorescence intensity of apoA-I-POLARIC in the dHDL fraction is well-correlated with the amount of apoA-I rather than the amount of secreted cholesterol. Furthermore, the ratio of fluorescence intensity to the amount of apoA-I-POLARIC was comparable between different populations of dHDL particles (supplementary Fig. VIIA). Therefore, it is highly likely that the fluorescence intensity change in apoA-I-POLARIC represents the HDL formation activity of ABCA1-expressing cells more directly than the measurement of secreted cholesterol. Therefore, the convenient measurement of apoA-I lipidation by apoA-I-POLARIC may provide a novel metric for the evaluation of the initial step of HDL formation.
The conventional methods for the measurement of HDL formation require the labeling of cellular cholesterol or the extraction of cholesterol from medium. On the other hand, our novel method simply involves the incubation of cells with apoA-I-POLARIC and the measurement of the fluorescence intensity of the medium. This simple protocol provides an extremely accurate measurement (Figs. 2, 4). To confirm whether apoA-I-POLARIC was suitable for the evaluation of an ABCA1 modulator, the effect of cyclosporine A, a well-known ABCA1 inhibitor, on HDL formation was evaluated by apoA-I-POLARIC (Fig. 5). Cyclosporine A treatment inhibited the ABCA1-dependent increase in fluorescence intensity of apoA-I-POLARIC in a concentration-dependent manner, whereas no effect was observed in ABCA1-non-expressing cells. We also demonstrated that HDL formation-independent lipid release induced by microparticle formation (Fig. 3) or cell death (Fig. 6) was not detected by apoA-I-POLARIC. Furthermore, HDL formation by endogenously expressing ABCA1 in THP-1 macrophages could be measured using apoA-I-POLARIC (Fig. 4). Although this method is not applicable to determination of the lipid efflux capacity of serum, these results demonstrate that apoA-I-POLARIC allows the specific detection of HDL formation by ABCA1 and is suitable for the high-throughput screening assay of ABCA1-dependent HDL formation modulators.
In conclusion, we have developed a novel method for the detection of HDL formation using the hydrophobicity-sensitive probe POLARIC. Efficacy and accuracy of this method were validated by dHDL formation assay in vitro and in ABCA1-expressing cells, and we showed that the increase in the fluorescence of apoA-I-POLARIC specifically represents ABCA1-dependent HDL formation. The advantage of this method is the simple protocol that does not require the labeling of cellular sterol and the extraction of lipids from culture medium. Furthermore, the fluorescence change in apoA-I-POLARIC represents the amount of apoA-I protein incorporated into HDL particles, whereas conventional methods measure the amount of secreted cholesterol into the medium. Our method provides a novel tool for the screening of HDL formation modulators and the study of the mechanism behind HDL formation.
Supplementary Material
Acknowledgments
The authors are indebted to Dr. Michael C. Phillips (The Children’s Hospital of Philadelphia) for his valuable comments.
Footnotes
Abbreviations:
- BHK
- baby hamster kidney
- CD
- circular dichroism
- dHDL
- discoidal HDL
- DMPC
- 1,2-di-myristoyl phosphatidylcholine
- DPPC
- 1,2-di-palmitoyl phosphatidylcholine
- LXR
- liver X receptor
- MLV
- multilamellar vesicle
- NBD
- 7-nitrobenz-2-oxa-1,3-diazole
This work was supported by grants-in-aid for scientific research 25293006 and 25670014 (H.S.), 25850070 (K.N.), and 25221203 (K.N., K.U.) from Japan Society for the Promotion of Science (JSPS).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of seven figures.
REFERENCES
- 1.Nagao K., Kimura Y., Mastuo M., Ueda K. 2010. Lipid outward translocation by ABC proteins. FEBS Lett. 584: 2717–2723. [DOI] [PubMed] [Google Scholar]
- 2.Rosenson R. S., Brewer H. B., Jr, Davidson W. S., Fayad Z. A., Fuster V., Goldstein J., Hellerstein M., Jiang X. C., Phillips M. C., Rader D. J., et al. 2012. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 125: 1905–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yokoyama S. 2006. Assembly of high-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 26: 20–27. [DOI] [PubMed] [Google Scholar]
- 4.Nagao K., Tomioka M., Ueda K. 2011. Function and regulation of ABCA1–membrane meso-domain organization and reorganization. FEBS J. 278: 3190–3203. [DOI] [PubMed] [Google Scholar]
- 5.Vedhachalam C., Duong P. T., Nickel M., Nguyen D., Dhanasekaran P., Saito H., Rothblat G. H., Lund-Katz S., Phillips M. C. 2007. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J. Biol. Chem. 282: 25123–25130. [DOI] [PubMed] [Google Scholar]
- 6.Oram J. F., Lawn R. M., Garvin M. R., Wade D. P. 2000. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J. Biol. Chem. 275: 34508–34511. [DOI] [PubMed] [Google Scholar]
- 7.Wang N., Silver D. L., Costet P., Tall A. R. 2000. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J. Biol. Chem. 275: 33053–33058. [DOI] [PubMed] [Google Scholar]
- 8.Bodzioch M., Orso E., Klucken J., Langmann T., Bottcher A., Diederich W., Drobnik W., Barlage S., Buchler C., Porsch-Ozcurumez M., et al. 1999. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 22: 347–351. [DOI] [PubMed] [Google Scholar]
- 9.Brooks-Wilson A., Marcil M., Clee S. M., Zhang L. H., Roomp K., van Dam M., Yu L., Brewer C., Collins J. A., Molhuizen H. O., et al. 1999. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 22: 336–345. [DOI] [PubMed] [Google Scholar]
- 10.Rust S., Rosier M., Funke H., Real J., Amoura Z., Piette J. C., Deleuze J. F., Brewer H. B., Duverger N., Denefle P., et al. 1999. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 22: 352–355. [DOI] [PubMed] [Google Scholar]
- 11.Khera A. V., Cuchel M., de la Llera-Moya M., Rodrigues A., Burke M. F., Jafri K., French B. C., Phillips J. A., Mucksavage M. L., Wilensky R. L., et al. 2011. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang N., Silver D. L., Thiele C., Tall A. R. 2001. ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J. Biol. Chem. 276: 23742–23747. [DOI] [PubMed] [Google Scholar]
- 13.Gillotte K. L., Zaiou M., Lund-Katz S., Anantharamaiah G. M., Holvoet P., Dhoest A., Palgunachari M. N., Segrest J. P., Weisgraber K. H., Rothblat G. H., et al. 1999. Apolipoprotein-mediated plasma membrane microsolubilization. Role of lipid affinity and membrane penetration in the efflux of cellular cholesterol and phospholipid. J. Biol. Chem. 274: 2021–2028. [DOI] [PubMed] [Google Scholar]
- 14.Hamon Y., Broccardo C., Chambenoit O., Luciani M. F., Toti F., Chaslin S., Freyssinet J. M., Devaux P. F., McNeish J., Marguet D., et al. 2000. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat. Cell Biol. 2: 399–406. [DOI] [PubMed] [Google Scholar]
- 15.Sankaranarayanan S., Kellner-Weibel G., de la Llera-Moya M., Phillips M. C., Asztalos B. F., Bittman R., Rothblat G. H. 2011. A sensitive assay for ABCA1-mediated cholesterol efflux using BODIPY-cholesterol. J. Lipid Res. 52: 2332–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linsel-Nitschke P., Jehle A. W., Shan J., Cao G., Bacic D., Lan D., Wang N., Tall A. R. 2005. Potential role of ABCA7 in cellular lipid efflux to apoA-I. J. Lipid Res. 46: 86–92. [DOI] [PubMed] [Google Scholar]
- 17.Abe-Dohmae S., Suzuki S., Wada Y., Aburatani H., Vance D. E., Yokoyama S. 2000. Characterization of apolipoprotein-mediated HDL generation induced by cAMP in a murine macrophage cell line. Biochemistry. 39: 11092–11099. [DOI] [PubMed] [Google Scholar]
- 18.Segrest J. P., Jones M. K., De Loof H., Brouillette C. G., Venkatachalapathi Y. V., Anantharamaiah G. M. 1992. The amphipathic helix in the exchangeable apolipoproteins: a review of secondary structure and function. J. Lipid Res. 33: 141–166. [PubMed] [Google Scholar]
- 19.Phillips M. C. 2013. New insights into the determination of HDL structure by apolipoproteins: Thematic review series: high density lipoprotein structure, function, and metabolism. J. Lipid Res. 54: 2034–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kono M., Okumura Y., Tanaka M., Nguyen D., Dhanasekaran P., Lund-Katz S., Phillips M. C., Saito H. 2008. Conformational flexibility of the N-terminal domain of apolipoprotein a-I bound to spherical lipid particles. Biochemistry. 47: 11340–11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito H., Dhanasekaran P., Nguyen D., Holvoet P., Lund-Katz S., Phillips M. C. 2003. Domain structure and lipid interaction in human apolipoproteins A-I and E, a general model. J. Biol. Chem. 278: 23227–23232. [DOI] [PubMed] [Google Scholar]
- 22.Munehira Y., Ohnishi T., Kawamoto S., Furuya A., Shitara K., Imamura M., Yokota T., Takeda S., Amachi T., Matsuo M., et al. 2004. Alpha1-syntrophin modulates turnover of ABCA1. J. Biol. Chem. 279: 15091–15095. [DOI] [PubMed] [Google Scholar]
- 23.Matz C. E., Jonas A. 1982. Micellar complexes of human apolipoprotein A-I with phosphatidylcholines and cholesterol prepared from cholate-lipid dispersions. J. Biol. Chem. 257: 4535–4540. [PubMed] [Google Scholar]
- 24.Sparks D. L., Lund-Katz S., Phillips M. C. 1992. The charge and structural stability of apolipoprotein A-I in discoidal and spherical recombinant high density lipoprotein particles. J. Biol. Chem. 267: 25839–25847. [PubMed] [Google Scholar]
- 25.Vaughan A. M., Oram J. F. 2003. ABCA1 redistributes membrane cholesterol independent of apolipoprotein interactions. J. Lipid Res. 44: 1373–1380. [DOI] [PubMed] [Google Scholar]
- 26.Amundson D. M., Zhou M. 1999. Fluorometric method for the enzymatic determination of cholesterol. J. Biochem. Biophys. Methods. 38: 43–52. [DOI] [PubMed] [Google Scholar]
- 27.Nagao K., Takahashi K., Azuma Y., Takada M., Kimura Y., Matsuo M., Kioka N., Ueda K. 2012. ATP hydrolysis-dependent conformational changes in the extracellular domain of ABCA1 are associated with apoA-I binding. J. Lipid Res. 53: 126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagao K., Hata M., Tanaka K., Takechi Y., Nguyen D., Dhanasekaran P., Lund-Katz S., Phillips M. C., Saito H. 2014. The roles of C-terminal helices of human apolipoprotein A-I in formation of high-density lipoprotein particles. Biochim. Biophys. Acta. 1841: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagao K., Kimura Y., Ueda K. 2012. Lysine residues of ABCA1 are required for the interaction with apoA-I. Biochim. Biophys. Acta. 1821: 530–535. [DOI] [PubMed] [Google Scholar]
- 30.Davidson W. S., Gillotte K. L., Lund-Katz S., Johnson W. J., Rothblat G. H., Phillips M. C. 1995. The effect of high density lipoprotein phospholipid acyl chain composition on the efflux of cellular free cholesterol. J. Biol. Chem. 270: 5882–5890. [DOI] [PubMed] [Google Scholar]
- 31.Sparks D. L., Frank P. G., Braschi S., Neville T. A., Marcel Y. L. 1999. Effect of apolipoprotein A-I lipidation on the formation and function of pre-beta and alpha-migrating LpA-I particles. Biochemistry. 38: 1727–1735. [DOI] [PubMed] [Google Scholar]
- 32.Jayaraman S., Abe-Dohmae S., Yokoyama S., Cavigiolio G. 2011. Impact of self-association on function of apolipoprotein A-I. J. Biol. Chem. 286: 35610–35623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duong P. T., Collins H. L., Nickel M., Lund-Katz S., Rothblat G. H., Phillips M. C. 2006. Characterization of nascent HDL particles and microparticles formed by ABCA1-mediated efflux of cellular lipids to apoA-I. J. Lipid Res. 47: 832–843. [DOI] [PubMed] [Google Scholar]
- 34.Lund-Katz S., Lyssenko N. N., Nickel M., Nguyen D., Chetty P. S., Weibel G., Phillips M. C. 2013. Mechanisms responsible for the compositional heterogeneity of nascent high density lipoprotein. J. Biol. Chem. 288: 23150–23160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nandi S., Ma L., Denis M., Karwatsky J., Li Z., Jiang X. C., Zha X. 2009. ABCA1-mediated cholesterol efflux generates microparticles in addition to HDL through processes governed by membrane rigidity. J. Lipid Res. 50: 456–466. [DOI] [PubMed] [Google Scholar]
- 36.Liu L., Bortnick A. E., Nickel M., Dhanasekaran P., Subbaiah P. V., Lund-Katz S., Rothblat G. H., Phillips M. C. 2003. Effects of apolipoprotein A-I on ATP-binding cassette transporter A1-mediated efflux of macrophage phospholipid and cholesterol: formation of nascent high density lipoprotein particles. J. Biol. Chem. 278: 42976–42984. [DOI] [PubMed] [Google Scholar]
- 37.Massey J. B., Pownall H. J. 2008. Cholesterol is a determinant of the structures of discoidal high density lipoproteins formed by the solubilization of phospholipid membranes by apolipoprotein A-I. Biochim. Biophys. Acta. 1781: 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laffitte B. A., Repa J. J., Joseph S. B., Wilpitz D. C., Kast H. R., Mangelsdorf D. J., Tontonoz P. 2001. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc. Natl. Acad. Sci. USA. 98: 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Goff W., Peng D. Q., Settle M., Brubaker G., Morton R. E., Smith J. D. 2004. Cyclosporin A traps ABCA1 at the plasma membrane and inhibits ABCA1-mediated lipid efflux to apolipoprotein A-I. Arterioscler. Thromb. Vasc. Biol. 24: 2155–2161. [DOI] [PubMed] [Google Scholar]
- 40.Nagao K., Maeda M., Manucat N. B., Ueda K. 2013. Cyclosporine A and PSC833 inhibit ABCA1 function via direct binding. Biochim. Biophys. Acta. 1831: 398–406. [DOI] [PubMed] [Google Scholar]
- 41.Davidson W. S., Thompson T. B. 2007. The structure of apolipoprotein A-I in high density lipoproteins. J. Biol. Chem. 282: 22249–22253. [DOI] [PubMed] [Google Scholar]
- 42.Chetty P. S., Mayne L., Lund-Katz S., Stranz D., Englander S. W., Phillips M. C. 2009. Helical structure and stability in human apolipoprotein A-I by hydrogen exchange and mass spectrometry. Proc. Natl. Acad. Sci. USA. 106: 19005–19010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S., Gulshan K., Brubaker G., Hazen S. L., Smith J. D. 2013. ABCA1 mediates unfolding of apolipoprotein AI N terminus on the cell surface before lipidation and release of nascent high-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 33: 1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mulya A., Lee J. Y., Gebre A. K., Thomas M. J., Colvin P. L., Parks J. S. 2007. Minimal lipidation of pre-beta HDL by ABCA1 results in reduced ability to interact with ABCA1. Arterioscler. Thromb. Vasc. Biol. 27: 1828–1836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.