Abstract
Objective:
To describe a cluster of progressive supranuclear palsy (PSP) in northern France. PSP has not been reported in geographical, temporal, or occupational clusters. A unit of Neurology and Neurogeriatrics opened in 2005 at the Centre Hospitalier de Wattrelos, serving the population of Wattrelos and Leers (combined population 51,551) and parts of neighboring towns. For most of the 20th century, this area was a center for chromate and phosphate ore processing, textile dyeing, and tanning. Significant industrial waste persists close to residential areas.
Methods:
From 2005 to 2014, 92 patients with PSP at Centre Hospitalier de Wattrelos were identified and studied. Detailed residential data were available in the medical records. Eighty cases have had magnetic resonance head scanning and 60 have died, of whom 13 have been examined neuropathologically.
Results:
The ratio of observed to expected PSP incidence over the period 2005 to 2012 was 12.3 (95% confidence interval: 7.4–35.9). Mean onset age was 74.3 years. The Richardson syndrome/PSP-parkinsonism ratio was 43%/42%. Four other phenotypes each occurred in 2% to 5%. Onset was gait/balance difficulty in 52%. None of the 92 affected patients were relatives and 7 were of North African ancestry. MRI was compatible with a clinical diagnostic of PSP in all cases. Histopathologic examination confirmed neurofibrillary degeneration and tufted astrocytes in all autopsied cases. Western blots revealed a typical tau 4R doublet. The tau H1 haplotype occurred in 95.8% of cases' chromosomes.
Conclusions:
We have identified a cluster of PSP in a geographical area with severe environmental contamination by industrial metals.
Progressive supranuclear palsy (PSP) is an uncommon sporadic disorder with no reports in the literature of geographical clusters. Its incidence in developed countries is approximately 1.2 per 100,000 per year and the prevalence of investigator-diagnosed cases is 5 to 6 per 100,000.1–4
The cause is unknown but several familial clusters have now been reported.5,6 Olfaction, affect, and reaction time assessments revealed more frequent dysfunction among relatives of individuals with PSP than among relatives of controls.7
Genetic variations in MAPT locus are a mild risk factor for PSP.8 An inversion of a 900-kb span of chromosome 17 defines the H1 haplotype.9–11 A subhaplotype of H1 designated H1c is responsible for the association between the H1 haplotype and PSP.12,13
Studies of nongenetic risk factors have found lesser educational attainment.14–16 Two geographical clusters of illnesses similar to PSP have been described on Guam17–20 and in Guadeloupe where a case-control survey21 revealed a strong association (odds ratio 8.3, p < 0.001) of Guadeloupean tauopathy with consumption of traditional medicines containing annonacin,22 a mitochondrial toxin that produces a tauopathy in a rodent model.23
Wattrelos is a town in northern France where textile dyeing plants and tanneries operated for most of the 20th century, using arsenic and chromate from the nearby chemical plants. Arsenic and hexavalent chromium contamination have been documented in the soils of the sites of former plants in Wattrelos and Leers,24 where the cluster of PSP cases described in this report live.
METHODS
Patients.
Wattrelos has one hospital, the Centre Hospitalier de Wattrelos (CHW). It is the only medical facility in the town and has an acute and chronic inpatient and outpatient service. In 2005, a Department of Geriatric Neurology was established in the hospital when one of the authors (D.C.-L.) was appointed to the staff. In April 2007, she diagnosed the first patient with PSP at CHW. By July 2014, 92 patients, all of whom had fulfilled the National Institute of Neurological Disorders and Stroke–Society for PSP criteria for clinically probable PSP, had been studied.25 Most patients manifested many of the additional supportive criteria for PSP including abnormal neck posture, signs of spastic face, poor or absent response of bradykinesia and axial and bulbar signs to levodopa therapy, early dysphagia and dysarthria, and early cognitive impairment with at least 2 of the following: apathy, abstract thought impairment, decreased verbal fluency, imitation behavior, or frontal release signs. Eighty-nine patients (97%) were referred by primary care physicians and 3 (3%) by neurologists or physiotherapists.
Clinical and imaging study.
Each patient received a trial of carbidopa/levodopa with evaluations at 3 months and 6 months using the Unified Parkinson's Disease Rating Scale, MRI, videotape records, olfactory testing, Folstein Mini-Mental State Examination, and detailed neuropsychological examination for patients with Mini-Mental State Examination score >19 of 30. The neuropsychological tests comprised the Grober and Buschke test,26 Frontal Assessment Battery,27 Cognitive, Trail Making Test (TMT), Stroop, images description (DO 80), and praxis analysis. Eye and eyelid movements were examined. Saccades, antisaccades, pursuit, and opticokinetic nystagmus (OKN) were tested at the first examination. OKN was evaluated using a handheld OKN tape. Command saccades were considered slow if the progress of the movement in response to direct the eyes to a peripheral target was perceptible to the examiner.
With assistance from family members, we recorded each residential street address since birth, occupations, job descriptions, and hobbies.
Olfactory testing was performed using the European Test of Olfactory Capacities.28 A speech therapist analyzed buccofacial apraxia29 and language (Boston Naming Test). Brain MRI study was performed in all patients except in the 12 with medical contraindications, in whom a CT was undertaken.
Standard protocol approvals, registrations, and patient consents.
We received approval from the institutional ethical standards committee on human experimentation, and we received written informed consent from all patients (none had guardians).
Epidemiologic study.
The rationale for defining the temporal limits of the cluster analysis to the period 2005 to 2012 is that before author D.C.-L. arrived at CHW in 2005, some cases with PSP would likely have been misdiagnosed. She diagnosed her first case of PSP in Wattrelos in 2007. We censored the incidence analysis in 2012 because cases with onset since then are unlikely to have reached clinical attention to date.
To calculate the expected incidence of PSP, we used the entire population of both Wattrelos and Leers although residents of those towns have the option of seeking neurologic care at the university center in Lille and elsewhere. This may have led us to underestimate the PSP incidence in those 2 towns despite the reports of neurologists in nearby towns that they saw little or no PSP. We obtained population data for 2006 and 2011 and used the mean of these figures to make our calculations. For the expected incidence, we averaged the results of all 4 formal studies of PSP frequency to date,1–4 arriving at a figure of approximately 1.2 cases per 100,000 per year. We did not age-adjust because the proportion of the Wattrelos-Leers population aged 60 years and older is similar to that in the areas studied in the 4 surveys.
Pathologic study.
Thirteen postmortem brains were obtained with consent from the patients' families. The left half was fixed in formaldehyde and the right was frozen at −80°C for biochemical and genetic study. Microscopic examination was performed on paraffin-embedded material after staining with hematoxylin and eosin, ubiquitin, α-synuclein, Gallyas silver, and anti-tau (polyclonal antibody; Dako, Carpinteria, CA; raised against the C-terminal part of the recombinant human protein, including the 4 repeats).
Biochemical study.
Frozen brain samples from the mesencephalon and Brodmann areas (BAs) 4, 10, and 18 were processed as previously described.30 Brain homogenates containing 30 μg of total proteins were loaded on sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Western blot analysis was processed using anti-pSer396, a monoclonal antibody against phosphorylated serine 396 on tau proteins (dilution 1:10,000; Invitrogen, Carlsbad, CA). Phosphorylation-independent antibody anti-tau Cter, directed against the last amino acids of tau sequence, was also used at the dilution of 1:10,000.31
Molecular genetic study.
DNA was prepared from frontal and occipital cortices by phenol-chloroform extraction. MAPT H1/H2 genotyping was performed on brain samples using the 238–base pair insertion/deletion polymorphism in intron 9 as described by Baker et al.9 Genotyping of SNP rs42557 was performed by standard PCR reaction and sequencing on an ABI 3130XL DNA analyzer (Applied Biosystems, Foster City, CA). Primers are available upon request.
RESULTS
Epidemiologic data.
We compiled a detailed clinical database of the 92 patients identified since 2007 as having PSP and living in Wattrelos, Leers, and nearby towns. The incidence of PSP elsewhere is 1.2 cases/105/y.1–4 The population of Wattrelos in 2011 was 41,538.32 The catchment area for its hospital includes all of that town and most of Leers, immediately to the southeast, with a 2011 population of 9,343,33 and smaller parts of other towns. Of the 92 incident PSP cases, 62 were living in Wattrelos or Leers at the time of diagnosis and experienced symptom onset between January 1, 2005, and December 31, 2012.
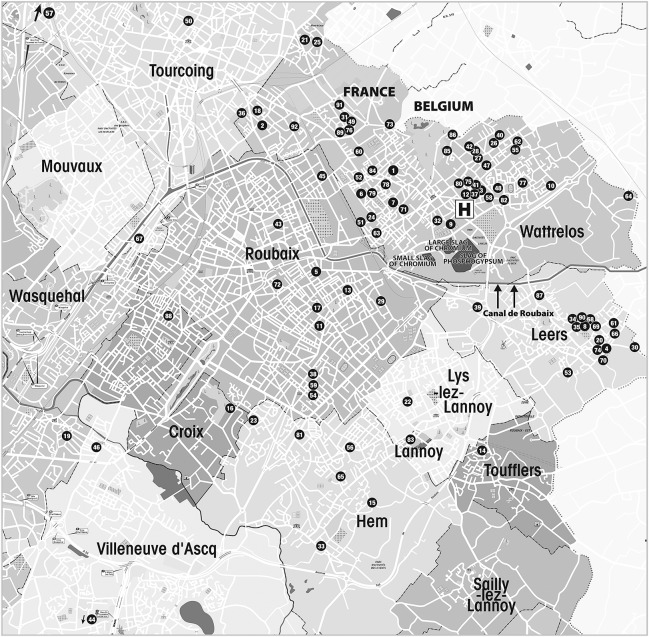
The geography of the cluster appears to be limited largely to Wattrelos and Leers. Inquiries of neurologists at the University of Lille, the closest referral center, revealed only the expected very low frequency of patients with PSP. The places of residence of the 92 patients at the time of PSP onset appear in the figure along with the hospital where they were diagnosed, the slag heaps, and the canal that receives runoff from the heaps. The numbers correspond to the patients' names in alphabetical order.
Figure. Places of residence of the 92 patients at the time of PSP onset.
Map showing the places of residence of the 92 patients at the time of PSP onset, along with the hospital where they were diagnosed, the slag heaps, and the canal that receives runoff from the heaps. The numbers correspond to the patients' names in alphabetical order. PSP = progressive supranuclear palsy.
The total population of Wattrelos and Leers averaged between 2006 and 2011 was 51,511. This gave an at-risk figure for the 8-year observation period of 412,088 person-years, for an expected absolute number of PSP cases of 4.95 over that period. As the observed number of incident cases was 62, the observed to expected (O/E) ratio is 12.3. The 95% confidence interval of the O/E ratio may be estimated by comparison with the interval of 2 to 10 for the prevalence figure (approximately 6 per 100,000) on which the incidence figures were calculated. This would give an interval of 0.4 to 2.0 for the incidence figure of 1.2/100,000/y and yield a 95% confidence interval for the O/E ratio of 7.4 to 35.9.
Clinical features of the patients.
For the 92 patients ascertained from April 2007 to July 2014, the male to female sex ratio was 1.11, with 43 women. The mean age at onset was 74.3 years (SD 7.89, range 50–93) (women, 75.9 years; men, 71.9 years). Onset age differed slightly between PSP in this cluster and sporadic PSP in New Jersey34 (67, SD 7.3 years) but was similar to the onset age in the UK study.2 The fraction of the population aged 60 years and older does not differ between Wattrelos (16.6%) and other studies. For the 60 patients known to be deceased, disease duration was 5.74 years (SD 2.2, range 2–12). At the first examination, phenotypic variants included Richardson syndrome in 30 patients (33%), PSP-parkinsonism in 49 (53%), progressive aphasia with PSP in 2 (2%), speech apraxia with PSP in 5 (5.4%), pure akinesia with gait freezing (PAGF) in 3 (3%), and frontotemporal dementia with PSP in 3 (3%).
The first symptom was gait or balance problems in 52%, very close to the 60% in the literature for PSP, axial and/or symmetric parkinsonism in 21%, cognitive decline in 14%, speech disorders in 8%, tremor in 2%, and visual difficulties in 1%. Eighty-nine percent reported falls at the first examination. Seventy-seven percent of patients experienced backward falls. All patients had prominent body bradykinesia and axial rigidity. Twenty percent had rest tremor. At first examination, vertical saccadic eye movements were slow in 85%, and down gaze was absent in 15%. Fifteen percent had square wave jerks.
As the disease progressed (3 years' follow-up), the ratio of phenotypic variants changed, and 40 patients (43%) had Richardson syndrome while 39 patients (42%) had PSP-parkinsonism. At the late stage of the disease, all patients fulfilled ocular motor criteria for PSP with restriction of vertical eye motion higher than 50% and slow vertical saccadic eye movements. Forty-three patients had complete vertical gaze palsy as demonstrated in the video on the Neurology® Web site at Neurology.org.
In patients with Richardson syndrome, eyelid symptoms, including eyelid apraxia and blink frequency below 1/min were present in all patients after 3 years of disease progression. In PSP-parkinsonism, eyelid motion was reduced but preserved, and blepharospasm was more common and seen in 5 patients. Fifty-six patients had eyelid apraxia (61%), 40 with Richardson syndrome (100%) and 16 with PSP-parkinsonism (40%). OKN quick phases were absent in all patients and antisaccades were impaired in all patients.
Only one patient experienced a significant and sustained response to carbidopa/levodopa, with a 40% improvement in the Unified Parkinson's Disease Rating Scale, during 3 years. The lack of levodopa responsiveness was obvious in all cases but one.
This cluster differs in its distribution of PSP's major clinical subtypes, Richardson syndrome and PSP-parkinsonism (43%:42% vs 54%:32%,35 respectively).
Thirteen patients to date have come to autopsy, 9 with Richardson syndrome (66%), 2 with speech apraxia (15%), one with PSP-parkinsonism, and one with PAGF.
Initial cognitive testing showed impaired episodic memory in 84 patients (91%) and biographic memory in 76 patients (83%). The Frontal Assessment Battery was performed in 47 patients, with mean score (of best possible 18) of 10.3 (SD 3.88). We administered the Grober and Buschke test to the 28 patients able to perform it. It showed reduced free recall (mean: 6.5 words/16; SD 2.5) and preserved cued remembering (mean: 14.5/16; SD 2). The mean results for TMT A was 83 seconds (SD 34; below 50th percentile) and for TMT B was 241 seconds (SD 120; 25th percentile 25). Patients had a mean of 2 errors on the Stroop test. Olfactory testing showed severe hyposmia in all patients, with a mean identification of 5 smells out of 16.
None of our patients or surviving relatives endorsed a family history of PSP. Family history of neurologic disorders was claimed by 13 patients (14%): dementia in 9 (10%) and parkinsonism in 4 (4%). Seven (8%) of our patients were of North African ethnic origin and were born in Algeria.
MRI was performed in 80 patients (87%). T1-weighted axial images showed midbrain atrophy in all cases, with loss of convexity of the lateral margin of the midbrain tegmentum, and widened interpeduncular angle. Thinning of the tectum and atrophy of the superior colliculi were common. A hummingbird sign was obvious in patients with Richardson syndrome, with normal pontine diameter.
Pathology.
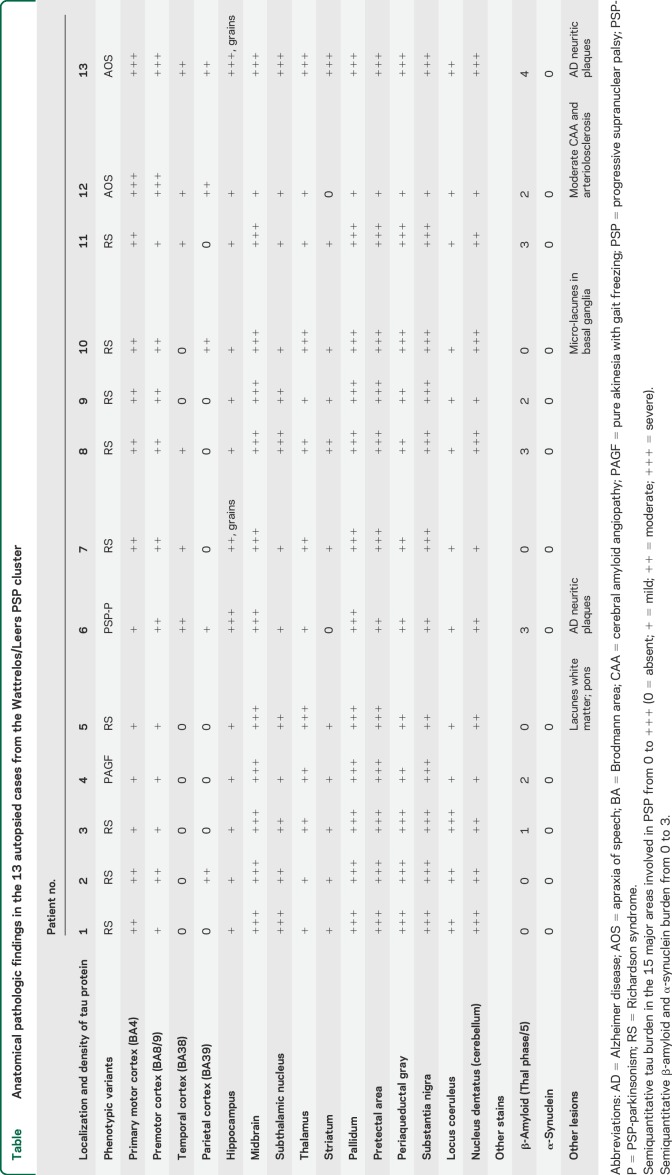
All 13 autopsied cases satisfied neuropathologic criteria for definite PSP.36 Macroscopic examination showed global atrophy disproportionately affecting brainstem. Microscopic study (table) showed severe neuronal loss involving substantia nigra and locus coeruleus. AD2 antibody showed diffuse tau protein deposits. Globoid neurofibrillary tangles and tufted astrocytes were prominent in the substantia nigra, subthalamic nucleus, locus coeruleus, midbrain tegmentum, cerebellar nuclei, cerebellar cortex, pallidum, and bulb. Neurofibrillary tangles were less abundant in entorhinal, temporal, and primary motor cortices. Tau deposits were less abundant in the subthalamic nucleus in 2 patients with PSP-parkinsonism and PAGF.
Table.
Anatomical pathologic findings in the 13 autopsied cases from the Wattrelos/Leers PSP cluster
In 2 patients with PSP and speech apraxia phenotype, neurofibrillary tangles were prominent in motor cortex and fronto-opercular cortex. In one patient with mild cognitive impairment, neurofibrillary tangles involved mainly the subcortical areas. Moderate β-amyloid deposition was observed in hippocampal cortex and frontal and motor cortex in older patients. It was observed in 8 brains (61%): moderate in 1 (8%) and more pronounced in 7 (53%). No brain revealed α-synuclein staining.
Vascular lesions were additionally seen in 3 patients (23%), and consisted of vascular lacunes, which appeared as hyperintensities in the white matter on fluid-attenuated inversion recovery MRI sequences, in 2 patients. The extent, intensity, and location of those lesions do not suggest that they would explain the neurologic findings that supported the diagnosis of PSP. One patient had a contraindication to MRI and correlations with pathology were not available.
Biochemical results.
Immunoblotting analysis of the different affected brain regions (mesencephalon and primary motor cortex [BA4]) of the first 12 autopsied PSP cases revealed, using the phosphorylation-dependent antibody anti-pSer396, the typical tau electrophoretic profile consisting of a main doublet at 64 and 69 kDa. However, heterogeneity in the signal intensity was observed among the different brain regions. Occipital cortex was never affected whereas frontal areas (BA10) were differentially affected among PSP cases. These findings were consistent with neuropathologic data obtained with AD2 antibodies (the latter is similar to pSer396 antibody).
Genetic results.
Of the 24 chromosome 17s in the first 12 autopsied cases, 23 (96%) bore the H1 haplotype. The frequency of allele A of SNP rs242557 occurred in 66.7%. All of the frequencies are similar to those previously described in PSP10–13 with an overrepresentation of the H1 haplotype and H1c subhaplotype.
DISCUSSION
Investigations to date suggest that this cluster of PSP is centered on Wattrelos and Leers, with very few patients living more than a few kilometers away. Some of the patients who died or discontinued medical care at CHW before the arrival of the first author at the hospital are likely to have been misdiagnosed as Parkinson disease or diffuse cerebrovascular disease.
The distribution of the residences shows high concentration of patients in the eastern part of Leers. A planned further study will include places of residence at multiple time points preceding PSP onset and equivalent time points for controls.
The pathologic and biochemical features of the 13 autopsied cases were typical of PSP. As reported by Williams et al.,35 tau burden was less important in the subthalamic nucleus in patients with PSP-parkinsonism and PAGF.
Vascular lacunes were observed in 3 cases of Richardson syndrome, but were judged to have no conclusive influence on the clinical picture with the possible exception of cognition.
The cause of the cluster will be the subject of subsequent studies. Suspicion must first be directed at the site just south of the center of Wattrelos, where large heaps of spent ore (slag) from now-defunct metals extraction industry are located. Other locations in the immediate area may have been contaminated by the numerous textile-dyeing and leather-tanning plants that used metals in their processes. Many residents of the area raise fruits and vegetables in vegetable gardens and allotments for family consumption and for sale at local markets. Some plant products such as thyme commonly grown in home gardens in Wattrelos are consumed by its residents and have been found to concentrate arsenic and other toxic metals.37
Arsenic, a potential neurotoxin, has been found in the soil at the sites of the former chemical plants in Wattrelos24 and is present in the phosphate ore formerly processed there, as described also in Pakistan.38 Contamination with chromium is common in Wattrelos, but we consider it to be a less likely candidate as a neurotoxin although a synergistic toxicity with arsenic is possible. Aluminum, suspected in the past of a causal link to Alzheimer disease, was not processed in this area.
A genetic founder effect must be considered. None of the patients knew of a relative with a diagnosis of PSP. The proportion with dementia or parkinsonism among family members was no greater than expected, but conclusions await a formal case-control survey. The genetic analysis of the most important genetic risk factors (haplotype H1 and subhaplotype H1c of MAPT) showed the expected distribution in the 12 autopsied cases. A future study should compare cases and local controls for genetic markers known to be associated with PSP and also seek still-unsuspected genomic and epigenetic etiologies using more advanced methods.
The pathology of PSP has similarities with postencephalitic parkinsonism although the clinical picture is very different. Our informal inquiries and our access to medical records failed to reveal evidence of any epidemic of encephalitis or of a history of such illness in any of the affected individuals.
If the cause of this cluster can be found, it will provide an important clue to the cause of PSP elsewhere and, by implication, a possible causative factor for other tauopathies.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge with their permission Caroline Destombes and Nathalie De Meulemeester Clorennec from RAVET-ANCEAU Department of Cartography and Infography; Patrice Gelé, engineer in charge of the brain bank in Lille; Sophie Lamballais, neuropsychologist at CHW; and Florence Levasseur, speech therapist. The authors thank John C. Steele, MD, for allowing the authors to produce the video from his examination of one of their patients with PSP.
GLOSSARY
- BA
Brodmann area
- CHW
Centre Hospitalier de Wattrelos
- O/E
observed to expected
- OKN
opticokinetic nystagmus
- PAGF
pure akinesia with gait freezing
- PSP
progressive supranuclear palsy
- TMT
Trail Making Test
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
D. Caparros-Lefebvre was involved in analysis and interpretation of data, drafting and revising the manuscript. L.I. Golbe was involved in analysis and interpretation of data, drafting and revising the manuscript. V. Deramecourt was involved in analysis and interpretation of data (neuropathology). C.-A. Maurage was involved in analysis and interpretation of data (neuropathology). V. Huin was involved in analysis and interpretation of data (genotyping analysis). L. Buée was involved in analysis and interpretation of data (Western blotting). V. Buée-Scherrer was involved in analysis and interpretation of data, and revising the manuscript for intellectual content. H. Obriot was involved in analysis and interpretation of data (Western blotting). B. Sablonnière was involved in analysis and interpretation of data, and revising the manuscript for intellectual content. F. Caparros was involved in analysis of data (MRI). A.J. Lees was involved in revising the manuscript for intellectual content.
STUDY FUNDING
The authors are grateful for financial support from the Movement Disorders Research Fund of Rutgers University.
DISCLOSURE
D. Caparros-Lefebvre reports no disclosures relevant to the manuscript. L. Golbe reports that he received grants from the Movement Disorders Research Fund of Rutgers Robert Wood Johnson Medical School (LIG), the Rainwater Charitable Foundation, and from a Center of Excellence grant from the American Parkinson's Disease Association (LIG). V. Deramecourt, C. Maurage, V. Huin, V. Buée-Scherrer, H. Obriot, B. Sablonnière, F. Caparros, L. Buée, and A. Lees report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Schrag A, Ben-Shlomo Y, Quinn N. Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet 1999;354:1771–1772. [DOI] [PubMed] [Google Scholar]
- 2.Nath U, Ben-Shlomo Y, Thomson RG, et al. The prevalence of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) in the UK. Brain 2001;124:1438–1449. [DOI] [PubMed] [Google Scholar]
- 3.Bower JH, Maraganore DM, McDonnell SK, Rossa WA. Incidence of progressive supranuclear palsy and multiple system atrophy in Olmsted County, Minnesota, 1976 to 1990. Neurology 1997;49:1284–1288. [DOI] [PubMed] [Google Scholar]
- 4.Kawashima M, Miyake M, Kusumi M, Adachi Y, Nakashima K. Prevalence of progressive supranuclear palsy in Yonago, Japan. Mov Disord 2004;19:1239–1240. [DOI] [PubMed] [Google Scholar]
- 5.Rojo A, Pernaute RS, Fontán A, et al. Clinical genetics of familial progressive supranuclear palsy. Brain 1999;122:1233–1245. [DOI] [PubMed] [Google Scholar]
- 6.Fujioka S, Algom AA, Murray ME, et al. Similarities between familial and sporadic autopsy-proven progressive supranuclear palsy. Neurology 2013;80:2076–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker KB, Montgomery EB., Jr Performance on the PD test battery by relatives of patients with progressive supranuclear palsy. Neurology 2001;56:25–30. [DOI] [PubMed] [Google Scholar]
- 8.Höglinger GU, Melhem NM, Dickson DW, et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet 2011;43:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker M, Litvan I, Houlden H, et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet 1999;8:711–715. [DOI] [PubMed] [Google Scholar]
- 10.Pittman AM, Myers AJ, Duckworth J, et al. The structure of the tau haplotype in controls and in progressive supranuclear palsy. Hum Mol Genet 2004;13:1267–1274. [DOI] [PubMed] [Google Scholar]
- 11.Houlden H, Baker M, Morris HR, et al. Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology 2001;56:1702–1706. [DOI] [PubMed] [Google Scholar]
- 12.Rademakers R, Melquist S, Cruts M, et al. High-density SNP haplotyping suggests altered regulation of tau gene expression in progressive supranuclear palsy. Hum Mol Genet 2005;14:3281–3292. [DOI] [PubMed] [Google Scholar]
- 13.Myers AJ, Pittman AM, Zhao AS, et al. The MAPT H1c risk haplotype is associated with increased expression of tau and especially of 4 repeat containing transcripts. Neurobiol Dis 2007;25:561–570. [DOI] [PubMed] [Google Scholar]
- 14.Golbe LI, Rubin RS, Cody RP, et al. Follow-up study of risk factors in progressive supranuclear palsy. Neurology 1996;47:148–154. [DOI] [PubMed] [Google Scholar]
- 15.Vidal JS, Vidailhet M, Derkinderen P, de Gaillarbois TD, Tzourio C, Alpérovitch A. Risk factors for progressive supranuclear palsy: a case-control study in France. J Neurol Neurosurg Psychiatry 2009;80:1271–1274. [DOI] [PubMed] [Google Scholar]
- 16.Golbe LI. The epidemiology of progressive supranuclear palsy. Handb Clin Neurol 2008;89:457–459. [DOI] [PubMed] [Google Scholar]
- 17.Lepore FE, Steele JC, Cox TA, et al. Supranuclear disturbances of ocular motility in Lytico-Bodig. Neurology 1988;38:1849–1853. [DOI] [PubMed] [Google Scholar]
- 18.Miklossy J, Steele JC, Yu S, et al. Enduring involvement of tau, beta-amyloid, alpha-synuclein, ubiquitin and TDP-43 pathology in the amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam (ALS/PDC). Acta Neuropathol 2008;116:625–637. [DOI] [PubMed] [Google Scholar]
- 19.Winton MJ, Joyce S, Zhukareva V, et al. Characterization of tau pathologies in gray and white matter of Guam parkinsonism-dementia complex. Acta Neuropathol 2006;111:401–412. [DOI] [PubMed] [Google Scholar]
- 20.Arif M, Kazim SF, Grundke-Iqbal I, Garruto RM, Iqbal K. Tau pathology involves protein phosphatase 2A in parkinsonism-dementia of Guam. Proc Natl Acad Sci USA 2014;111:1144–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caparros-Lefebvre D, Sergeant N, Lees A, et al. Guadeloupean parkinsonism: a cluster of progressive supranuclear palsy-like tauopathy. Brain 2002;125:801–811. [DOI] [PubMed] [Google Scholar]
- 22.Caparros-Lefebvre D, Elbaz A. Possible relation of atypical parkinsonism in the French West Indies with consumption of tropical plants: a case-control study. Caribbean Parkinsonism Study Group. Lancet 1999;354:281–286. [DOI] [PubMed] [Google Scholar]
- 23.Höllerhage M, Matusch A, Champy P, et al. Natural lipophilic inhibitors of mitochondrial complex I are candidate toxins for sporadic neurodegenerative tau pathologies. Exp Neurol 2009;220:133–142. [DOI] [PubMed] [Google Scholar]
- 24.Basol Recherche. Available at: http://basol.developpement-durable.gouv.fr/politique-nationale.htm. Accessed April 12, 2015.
- 25.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP International Workshop. Neurology 1996;47:1–9. [DOI] [PubMed] [Google Scholar]
- 26.Grober E, Buschke H, Crystal H, Bang S, Dresner R. Screening for dementia by memory testing. Neurology 1988;38:900–903. [DOI] [PubMed] [Google Scholar]
- 27.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology 2000;55:1621–1626. [DOI] [PubMed] [Google Scholar]
- 28.Thomas-Danguin T, Rouby C, Sicard G, et al. Development of the ETOC: a European test of olfactory capabilities. Rhinology 2003;41:142–151. [PubMed] [Google Scholar]
- 29.Lhermitte F, Ducarne B. Plan of clinical examination of aphasic subjects. Rev Prat 1965;15:2365–2374. [PubMed] [Google Scholar]
- 30.Sergeant N, David JP, Lefranc D, Vermersch P, Wattez A, Delacourte A. Different distribution of phosphorylated tau protein isoforms in Alzheimer's and Pick's diseases. FEBS Lett 1997;412:578–582. [DOI] [PubMed] [Google Scholar]
- 31.Sergeant N, Sablonnière B, Schraen-Maschke S, et al. Dysregulation of human brain microtubule-associated tau mRNA maturation in myotonic dystrophy type 1. Hum Mol Genet 2001;10:2143–2155. [DOI] [PubMed] [Google Scholar]
- 32.Institut national de la statistique et des études économiques. Base de donees: Commune de Wattrelos. Available at: http://www.insee.fr/fr/themes/comparateur.asp?codgeo=com-59650. Accessed April 12, 2015.
- 33.Institut national de la statistique et des études économiques. Base de donees: Commune de Leers. Available at: http://www.insee.fr/fr/themes/comparateur.asp?codgeo=com-59339. Accessed April 12, 2015.
- 34.Golbe LI, Ohman-Strickland PA. A clinical rating scale for progressive supranuclear palsy. Brain 2007;130:1552–1565. [DOI] [PubMed] [Google Scholar]
- 35.Williams DR, de Silva R, Paviour DC, et al. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson's syndrome and PSP-parkinsonism. Brain 2005;128:1247–1258. [DOI] [PubMed] [Google Scholar]
- 36.Litvan I, Hauw JJ, Bartko JJ, et al. Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol 1996;55:97–105. [DOI] [PubMed] [Google Scholar]
- 37.Arpadjan S, Celik G, Taşkesen S, Güçer S. Arsenic, cadmium and lead in medicinal herbs and their fractionation. Food Chem Toxicol 2008;46:2871–2875. [DOI] [PubMed] [Google Scholar]
- 38.Javied S, Waheed S, Siddique N, Chaudhry MM, Irfan N, Tufail M. Arsenic pollution from phosphogypsum produced at Multan, Pakistan. Nucleus 2009;46:219–224. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.