Abstract
Objective:
To contrast the relationships of hormonal eating peptides and hypothalamic volumes to eating behavior and metabolic changes (body mass index [BMI]) in behavioral variant frontotemporal dementia (bvFTD) and semantic variant primary progressive aphasia (svPPA).
Methods:
Seventy-five patients with dementia (19 bvFTD, 26 svPPA, and 30 Alzheimer disease dementia) and 23 controls underwent fasting blood analyses of leptin, ghrelin, cholecystokinin, peptide tyrosine tyrosine (PYY), and agouti-related peptide (AgRP) levels. On brain MRI anterior, posterior, and total hypothalamic volumes were measured. Relationships between endocrine measures, hypothalamic volumes, eating behaviors, and BMI were investigated.
Results:
Levels of AgRP were higher in patients with bvFTD (69 ± 89 pg/mL) and svPPA (62 ± 81 pg/mL) compared with controls (23 ± 19 pg/mL, p < 0.01). No differences were found for leptin, oxytocin, cholecystokinin, ghrelin, and PYY levels. Patients with bvFTD and svPPA had higher scores on questionnaires measuring eating behaviors. Atrophy of the posterior and total hypothalamus was observed in the bvFTD group only. Linear regression modeling revealed that leptin and AgRP levels predicted BMI.
Conclusion:
Eating abnormalities are multifactorial in FTD. In bvFTD, they are in part related to hypothalamic degeneration, with potential disintegration of the network connections between the hypothalamus and orbitofrontal cortex/reward pathways. In svPPA, although hypothalamic volumes are preserved, this group experiences elevated AgRP levels similar to bvFTD, which predicts BMI in both groups. This finding highlights the potential key role of AgRP in eating and metabolic changes and provides a potential target for treatment to modify disease progression.
Alterations in eating behavior are one of the diagnostic criteria for behavioral variant frontotemporal dementia (bvFTD).1–3 It is increasingly recognized that such changes are also present in semantic variant primary progressive aphasia (svPPA).1,2
Eating changes in FTD have effects on metabolism and may affect disease progression and prognosis.4 The underlying pathophysiology of these eating changes and the possibility of differences between bvFTD and svPPA have not been systematically investigated. One study5 has suggested that low ghrelin and cortisol levels and high leptin levels observed in bvFTD represent compensatory mechanisms to the excess eating triggered by brain pathology involving the orbitofrontal cortex and the posterior hypothalamus.6,7
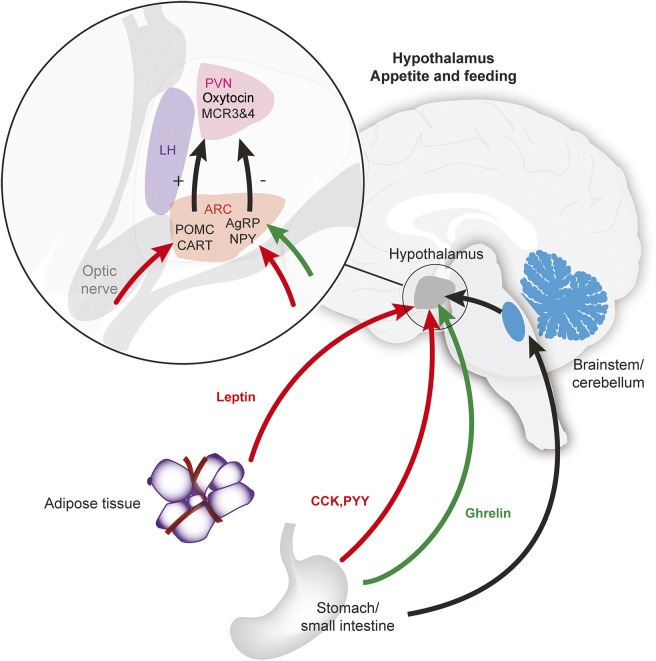
The hypothalamus is involved in 2 major pathways that mediate appetite and eating behavior (figure 1): an appetite-stimulating pathway, with key peptides including ghrelin and agouti-related peptide (AgRP), and an appetite-suppressing pathway, with key peptides including leptin.8 Oxytocin, which is produced in the hypothalamus, is an important component of the pathways activated by leptin, and is believed to decrease food intake.9 Other peptides released peripherally include peptide tyrosine tyrosine (PYY) and cholecystokinin (CCK), and participate by inhibiting food intake. In contrast, AgRP has been found to be a stimulator of increased food intake, by likely antagonism of melanocortin receptor subtypes 3 and 4.10
Figure 1. Peripheral and central pathways and the hypothalamus, controlling appetite and feeding behavior.
Interaction of peripheral and central factors controlling appetite. Appetite-stimulating pathway (shown in green) results from ghrelin being released peripherally and targeting neurons of the arcuate nucleus (ARC) of the hypothalamus that contain neuropeptide Y (NPY) and agouti-related peptide (AgRP). An appetite-suppressing pathway involves leptin (shown in red) being released from peripheral adipocytes, which then acts on pro-opiomelanocortin (POMC) and the cocaine- and amphetamine-related transcript (CART) neurons. Peptide tyrosine tyrosine (PYY) and cholecystokinin (CCK), released peripherally, also suppress appetite. AgRP, NPY, POMC, and CART neurons project to act on melanocortin receptors (MCR). POMC is cleaved into α- and β-melanocyte-stimulating hormones that act on melanocortin receptor subtypes 3 and 4 (MCR 3 and 4) to decrease food intake. AgRP stimulates food intake by antagonism of MCR 3 and 4 receptors. Autonomic pathways (black arrow) are also involved in food intake through projections via the brainstem and cerebellum to the hypothalamus. LH = lateral hypothalamus; PVN = paraventricular nucleus.
An approximately 7-fold increase in CSF AgRP has previously been identified in patients with FTD who have TAR DNA-binding protein 43 (TDP-43) pathology compared to those with tau pathology, an increase associated with the svPPA phenotype.11 Changes in peripheral eating hormones have not been comprehensively investigated in svPPA, despite the increasing evidence of eating and metabolic abnormalities in this subtype.1,4 In this study, we examined peripheral eating hormonal peptides and hypothalamic volumes in relation to eating behavior and metabolic changes (body mass index [BMI]) in bvFTD vs svPPA. We hypothesized that eating disturbance would be related to abnormal hormonal peptide levels and hypothalamic atrophy. Understanding such pathophysiologic changes is necessary for developing potential therapies for abnormal eating behaviors.
METHODS
Patients.
Seventy-five patients with dementia (19 bvFTD, 26 svPPA, 30 Alzheimer disease [AD] dementia) were recruited from FRONTIER, the frontotemporal dementia clinic at Neuroscience Research Australia. All patients underwent a comprehensive assessment (neurologic and cognitive assessment, structural brain MRI) and met current clinical diagnostic criteria for probable bvFTD, svPPA, or AD dementia.3,12–14 Disease severity was established using the Frontotemporal Dementia Rating Scale (FRS)15 and the Clinical Dementia Rating (CDR).16 In addition, 23 healthy controls (spouses of patients or recruited from a panel of healthy study volunteers) were included in the study. Healthy controls scored above 88 of 100 on the Addenbrooke's Cognitive Examination–Revised17 and 0 on the CDR.16 Exclusion criteria included significant extrapyramidal features, history of stroke, epilepsy, alcoholism, significant traumatic brain injury, or presence of ferrous metal implants in the body. Patients with an uncertain diagnosis, or where a carer was not available, were excluded.
Eating behavior and physical measurements.
Eating behavior.
Carers completed the Appetite and Eating Habits Questionnaire (APEHQ)1,2 and Cambridge Behavioural Inventory (CBI).18 The APEHQ comprises 34 questions examining changes in eating behaviors (swallowing, appetite, eating habits, food preference, and other oral behaviors). Carers rated frequency on a 5-point Likert scale, ranging from 0 (never) to 4 (daily or continuously), and severity on a 4-point Likert scale, ranging from 0 (not applicable) to 3 (marked) for each behavior. A composite frequency × severity score was calculated for each question, as well as an overall score. The 4 questions from the CBI related to eating behavior (sweet preference, same foods, change in appetite, and table manners) were also analyzed.
Clinical assessment.
Height and weight were measured (shoes removed) and BMI (in kg/m2) was derived. Blood samples were obtained following a 10-hour fast. Sixteen milliliters of blood was collected in 2 serum separating tubes, centrifuged at 3,500 rpm for 10 minutes after resting for 30 minutes. A portion of the serum sample was frozen at −80°C for batch analysis using ELISA technique for leptin, CCK, ghrelin, and AgRP.
An additional sample of 4 mL of blood was collected in an ethylenediaminetetraacetic acid tube. To inhibit protein degradation, 260 μL of aprotinin–bovine (serine protease inhibitor) was added to the whole blood, as was 40 μL of Ile-Pro-Ile (an inhibitor of dipeptidyl peptidase IV). The sample with inhibitors was centrifuged immediately at 3,500 rpm for 10 minutes, then the plasma was extracted and snap-frozen by immersion in liquid nitrogen. The sample was then stored frozen at −80°C for batch analysis exclusively of PYY and oxytocin. The samples were assayed using quantitative ELISA and enzyme immunoassay techniques. Ten percent of the cohort was duplicated in each assay to account for intra-assay variations. Percent coefficient of variation <10% was accepted as a valid assay.
Measurement of eating peptides.
The concentration of leptin in human serum was measured using quantitative sandwich ELISA techniques (Quantikine; R&D Systems, Minneapolis, MN). The optical density of the peptide was measured at 450 nm and the concentration of human leptin was obtained in pg/mL. CCK, ghrelin, and human PYY peptides in human serum was measured using competitive enzyme immunoassay techniques (Sigma-Aldrich, St. Louis, MO). The absorbance was measured at 450 nm and the concentration of the peptide in serum was measured in pg/mL for CCK and PYY and ng/mL for ghrelin. The concentration of oxytocin in human plasma was measured using competitive ELISA techniques (Enzo Life Sciences, Farmingdale, NY). A polyclonal antibody was used to bind to oxytocin in order to determine the concentrations. The absorbance of the end product was measured at 450 nm with wavelength correction at 590 nm. The concentration of oxytocin was measured in pg/mL.
Human AgRP was measured using quantitative “sandwich” ELISA techniques (Aviva Systems Biology, San Diego, CA). The optical density of the end product was measured at 450 nm. The concentration of the AgRP peptide in human serum was measured in pg/mL.
Imaging methods.
High-resolution (voxel size 1 mm3), 3-dimensional, T1 MRIs were acquired to perform morphometric analyses of the hypothalamus. A second sequence (dual T2 images) was collected to measure intracranial volume to correct for interindividual head size difference. The hypothalamus was manually traced on the T1 images in the coronal plane using previously published, well-defined boundaries.7 The hypothalamus was defined into 2 equal volumes in the anterior and posterior axis. Anterior and posterior hypothalamus volumes were expressed as a proportion of intracranial volume to correct for individual and sex differences in brain size.
Standard protocols approvals, registrations, and patient consents.
This study was approved by the South Eastern Sydney Area Health District and the University of New South Wales human ethics committees. Written informed consent was obtained from the participants and/or primary carers.
Data analysis.
Data were analyzed using SPSS statistics version 21.0 (IBM Corp., Armonk, NY). Kolmogorov-Smirnov tests were run to determine suitability of variables for parametric analyses. Analyses of variance, followed by Tukey post hoc tests, were used to determine group differences for the demographic/clinical (age, Addenbrooke's Cognitive Examination–Revised [ACE-R], FRS), eating (APEHQ, CBI eating, BMI), and brain volumetric (anterior, posterior, and total hypothalamic volumes) variables. Because of nonnormal distribution, group differences in blood concentration of the various peptides were analyzed using Kruskal-Wallis tests followed by post hoc Mann–Whitney U tests corrected for multiple comparisons (p ≤ 0.01 regarded as significant). Spearman correlations were performed to examine the relations of blood peptides, hypothalamic volumes, BMI, eating questionnaire scores, and FRS scores. The relationships between key peptides and hypothalamic volumes with eating behavior and BMI were further explored using multiple linear regression analyses, using hierarchical and enter regression models. Differences in frequency patterns of categorical variables (e.g., sex and global CDR scores) were examined with χ2 tests and post hoc Fisher exact tests (p < 0.05 regarded as significant).
RESULTS
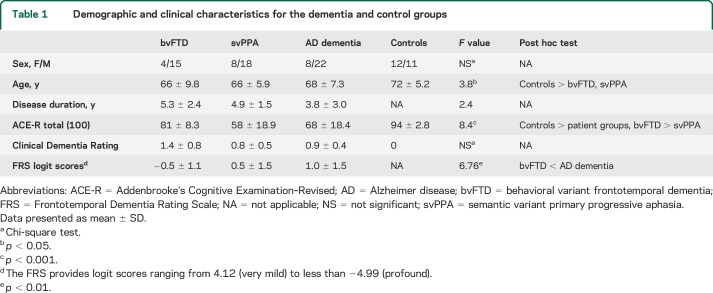
Participant groups did not differ in sex distribution or disease duration (table 1). The control group was older than the bvFTD (p = 0.040) and svPPA (p = 0.014) groups. On the ACE-R, patient groups scored lower than controls, as did the svPPA compared with the bvFTD group (p < 0.001). No group differences were detected on global scores of the CDR (p = 0.102). Finally, the bvFTD group was more functionally impaired relative to the AD dementia group (FRS; p < 0.001).
Table 1.
Demographic and clinical characteristics for the dementia and control groups
Eating behavior and physical measurements.
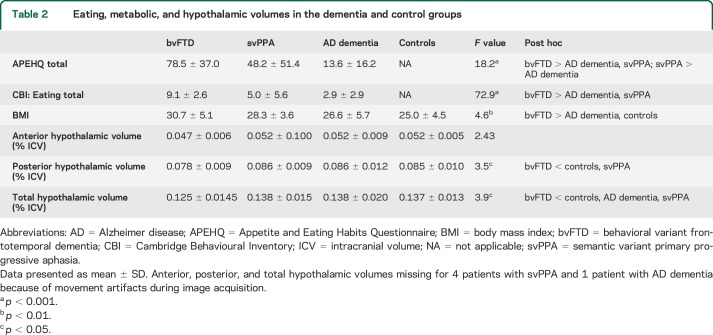
Group differences were present on measures of eating behavior (table 2). The patients with bvFTD showed higher eating disturbance on the APEHQ relative to the AD dementia (p < 0.001) and svPPA (p = 0.029) groups. The svPPA group also scored higher than the AD dementia group (p = 0.003). Similarly, on the CBI eating subscale, the bvFTD group scored higher compared with AD dementia (p < 0.001) and svPPA (p = 0.007) groups. BMI differed across groups, with higher BMI in patients with bvFTD compared to patients with AD dementia (p = 0.033) and to controls (p = 0.003) but there was no difference between bvFTD and svPPA groups.
Table 2.
Eating, metabolic, and hypothalamic volumes in the dementia and control groups
Eating peptide levels.
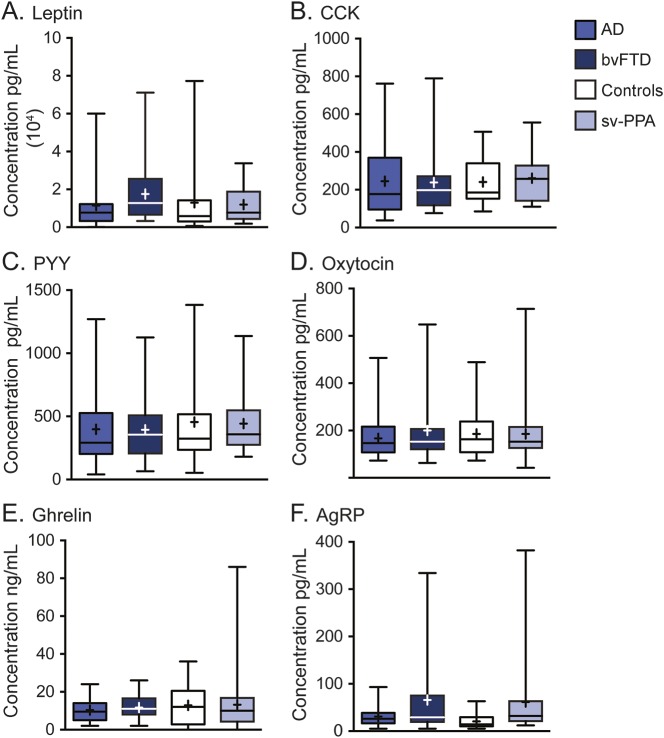
The blood levels for leptin, CCK, ghrelin, PYY, and oxytocin did not differ across groups (figure 2, table e-1 on the Neurology® Web site at Neurology.org). In contrast, group differences were present for AgRP (H [3] = 9.2, p = 0.026), with the bvFTD (mean = 69 ± 89 pg/mL; U = 129, p = 0.010) and svPPA (mean = 62 ± 81 pg/mL; U = 125.0, p = 0.004) groups having elevated levels compared with controls (mean = 23 ± 19). The level of AgRP was also elevated compared with the AD dementia group (33 ± 25) but did not reach significance.
Figure 2. Eating hormone levels in patient and control groups.
Serum leptin (A), serum CCK (B), plasma PYY (C), plasma oxytocin (D), serum ghrelin (E), and serum AgRP levels (F). Results shown as box plots: line = median; + = mean; whiskers = maximum and minimum values. AD = Alzheimer disease; AgRP = agouti-related peptide; bvFTD = behavioral variant frontotemporal dementia; CCK = cholecystokinin; PYY = peptide tyrosine tyrosine; svPPA = semantic variant primary progressive aphasia.
Hypothalamic volumes.
Posterior (F3,87 = 3.47, p = 0.020) and total (F3,87 = 3.92, p = 0.01) hypothalamic volumes differed across groups, but not the anterior hypothalamic volume (F3,87 = 2.43, p = 0.076) (table 2). The bvFTD group had smaller posterior and total hypothalamic volumes than the control (p = 0.036) and svPPA groups (p = 0.025), and smaller total volume than the AD dementia group (p = 0.046).
Correlations.
For all participants combined, smaller posterior hypothalamic volumes correlated with increasing CBI eating scores (rs = −0.271, p = 0.035). Leptin levels correlated with BMI values (rs = 0.579, p < 0.001). AgRP levels correlated with BMI (rs = 0.214, p = 0.048) and functional impairment on the FRS (rs = −0.266, p = 0.032). Total APEHQ (rs = −0.652, p < 0.001) and total CBI eating scores (rs = −0.629, p < 0.001) also correlated with the FRS with functional impairment associated with greater abnormal eating behavior. Total eating CBI scores also correlated with BMI (rs = 0.279, p = 0.022). The APEHQ correlated to the CBI eating scores (rs = 0.801, p < 0.001).
Linear regression modeling.
In a model considering all study participants, leptin (β = 0.642, p < 0.001) and AgRP (β = 0.191, p = 0.048) emerged as predictors of BMI, explaining 45% of the score variance. No other endocrine or hypothalamic volumes contributed to the model. A diagnosis of bvFTD or svPPA did not modify the contribution to the model. In a second model, the endocrine and hypothalamic measures did not predict eating behaviors (CBI eating and APEHQ); nevertheless, for CBI eating scores, a diagnosis of bvFTD was associated with an increase (β = 0.332, p = 0.005) in eating disturbance, explaining 19% of the score variance. This effect was not observed for APEHQ.
DISCUSSION
In this study examining changes in eating peptides and hypothalamic volumes and their relations to eating behavior and BMI in FTD, elevated levels of fasting AgRP were found in both bvFTD and svPPA. In contrast, levels of other peptides involved in the regulation of eating behavior (fasting leptin, oxytocin, CCK, PYY, and ghrelin) did not differ across groups. While an elevated leptin concentration was observed in the bvFTD group, this did not reach statistical significance. Leptin and AgRP levels were found to predict BMI levels, and smaller posterior hypothalamic volumes were found to correlate with higher total CBI eating scores.
Existing studies investigating eating behavior in bvFTD have implicated a number of important brain regions: the lateral orbitofrontal cortex,19 right orbitofrontal-insular-striatal structures,20 right hemisphere reward circuit regions, and the hypothalamus.6 Here, we found marked atrophy in posterior and total hypothalamic volumes in bvFTD. These findings suggest a potential contribution of the hypothalamus in regulating eating behavior in bvFTD previously uncovered in a combined in vivo and postmortem investigation.7 That study showed that posterior hypothalamic atrophy with associated neuronal loss, but intact eating peptide neurons, was most pronounced in bvFTD cases with TDP-43 deposition and not in the cases with tau deposition.7
The current study is the first to demonstrate that posterior hypothalamic atrophy is specific to bvFTD only, and is not present in svPPA. This finding indicates that hypothalamic atrophy is therefore not directly related to TDP-43 pathology per se, the predominant pathology in svPPA,21 but may relate to the cellular structures depositing the TDP-43 (affecting neuronal cell bodies in bvFTD but only large processes, such as dendrites or axons, in svPPA).22 This concept aligns with the APEHQ and CBI eating scores, showing that while abnormal eating behaviors are present in svPPA, they are not as pronounced as in bvFTD.1
Peptides that control eating behavior have received limited attention in dementia. Decreased ghrelin and cortisol levels, as well as high insulin levels, have been previously reported in bvFTD.5 Insulin levels, however, were not related to BMI, which could reflect a state of insulin resistance and metabolic change,4 rather than a role in controlling satiety. High leptin levels were also observed in a small subset of patients with bvFTD (n = 5) who overate.5 Another study failed to find significant differences in leptin levels between patients with FTD and those with AD dementia, although the authors suggested that hyperphagic females with FTD may have higher leptin levels.23 This finding seems paradoxical to the eating behaviors seen in bvFTD, as leptin inhibits food intake.24 Thus, this evidence, together with our findings relating leptin to BMI, indicate that leptin levels may be a marker of increased adipocyte mass,25 a consequence of the eating behavior, rather than promoting abnormal eating behavior.
Both bvFTD and svPPA showed elevated levels of AgRP, with AgRP levels being associated with BMI. AgRP is known to be a strong promoter of food intake.10 Administration of AgRP intracerebroventricularly in rats results in hyperphagia that can last up to 7 days,26 potentially resulting from inhibition of melanocortin receptors that mediate the activity of the appetite-suppressing pathway. In addition to increasing total food intake in rats, AgRP may also lead to a preference for fat-enriched food,27 and sucrose in the setting of a high-fat diet.28 One previous study11 in FTD has suggested that elevated AgRP in CSF may be related to TDP-43 pathology, a finding likely driven by the numbers of patients with svPPA within that cohort; however, this study did not examine the relationship of this peptide to eating behavior or effect on BMI. The current study points toward an important role of AgRP in the eating abnormalities and metabolic changes4 possibly by promoting hyperphagia, particularly affecting BMI. This provides a potential avenue of research to target this peptide through the use of biological agents. Of note, while AgRP levels predicted BMI, they were not directly associated with eating behavior per se, possibly reflecting the relatively small sample size and large measure variance. This finding also suggests that its site of action is at a central level driving peripheral biological factors such as BMI, and through interactions with other behavioral variables (e.g., disinhibition) and brain structures (e.g., orbitofrontal cortex), affects eating behavior.
The findings of high AgRP levels along with posterior and total hypothalamic atrophy in bvFTD provide important insights into the different pathologic processes underlying eating behavior in bvFTD and svPPA. In bvFTD, hypothalamic atrophy suggests a number of possible mechanisms that involve the hypothalamic nuclei controlling feeding behavior, including the arcuate and paraventricular nuclei, which both contain AgRP neurons and lateral hypothalamic nuclei. The posterior hypothalamus also contains nuclei controlling autonomic function. Autonomic dysregulation, which is associated with altered responses to hunger and satiety,29 has been found to be increased in bvFTD.30,31 In addition, the hypothalamus has functional projections to the striatum, thalamus, brainstem, orbitofrontal cortex, middle and posterior cingulum, and temporal brain regions.32 Recent animal studies have also shown that the lateral hypothalamus connects to the reward centers in the ventral tegmental area, which may mediate sucrose preference.33 The hypothalamus is therefore densely connected to many of the areas involved in reward processing that may also influence eating behaviors.32 Many of these brain regions also undergo pathologic changes in bvFTD,32,34 potentially disrupting reward feedback between these centers and the hypothalamus, leading to changes in eating behavior. The hypothalamus is also involved in a number of other functions including pain, temperature regulation,30 and sleep,35 all of which are dysregulated in bvFTD, reinforcing the pivotal role for the hypothalamus underlying bvFTD symptomatology.
In svPPA, the absence of hypothalamic atrophy suggests that the high AgRP levels interact with intact hypothalamic structures and that changes in eating behavior are peripherally driven or may involve additional brain structures and reward centers. This may account for the differences observed in eating behavior between bvFTD and svPPA, including the rigid food choices. These findings highlight the multifactorial nature of eating abnormalities in FTD.
Further research is required to delineate the functional architecture of these neural networks and how differential disruption of the networks relates to eating disturbances in FTD. Future studies will need to examine changes in hormonal peptide levels and hypothalamic volumes over the course of the disease. Similarly, research will be required to determine the relationships between AgRP and other metabolic variables including glycemic control and diurnal variation, which may have contributed to the large within-group variances in AgRP levels observed in this study. We suggest that convergent approaches synthesizing ecologically valid tasks with neuroimaging metrics will prove pivotal in clarifying neural substrates of eating network deficits. The changes in peripheral hormones (AgRP) and the direct relationship to high BMI (AgRP and leptin) suggest that biological agents targeting AgRP and/or leptin may assist in modifying metabolic changes associated with overeating.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge the assistance of Prof. Sadaf Farooqi, Department of Metabolic Medicine, University of Cambridge, for helpful discussions, Ms. Heidi Cartwright for assistance with figures, Dr. Yue Huang and Ms. Mia McMillan for assistance with blood processing and laboratory aspects, and Prof. Katherine Samaras for assistance with assays.
GLOSSARY
- ACE-R
Addenbrooke's Cognitive Examination–Revised
- AD
Alzheimer disease
- AgRP
agouti-related peptide
- APEHQ
Appetite and Eating Habits Questionnaire
- BMI
body mass index
- bvFTD
behavioral variant frontotemporal dementia
- CBI
Cambridge Behavioural Inventory
- CCK
cholecystokinin
- CDR
Clinical Dementia Rating
- FRS
Frontotemporal Dementia Rating Scale
- FTD
frontotemporal dementia
- PYY
peptide tyrosine tyrosine
- svPPA
semantic variant primary progressive aphasia
- TDP-43
TAR DNA-binding protein 43
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Rebekah M. Ahmed: study concept, data analyses, manuscript preparation, and writing. Sahar Latheef: blood analyses, manuscript preparation, and writing. Lauren Bartley: data acquisition and manuscript preparation. Muireann Irish: data analyses, manuscript preparation, and writing. Glenda Halliday: data analyses, manuscript preparation. Matthew Kiernan: manuscript preparation and writing. John R. Hodges: manuscript preparation, writing, and study concept. Olivier Piguet: study concept, data analyses, manuscript preparation, and writing.
STUDY FUNDING
This work was supported by funding to ForeFront, a collaborative research group dedicated to the study of frontotemporal dementia and motor neuron disease, from the National Health and Medical Research Council of Australia (NHMRC) program grant (1037746 to G.H., M.K., and J.H.) and the Australian Research Council Centre of Excellence in Cognition and Its Disorders Memory Node (CE110001021 to O.P. and J.H.) and other grants/sources (NHMRC project grant 1003139). The authors are grateful to the research participants involved with the ForeFront research studies. R.M.A. is a Royal Australasian College of Physicians PhD scholar and Motor Neurone Disease Research Institute of Australia (MNDRIA) PhD scholar. G.H. is an NHMRC Senior Principal Research Fellow (630434). M.I. is an ARC Discovery Early Career Researcher Award Fellow (DE130100463). O.P. is an NHMRC Career Development Research Fellow (1022684).
DISCLOSURE
R. Ahmed, S. Latheef, L. Bartley, M. Irish, and G. Halliday report no disclosures relevant to the manuscript. M. Kiernan is editor of the Journal of Neurology, Neurosurgery & Psychiatry. J. Hodges and O. Piguet report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Ahmed RM, Irish M, Kam J, et al. Quantifying the eating abnormalities in frontotemporal dementia. JAMA Neurol 2014;71:1540–1546. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda M, Brown J, Holland AJ, Fukuhara R, Hodges JR. Changes in appetite, food preference, and eating habits in frontotemporal dementia and Alzheimer's disease. J Neurol Neurosurg Psychiatry 2002;73:371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed RM, MacMillan M, Bartley L, et al. Systemic metabolism in frontotemporal dementia. Neurology 2014;83:1812–1818. [DOI] [PubMed] [Google Scholar]
- 5.Woolley JD, Khan BK, Natesan A, et al. Satiety-related hormonal dysregulation in behavioral variant frontotemporal dementia. Neurology 2014;82:512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perry DC, Sturm VE, Seeley WW, Miller BL, Kramer JH, Rosen HJ. Anatomical correlates of reward-seeking behaviours in behavioural variant frontotemporal dementia. Brain 2014;137:1621–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piguet O, Petersen A, Yin Ka Lam B, et al. Eating and hypothalamus changes in behavioral-variant frontotemporal dementia. Ann Neurol 2011;69:312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaptini L, Peikin S. Neuroendocrine regulation of food intake. Curr Opin Gastroenterol 2008;24:223–229. [DOI] [PubMed] [Google Scholar]
- 9.Sabatier N, Leng G, Menzies J. Oxytocin, feeding, and satiety. Front Endocrinol 2013;4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stutz AM, Morrison CD, Argyropoulos G. The agouti-related protein and its role in energy homeostasis. Peptides 2005;26:1771–1781. [DOI] [PubMed] [Google Scholar]
- 11.Hu WT, Chen-Plotkin A, Grossman M, et al. Novel CSF biomarkers for frontotemporal lobar degenerations. Neurology 2010;75:2079–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKhann GM, Albert MS, Grossman M, et al. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol 2001;58:1803–1809. [DOI] [PubMed] [Google Scholar]
- 14.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mioshi E, Hsieh S, Savage S, Hornberger M, Hodges JR. Clinical staging and disease progression in frontotemporal dementia. Neurology 2010;74:1591–1597. [DOI] [PubMed] [Google Scholar]
- 16.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 17.Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrooke's Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry 2006;21:1078–1085. [DOI] [PubMed] [Google Scholar]
- 18.Bozeat S, Gregory CA, Ralph MA, Hodges JR. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer's disease? J Neurol Neurosurg Psychiatry 2000;69:178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitwell JL, Sampson EL, Loy CT, et al. VBM signatures of abnormal eating behaviours in frontotemporal lobar degeneration. Neuroimage 2007;35:207–213. [DOI] [PubMed] [Google Scholar]
- 20.Woolley JD, Gorno-Tempini ML, Seeley WW, et al. Binge eating is associated with right orbitofrontal-insular-striatal atrophy in frontotemporal dementia. Neurology 2007;69:1424–1433. [DOI] [PubMed] [Google Scholar]
- 21.Josephs KA, Hodges JR, Snowden JS, et al. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol 2011;122:137–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rohrer JD, Lashley T, Schott JM, et al. Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain 2011;134:2565–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alberici A, Bocchio L, Geroldi C, et al. Serum leptin levels are higher in females affected by frontotemporal lobar degeneration than Alzheimer's disease. J Neurol Neurosurg Psychiatry 2008;79:712–715. [DOI] [PubMed] [Google Scholar]
- 24.Coll AP, Farooqi IS, O'Rahilly S. The hormonal control of food intake. Cell 2007;129:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruhl CE, Harris TB, Ding J, et al. Body mass index and serum leptin concentration independently estimate percentage body fat in older adults. Am J Clin Nutr 2007;85:1121–1126. [DOI] [PubMed] [Google Scholar]
- 26.Hagan MM, Rushing PA, Pritchard LM, et al. Long-term orexigenic effects of AgRP-(83–132) involve mechanisms other than melanocortin receptor blockade. Am J Physiol Regul Integr Comp Physiol 2000;279:R47–R52. [DOI] [PubMed] [Google Scholar]
- 27.Tracy AL, Clegg DJ, Johnson JD, Davidson TL, Benoit SC. The melanocortin antagonist AgRP (83–132) increases appetitive responding for a fat, but not a carbohydrate, reinforcer. Pharmacol Biochem Behav 2008;89:263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Figlewicz DP, Jay JL, Acheson MA, et al. Moderate high fat diet increases sucrose self-administration in young rats. Appetite 2013;61:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cong ND, Saikawa T, Ogawa R, Hara M, Takahashi N, Sakata T. Reduced 24 hour ambulatory blood pressure and abnormal heart rate variability in patients with dysorexia nervosa. Heart 2004;90:563–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed RM, Iodice V, Daveson N, Kiernan MC, Piguet O, Hodges JR. Autonomic dysregulation in frontotemporal dementia. J Neurol Neurosurg Psychiatry 2015;86:1048–1049. [DOI] [PubMed] [Google Scholar]
- 31.Struhal W, Javor A, Brunner C, et al. The phoenix from the ashes: cardiovascular autonomic dysfunction in behavioral variant of frontotemporal dementia. J Alzheimers Dis 2014;42:1041–1046. [DOI] [PubMed] [Google Scholar]
- 32.Kullmann S, Heni M, Linder K, et al. Resting-state functional connectivity of the human hypothalamus. Hum Brain Mapp 2014;35:6088–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nieh EH, Matthews GA, Allsop SA, et al. Decoding neural circuits that control compulsive sucrose seeking. Cell 2015;160:528–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hornberger M, Savage S, Hsieh S, Mioshi E, Piguet O, Hodges JR. Orbitofrontal dysfunction discriminates behavioral variant frontotemporal dementia from Alzheimer's disease. Dement Geriatr Cogn Disord 2010;30:547–552. [DOI] [PubMed] [Google Scholar]
- 35.Bonakis A, Economou NT, Paparrigopoulos T, et al. Sleep in frontotemporal dementia is equally or possibly more disrupted, and at an earlier stage, when compared to sleep in Alzheimer's disease. J Alzheimers Dis 2014;38:85–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.