Abstract
Background:
The incidence of urinary stone disease has shown a steep rise in recent decades along with marked modifications in dietary habits and life- style. There has been an increased prevalence of urinary stone disease in patients with diabetes. We took up this study to determine the association of diabetes mellitus with kidney stones in patients undergoing surgical treatment.
Materials and Methods:
Patients presenting with renal stones for surgical management formed the study group. Body mass index (BMI) was calculated by noting the weight and height of the patient. The extracted stone/stone fragments were analyzed to determine the chemical composition. Urinary pH was similarly noted in all.
Results:
The mean BMI among the diabetics was 26.35 ± 5.20 (range 17.75-35.60), whereas the mean BMI among the non-diabetics was 23.41 ± 2.85 (range 17.71-31.62) (P < 0.0004). The incidence of uric acid calculi in the diabetics was significantly high (P < 0.03). The mean urinary pH among the diabetics was 5.61 ± 0.36 and among the non-diabetics was 6.87 ± 0.32, which was significantly lower (P < 0.000044).
Conclusions:
There is a strong association between type 2 diabetes and uric acid stone formation. There is also a strong association between diabetes mellitus, BMI, and also with lower urinary pH.
Keywords: Diabetes mellitus, renal stones, uric acid stones, urolithiasis
INTRODUCTION
The incidence of urinary stone disease has shown a steep rise in recent decades in all industrialized countries,[1,2] as did the incidence of obesity, the metabolic syndrome, and type 2 diabetes.[3,4,5,6] These epidemiologic changes have occurred along with marked modifications in dietary habits and life- style that occurred in all Western and westernized populations, characterized by a high calorie intake coupled with reduced physical activity.[7,8,9] Similar rise in incidence of urinary stones has been subjectively noted in many Indian centers, though the correct data are not available. This rise in incidence could probably be explained by the association that exists among diabetes, obesity, and urinary stone disease. Two recent studies have revealed an increased prevalence of nephrolithiasis in patients with diabetes mellitus (DM) as compared with patients without diabetes,[10,11] though the exact chemical composition of the stone was not identified. It has not been well defined whether calcium (Ca) or uric acid (UA) stones or both contributed to the increased prevalence of urinary stone disease in patients with diabetes, as alterations in urine biochemistry associated with obesity and type 2 diabetes may favor the formation of UA as well as of Ca stones.[12,13,14,15]
We took up this study to determine the association of diabetes mellitus with kidney stones in patients undergoing surgical treatment.
MATERIALS AND METHODS
All patients presenting with renal stones for surgical management at our hospital formed the study group. A detailed history of these patients was recorded which included residential address, source of water, details of diet, previous history of stones and their treatment. All patients underwent routine blood and urinary examination. Urinary pH was noted with the pH meter and urine was also sent for culture and sensitivity. Imaging of stones was done both with ultrasonography and X-rays. CT (Computed tomography) was done whenever felt necessary. Body mass index [BMI = mass (kg)/(height (m))2] was calculated by noting the weight and height of the patient. All these patients underwent surgical treatment to extract the renal calculi. Surgical procedures included open surgery, PCNL (Percutaneous nephrolithotomy) and RIRS (Retrograde intrarenal surgery). The extracted stone/stone fragments were analyzed to note the chemical composition. Measures of central tendency, standard deviation and Students t-test statistical analysis were done using SPSS software version 16.
RESULTS
During the study period July 2011 to June 2013, 250 patients with renal urolithiasis and 30 patients with diabetes as co-morbidity with renal urolithiasis underwent surgical extraction of the calculi. Among the diabetics 60% were males and 40% were females, whereas among the non-diabetics 70.09% were males and 29.1% were females. The mean age of the diabetics was 50.8 ± 12.5 years and in the non-diabetics was 41.48 ± 11.7 years. The mean BMI among the diabetics was 26.35 ± 5.21 (range 17.75-35.60), whereas the mean BMI among the non-diabetics was 23.40 ± 2.85 (range 17.71-31.62). There was a significant difference in the BMI between these groups (P < 0.0004).
Nearly all of the stones were mixed stones; however the dominant component such as calcium and uric acid was noted to classify the stones. Among the diabetics, 70% of the stones were calcium oxalate [Figure 1] and 30% were uric acid calculi [Figure 2], whereas among the non-diabetics 89% of the stones were calcium stones and 11% were uric acid calculi. The incidence of uric acid calculi in the diabetics was significantly high (P < 0.03). The mean urinary pH among the diabetics was 5.61 ± 0.34 and among the non-diabetics was 6.87 ± 0.32, which was significantly lower (P < 0.0000). The small sample size is the major limitation of this study.
Figure 1.
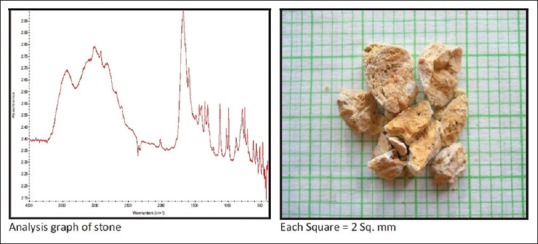
Uric acid calculi
Figure 2.
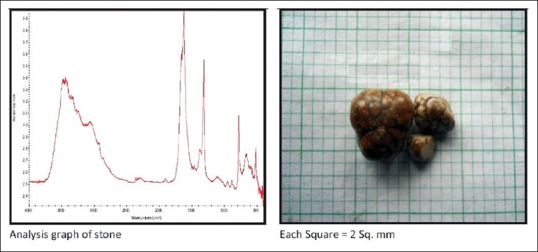
Calcium oxalate calculi
DISCUSSION
Urolithiasis is a major cause of morbidity worldwide. Over $2 billion is spent annually toward the treatment of stone disease in United States alone.[16,17] Identifying common systemic diseases that increase the risk of renal stone formation would help in preventing the occurrence as well as the recurrence of these stones. It is well known that Type 2 diabetes mellitus is characterized by insulin resistance, a metabolic derangement that may increase the risk of kidney stone formation.[18] Insulin resistance is associated with defects in renal ammonium production,[19,20] and stone formers with diabetes may have more acidic urine than stone formers without diabetes.[21] Although a low urinary pH plays a major role in the formation of uric acid kidney stones,[22,23] a defect in renal acid excretion also could lead to hypocitraturia, an important risk factor for calcium stones.[24,25] In addition, the compensatory hyperinsulinemia of insulin resistance[18] may increase the urinary excretion of calcium.[26,27,28]
Despite the compelling evidence of insulin resistance on urine composition, data on the potential association between diabetes and nephrolithiasis are sparse. Meydan et al. reported that the prevalence of stone disease in subjects with diabetes was 21%, compared to 8% (P < 0.05) in non-diabetic controls.[10] Family history and male gender were significant risk factors for the development of urinary stones in the diabetic patients. Alcohol consumption was a significant risk factor for the development of urinary stones in diabetics (odds ratio 3.68; 95% confidence interval 1.29-10.45; P < 0.05). However, this study did not adjust for body mass index, an important risk factor for both diabetes and nephrolithiasis.[15] Very few prospective studies have evaluated the association between diabetes mellitus and the risk of kidney stones. Taylor et al.[11] evaluated the relation between DM and prevalent kidney stones, in a cross-sectional study of three large cohorts including over 200,000 participants: The Nurses’ Health Study I (older women), the Nurses’ Health Study II (younger women), and the Health Professionals Follow-up Study (men). They also prospectively studied the association between DM and incident nephrolithiasis over a combined 44 years of follow-up. They reported that at baseline, the multivariate relative risk of prevalent stone disease in individuals with DM compared to individuals without was 1.38 (95% CI 1.06-1.79) in older women, 1.67 (95% CI 1.28-2.20) in younger women, and 1.31 (95% CI 1.11-1.54) in men. Prospectively, the multivariate relative risk of incident kidney stone formation in participants with DM compared to participants without was 1.29 (95% CI 1.05-1.58) in older women, 1.60 (95% CI 1.16-2.21) in younger women, and 0.81 (95% CI 0.59-1.09) in men. The multivariate relative risk of incident DM in participants with a history of kidney stones compared to participants without was 1.33 (95% CI 1.18-1.50) in older women, 1.48 (95% CI 1.14-1.91) in younger women, and 1.49 (95% CI 1.29-1.72) in men. They concluded that DM was a risk factor for the development of kidney stones, independent of age, BMI, thiazide diuretic use, and diet.
There has been a general hypothesis that type 2 diabetes may be associated with an increased risk for formation of uric acid (UA) stones. Insulin resistance, characteristic of the metabolic syndrome and type 2 diabetes results in lower urine pH through impaired kidney ammoniagenesis. A low urine pH is the main factor of uric acid (UA) stone formation, and it is for this reason that type 2 diabetes should favor the formation of UA stones.
Our study shows an increased incidence of uric acid calculi among the diabetics as compared to non-diabetics (P < 0.03). Daudon et al.[29] analyzed a series of 2464 calculi from 272 (11%) patients with type 2 diabetes and 2192 without type 2 diabetes. The proportion of UA stones was 35.7% in patients with type 2 diabetes and 11.3% in patients without type 2 diabetes (P < 0.0001). Reciprocally, the proportion of patients with type 2 diabetes was significantly higher among UA than among calcium stone formers (27.8 versus 6.9%; P < 0.0001). Stepwise regression analysis identified type 2 diabetes as the strongest factor that was independently associated with the risk for UA stones (odds ratio 6.9; 95% confidence interval 5.5 to 8.8). The proper influence of type 2 diabetes was the most apparent in women and in patients in the lowest age and body mass index classes. The authors concluded that their data provided epidemiologic evidence that type 2 diabetes was significantly associated with an increased risk for UA stone formation, because the proportion of UA stones was strikingly higher in stone formers with than without diabetes. These findings suggest that UA nephrolithiasis should be considered as possibly reflecting a state of insulin resistance rather than simply UA stone formation. Accordingly, onset of UA nephrolithiasis in a patient should prompt a check for type 2 diabetes and the components of the metabolic syndrome, especially in overweight patients.
Cameron et al.[30] took up a study to identify the metabolic features that placed patients with type 2 diabetes at increased risk for uric acid nephrolithiasis. Three groups of individuals were recruited for this outpatient study: Patients who had type 2 diabetes and were not stone formers (n = 24), patients who did not have diabetes and were uric acid stone formers (UASF; n = 8), and normal volunteers (NV; n = 59). Participants provided a fasting blood sample and a single 24-h urine collection for stone risk analysis. Twenty-four hour urine volume and total uric acid did not differ among the three groups. Patients with type 2 diabetes and UASF had lower 24-h urine pH than NV. Urine pH inversely correlated with both body weight and 24-h urine sulfate in all groups. Urine pH remained significantly lower in patients with type 2 diabetes and UASF than NV after adjustment for weight and urine sulfate (P < 0.01). Our study too revealed a lower urinary pH among diabetics as compared to non-diabetics (P < 0.000). For a given urine sulfate, urine net acid excretion tended to be higher in patients with type 2 diabetes versus NV. With increasing urine sulfate, NV and patients with type 2 diabetes had a similar rise in urine ammonium, whereas in UASF, ammonium excretion remained unchanged. The authors concluded that the main risk factor for uric acid nephrolithiasis in patients with type 2 diabetes was a low urine pH. Higher body mass and increased acid intake could contribute to but cannot entirely account for the lower urine pH in patients with type 2 diabetes.
CONCLUSIONS
There is a strong association between type 2 diabetes and uric acid stone formation. There is also a strong association between diabetes mellitus, BMI, and also with lower urinary pH. It is proposed that uric acid urolithiasis may be added to the conditions that potentially are associated with insulin resistance. It would also make it necessary to screen all patients with uric acid urolithiasis, especially if overweight for type 2 diabetes or components of the metabolic syndrome.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Trinchieri A, Coppi F, Montanari E, Del Nero A, Zanetti G, Pisani E. Increase in the prevalence of symptomatic upper urinary tract stones during the last ten years. Eur Urol. 2000;37:23–5. doi: 10.1159/000020094. [DOI] [PubMed] [Google Scholar]
- 2.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int. 2003;63:1817–23. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288:1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 5.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 6.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen SJ, Popkin BM. Patterns and trends in food portion sizes, 1977-1998. JAMA. 2003;289:450–3. doi: 10.1001/jama.289.4.450. [DOI] [PubMed] [Google Scholar]
- 8.Després JP. Our passive lifestyle, our toxic diet, and the atherogenic/diabetogenic metabolic syndrome: Can we afford to be sedentary and unfit? Circulation. 2005;112:453–5. doi: 10.1161/CIRCULATIONAHA.105.553289. [DOI] [PubMed] [Google Scholar]
- 9.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, et al. Origins and evolution of the Western diet: Health implications for the 21st century. Am J Clin Nutr. 2005;81:341–54. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 10.Meydan N, Barutca S, Caliskan S, Camsari T. Urinary stone disease in diabetes mellitus. Scand J Urol Nephrol. 2003;37:64–70. doi: 10.1080/00365590310008730. [DOI] [PubMed] [Google Scholar]
- 11.Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005;68:1230–5. doi: 10.1111/j.1523-1755.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 12.Curhan GC, Willett WC, Rimm EB, Speizer FE, Stampfer MJ. Body size and risk of kidney stones. J Am Soc Nephrol. 1998;9:1645–52. doi: 10.1681/ASN.V991645. [DOI] [PubMed] [Google Scholar]
- 13.Powell CR, Stoller ML, Schwartz BF, Kane C, Gentle DL, Bruce JE, et al. Impact of body weight on urinary electrolytes in urinary stone formers. Urology. 2000;55:825–30. doi: 10.1016/s0090-4295(99)00617-2. [DOI] [PubMed] [Google Scholar]
- 14.Siener R, Glatz S, Nicolay C, Hesse A. The role of overweight and obesity in calcium oxalate stone formation. Obes Res. 2004;12:106–13. doi: 10.1038/oby.2004.14. [DOI] [PubMed] [Google Scholar]
- 15.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455–62. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 16.Pearle MS, Calhoun EA, Curhan GC. Urolithiasis. In: Litwin M, Saigal C, editors. Urologic Diseases in America. United States Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Washington, DC: US Government Printing Office; 2004. pp. 3–42. [Google Scholar]
- 17.Lingeman JE, Saywell RM, Jr, Woods JR, Newman DM. Cost analysis of extracorporeal shock wave lithotripsy relative to other surgical and nonsurgical treatment alternatives for urolithiasis. Med Care. 1986;24:1151–60. doi: 10.1097/00005650-198612000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Beck-Nielsen H, Groop LC. Metabolic and genetic characterization of prediabetic states. Sequence of events leading to non-insulin-dependent diabetes mellitus. J Clin Invest. 1994;94:1714–21. doi: 10.1172/JCI117518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakhaee K, Adams-Huet B, Moe OW, Pak CY. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002;62:971–9. doi: 10.1046/j.1523-1755.2002.00508.x. [DOI] [PubMed] [Google Scholar]
- 20.Abate N, Chandalia M, Cabo-Chan AV, Jr, Moe OW, Sakhaee K. The metabolic syndrome and uric acid nephrolithiasis: Novel features of renal manifestation of insulin resistance. Kidney Int. 2004;65:386–92. doi: 10.1111/j.1523-1755.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 21.Pak CY, Sakhaee K, Moe O, Preminger GM, Poindexter JR, Peterson RD, et al. Biochemical profile of stone-forming patients with diabetes mellitus. Urology. 2003;61:523–7. doi: 10.1016/s0090-4295(02)02421-4. [DOI] [PubMed] [Google Scholar]
- 22.Asplin JR. Uric acid stones. Semin Nephrol. 1996;16:412–24. [PubMed] [Google Scholar]
- 23.Riese RJ, Sakhaee K. Uric acid nephrolithiasis: Pathogenesis and treatment. J Urol. 1992;148:765–71. doi: 10.1016/s0022-5347(17)36715-0. [DOI] [PubMed] [Google Scholar]
- 24.Hamm LL. Renal handling of citrate. Kidney Int. 1990;38:728–35. doi: 10.1038/ki.1990.265. [DOI] [PubMed] [Google Scholar]
- 25.Coe FL, Parks JH, Asplin JR. The pathogenesis and treatment of kidney stones. N Engl J Med. 1992;327:1141–52. doi: 10.1056/NEJM199210153271607. [DOI] [PubMed] [Google Scholar]
- 26.Kerstetter J, Caballero B, O’Brien K, Wurtman R, Allen L. Mineral homeostasis in obesity: Effects of euglycemic hyperinsulinemia. Metabolism. 1991;40:707–13. doi: 10.1016/0026-0495(91)90088-e. [DOI] [PubMed] [Google Scholar]
- 27.Shimamoto K, Higashiura K, Nakagawa M, Masuda A, Shiiki M, Miyazaki Y, et al. Effects of hyperinsulinemia under the euglycemic condition on calcium and phosphate metabolism in non-obese normotensive subjects. Tohoku J Exp Med. 1995;177:271–8. doi: 10.1620/tjem.177.271. [DOI] [PubMed] [Google Scholar]
- 28.Nowicki M, Kokot F, Surdacki A. The influence of hyperinsulinaemia on calcium-phosphate metabolism in renal failure. Nephrol Dial Transplant. 1998;13:2566–71. doi: 10.1093/ndt/13.10.2566. [DOI] [PubMed] [Google Scholar]
- 29.Daudon M, Traxer O, Conort P, Lacour B, Jungers P. Type 2 diabetes increases the risk for uric acid stones. J Am Soc Nephrol. 2006;17:2026–33. doi: 10.1681/ASN.2006030262. [DOI] [PubMed] [Google Scholar]
- 30.Cameron MA, Maalouf NM, Adams-Huet B, Moe OW, Sakhaee K. Urine composition in type 2 diabetes: Predisposition to uric acid nephrolithiasis. J Am Soc Nephrol. 2006;17:1422–8. doi: 10.1681/ASN.2005121246. [DOI] [PubMed] [Google Scholar]


